The Natural History of CNGB1-Related Retinopathy: A Longitudinal Phenotypic Analysis
Abstract
:1. Introduction
2. Results
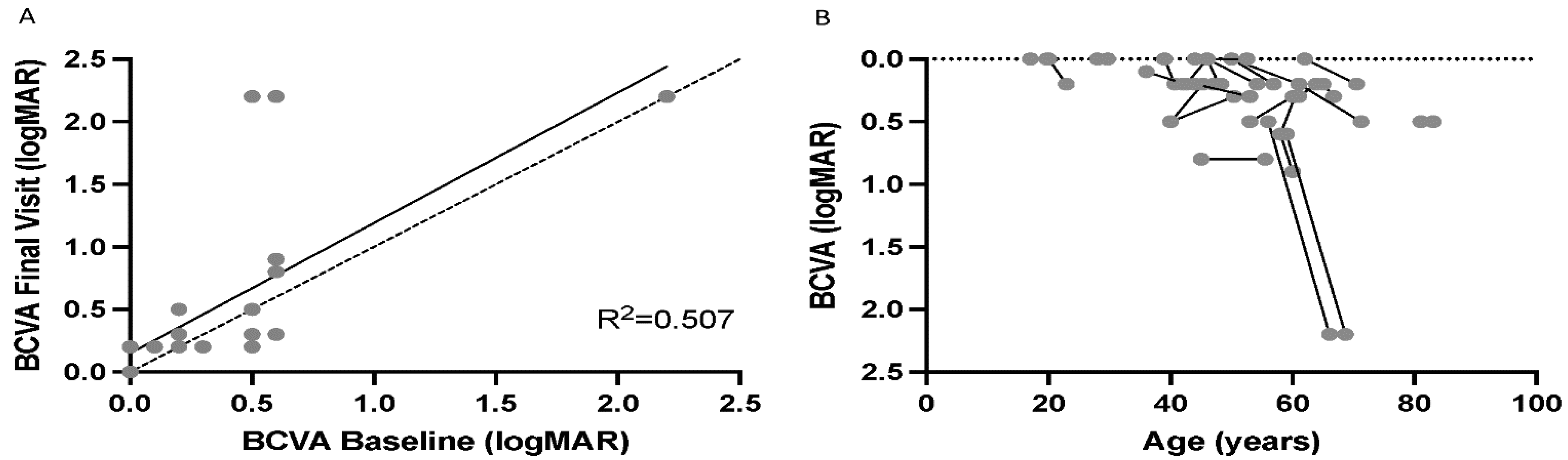
2.1. Demographics and Visual Acuity
2.2. SD-OCT Ellipsoid Zone and Associated Features
2.3. HyperAF Area
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Retinal Imaging
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Trudeau, M.C.; Zagotta, W.N. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 2002, 36, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Rich, E.D.; Varnum, M.D. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron 2004, 42, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Shuart, N.G.; Haitin, Y.; Camp, S.S.; Black, K.D.; Zagotta, W.N. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat. Commun. 2011, 2, 457. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bareil, C.; Hamel, C.P.; Delague, V.; Arnaud, B.; Demaille, J.; Claustres, M. Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum. Genet. 2001, 108, 328–334. [Google Scholar] [CrossRef]
- Ge, Z.; Bowles, K.; Goetz, K.; Scholl, H.P.; Wang, F.; Wang, X.; Xu, S.; Wang, K.; Wang, H.; Chen, R. NGS-based Molecular diagnosis of 105 eyeGENE(®) probands with Retinitis Pigmentosa. Sci. Rep. 2015, 5, 18287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Guan, L.; Shen, T.; Zhang, J.; Xiao, X.; Jiang, H.; Li, S.; Yang, J.; Jia, X.; Yin, Y.; et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum. Genet. 2014, 133, 1255–1271. [Google Scholar] [CrossRef]
- Dryja, T.P.; Finn, J.T.; Peng, Y.W.; McGee, T.L.; Berson, E.L.; Yau, K.W. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc. Natl. Acad Sci. USA 1995, 92, 10177–10181. [Google Scholar] [CrossRef] [Green Version]
- Hull, S.; Attanasio, M.; Arno, G.; Carss, K.; Robson, A.G.; Thompson, D.A.; Plagnol, V.; Michaelides, M.; Holder, G.E.; Henderson, R.H.; et al. Clinical Characterization of CNGB1-Related Autosomal Recessive Retinitis Pigmentosa. JAMA Ophthalmol. 2017, 135, 137–144. [Google Scholar] [CrossRef]
- Nassisi, M.; Smirnov, V.M.; Solis Hernandez, C.; Mohand-Saïd, S.; Condroyer, C.; Antonio, A.; Kühlewein, L.; Kempf, M.; Kohl, S.; Wissinger, B.; et al. CNGB1-related rod-cone dystrophy: A mutation review and update. Hum. Mutat. 2021, 42, 641–666. [Google Scholar] [CrossRef]
- Petersen-Jones, S.M.; Occelli, L.M.; Winkler, P.A.; Lee, W.; Sparrow, J.R.; Tsukikawa, M.; Boye, S.L.; Chiodo, V.; Capasso, J.E.; Becirovic, E.; et al. Patients and animal models of CNGβ1-deficient retinitis pigmentosa support gene augmentation approach. J. Clin. Investig. 2018, 128, 190–206. [Google Scholar] [CrossRef] [Green Version]
- Charbel Issa, P.; Reuter, P.; Kühlewein, L.; Birtel, J.; Gliem, M.; Tropitzsch, A.; Whitcroft, K.L.; Bolz, H.J.; Ishihara, K.; MacLaren, R.E.; et al. Olfactory Dysfunction in Patients with CNGB1-Associated Retinitis Pigmentosa. JAMA Ophthalmol. 2018, 136, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Ba-Abbad, R.; Holder, G.E.; Robson, A.G.; Neveu, M.M.; Waseem, N.; Arno, G.; Webster, A.R. Isolated rod dysfunction associated with a novel genotype of CNGB1. Am. J. Ophthalmol. Case Rep. 2019, 14, 83–86. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Raddadi, O.; Schatz, P.; Lenzner, S.; Neuhaus, C.; Azzam, E.; Abdelkader, E. Severe retinitis pigmentosa phenotype associated with novel CNGB1 variants. Am. J. Ophthalmol. Case Rep. 2020, 19, 100780. [Google Scholar] [CrossRef]
