The Value of FET PET/CT in Recurrent Glioma with a Different IDH Mutation Status: The Relationship between Imaging and Molecular Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Determination of IDH Genotype
2.3. 18F-FET PET Imaging
2.4. 18F-FET PET Image Analysis
- TAC score of −1: lesions with an early peak in SUV, followed by a constant descent of activity;
- TAC score of 0: lesions with ascending SUV reaching an early peak before 22.5 min, followed by a plateau or small descent of less than 5%;
- TAC score of 1: lesions with constantly increasing SUV without an identifiable peak.
2.5. Diagnosis of TP
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.B. Predicting Clinical Outcomes Using Molecular Biomarkers. Biomark. Cancer 2016, 8, 89–99. [Google Scholar] [CrossRef]
- Huang, J.; Yu, J.; Tu, L.; Huang, N.; Li, H.; Luo, Y. Isocitrate Dehydrogenase Mutations in Glioma: From Basic Discovery to Therapeutics Development. Front. Oncol. 2019, 9, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminska, B.; Czapski, B.; Guzik, R.; Król, S.K.; Gielniewski, B. Consequences of IDH1/2 Mutations in Gliomas and an Assessment of Inhibitors Targeting Mutated IDH Proteins. Molecules 2019, 24, 968. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.Y.; Packer, R.J. The 2021 WHO Classification of Tumors of the Central Nervous System: Clinical implications. Neuro Oncol. 2021, 23, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current Clinical Brain Tumor Imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef] [Green Version]
- Chourmouzi, D.; Papadopoulou, E.; Marias, K.; Drevelegas, A. Imaging of brain tumors. Surg. Oncol. Clin. N. Am. 2014, 23, 629–684. [Google Scholar] [CrossRef]
- Verma, N.; Cowperthwaite, M.C.; Burnett, M.G.; Markey, M.K. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro Oncol. 2013, 15, 515–534. [Google Scholar] [CrossRef] [Green Version]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant glioma. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Vellayappan, B.; Tan, C.L.; Yong, C.; Khor, L.K.; Koh, W.Y.; Yeo, T.T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients with Brain Metastases. Front. Oncol. 2018, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Munier, S.; Ginalis, E.E.; Patel, N.V.; Danish, S.; Hanft, S. Radiation Necrosis in Intracranial Lesions. Cureus 2020, 12, e7603. [Google Scholar] [CrossRef] [Green Version]
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015, 17, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Santo, G.; Laudicella, R.; Linguanti, F.; Nappi, A.G.; Abenavoli, E.; Vergura, V.; Rubini, G.; Sciagrà, R.; Arnone, G.; Schillaci, O.; et al. The Utility of