- Kondo, H.; Qin, M.; Mizota, A.; Kondo, M.; Hayashi, H.; Hayashi, K.; Oshima, K.; Tahira, T.; Hayashi, K. A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4433–4439. [Google Scholar] [CrossRef]
- Bocquet, B.; Marzouka, N.A.; Hebrard, M.; Manes, G.; Sénéchal, A.; Meunier, I.; Hamel, C.P. Homozygosity mapping in autosomal recessive retinitis pigmentosa families detects novel mutations. Mol. Vis. 2013, 19, 2487–2500. [Google Scholar]
- Maranhao, B.; Biswas, P.; Gottsch, A.D.; Navani, M.; Naeem, M.A.; Suk, J.; Chu, J.; Khan, S.N.; Poleman, R.; Akram, J.; et al. Investigating the Molecular Basis of Retinal Degeneration in a Familial Cohort of Pakistani Decent by Exome Sequencing. PLoS ONE 2015, 10, e0136561. [Google Scholar] [CrossRef]
- Hüttl, S.; Michalakis, S.; Seeliger, M.; Luo, D.G.; Acar, N.; Geiger, H.; Hudl, K.; Mader, R.; Haverkamp, S.; Moser, M.; et al. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J. Neurosci. 2005, 25, 130–138. [Google Scholar] [CrossRef]
- Winkler, P.A.; Ekenstedt, K.J.; Occelli, L.M.; Frattaroli, A.V.; Bartoe, J.T.; Venta, P.J.; Petersen-Jones, S.M. A large animal model for CNGB1 autosomal recessive retinitis pigmentosa. PLoS ONE 2013, 8, e72229. [Google Scholar] [CrossRef] [Green Version]
- Koch, S.; Sothilingam, V.; Garcia Garrido, M.; Tanimoto, N.; Becirovic, E.; Koch, F.; Seide, C.; Beck, S.C.; Seeliger, M.W.; Biel, M.; et al. Gene therapy restores vision and delays degeneration in the CNGB1(−/−) mouse model of retinitis pigmentosa. Hum. Mol. Genet. 2012, 21, 4486–4496. [Google Scholar] [CrossRef] [Green Version]
- Toms, M.; Dubis, A.M.; de Vrieze, E.; Tracey-White, D.; Mitsios, A.; Hayes, M.; Broekman, S.; Baxendale, S.; Utoomprurkporn, N.; Bamiou, D.; et al. Clinical and preclinical therapeutic outcome metrics for USH2A-related disease. Hum. Mol. Genet. 2020, 29, 1882–1899. [Google Scholar] [CrossRef] [Green Version]
- Hagag, A.M.; Mitsios, A.; Narayan, A.; Abbouda, A.; Webster, A.R.; Dubis, A.M.; Moosajee, M. Prospective deep phenotyping of choroideremia patients using multimodal structure-function approaches. Eye 2021, 35, 838–852. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: Literature review and model. Retina 2011, 31, 1609–1619. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.W.; Wu, Z.; Guymer, R.H.; Luu, C.D. Ellipsoid zone on optical coherence tomography: A review. Clin. Exp. Ophthalmol. 2016, 44, 422–430. [Google Scholar] [CrossRef]
- Birch, D.G.; Locke, K.G.; Wen, Y.; Locke, K.I.; Hoffman, D.R.; Hood, D.C. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013, 131, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Strampe, M.R.; Huckenpahler, A.L.; Higgins, B.P.; Tarima, S.; Visotcky, A.; Stepien, K.E.; Kay, C.N.; Carroll, J. Intraobserver Repeatability and Interobserver Reproducibility of Ellipsoid Zone Measurements in Retinitis Pigmentosa. Transl. Vis. Sci. Technol. 2018, 7, 13. [Google Scholar] [CrossRef]
- Lima, L.H.; Burke, T.; Greenstein, V.C.; Chou, C.L.; Cella, W.; Yannuzzi, L.A.; Tsang, S.H. Progressive constriction of the hyperautofluorescent ring in retinitis pigmentosa. Am. J. Ophthalmol. 2012, 153, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Csaky, K.; Ferris, F., 3rd; Chew, E.Y.; Nair, P.; Cheetham, J.K.; Duncan, J.L. Report From the NEI/FDA Endpoints Workshop on Age-Related Macular Degeneration and Inherited Retinal Diseases. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3456–3463. [Google Scholar] [CrossRef]
- Liew, G.; Strong, S.; Bradley, P.; Severn, P.; Moore, A.T.; Webster, A.R.; Mitchell, P.; Kifley, A.; Michaelides, M. Prevalence of cystoid macular oedema, epiretinal membrane and cataract in retinitis pigmentosa. Br. J. Ophthalmol. 2019, 103, 1163–1166. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Akimoto, M.; Ooto, S.; Suzuki, T.; Ikeda, H.; Kawagoe, N.; Takahashi, M.; Yoshimura, N. Association between abnormal autofluorescence and photoreceptor disorganization in retinitis pigmentosa. Am. J. Ophthalmol. 2008, 145, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Khateb, S.; Nassisi, M.; Bujakowska, K.M.; Méjécase, C.; Condroyer, C.; Antonio, A.; Foussard, M.; Démontant, V.; Mohand-Saïd, S.; Sahel, J.A.; et al. Longitudinal Clinical Follow-up and Genetic Spectrum of Patients with Rod-Cone Dystrophy Associated with Mutations in PDE6A and PDE6B. JAMA Ophthalmol. 2019, 137, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Wang, Z.; Wang, J.; Pennesi, M.E.; Yang, P.; Huang, D.; Li, D.; Jia, Y. Deep learning for the segmentation of preserved photoreceptors on en face optical coherence tomography in two inherited retinal diseases. Biomed. Opt. Express 2018, 9, 3092–3105. [Google Scholar] [CrossRef] [PubMed]
- Anikina, E.; Georgiou, M.; Tee, J.; Webster, A.R.; Weleber, R.G.; Michaelides, M. Characterization of Retinal Function Using Microperimetry-Derived Metrics in Both Adults and Children with RPGR-Associated Retinopathy. Am. J. Ophthalmol. 2021, 234, 81–90. [Google Scholar] [CrossRef]
- Buckley, T.M.W.; Jolly, J.K.; Menghini, M.; Wood, L.J.; Nanda, A.; MacLaren, R.E. Test-retest repeatability of microperimetry in patients with retinitis pigmentosa caused by mutations in RPGR. Clin. Exp. Ophthalmol. 2020, 48, 714–715. [Google Scholar] [CrossRef]
- Roman, A.J.; Cideciyan, A.V.; Wu, V.; Garafalo, A.V.; Jacobson, S.G. Full-field stimulus testing: Role in the clinic and as an outcome measure in clinical trials of severe childhood retinal disease. Prog. Retin. Eye Res. 2022, 87, 101000. [Google Scholar] [CrossRef]
- Roman, A.J.; Cideciyan, A.V.; Aleman, T.S.; Jacobson, S.G. Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol. Meas. 2007, 28, N51–N56. [Google Scholar] [CrossRef]
- Gill, J.S.; Theofylaktopoulos, V.; Mitsios, A.; Houston, S.; Hagag, A.M.; Dubis, A.M.; Moosajee, M. Investigating Biomarkers for USH2A Retinopathy Using Multimodal Retinal Imaging. Int. J. Mol. Sci. 2022, 23, 4198. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, D.J.; Dubis, A.M.; Moosajee, M. The Natural History of CNGB1-Related Retinopathy: A Longitudinal Phenotypic Analysis. Int. J. Mol. Sci. 2022, 23, 6785. https://doi.org/10.3390/ijms23126785
Jackson DJ, Dubis AM, Moosajee M. The Natural History of CNGB1-Related Retinopathy: A Longitudinal Phenotypic Analysis. International Journal of Molecular Sciences. 2022; 23(12):6785. https://doi.org/10.3390/ijms23126785
Chicago/Turabian StyleJackson, Daniel J., Adam M. Dubis, and Mariya Moosajee. 2022. "The Natural History of CNGB1-Related Retinopathy: A Longitudinal Phenotypic Analysis" International Journal of Molecular Sciences 23, no. 12: 6785. https://doi.org/10.3390/ijms23126785
APA StyleJackson, D. J., Dubis, A. M., & Moosajee, M. (2022). The Natural History of CNGB1-Related Retinopathy: A Longitudinal Phenotypic Analysis. International Journal of Molecular Sciences, 23(12), 6785. https://doi.org/10.3390/ijms23126785






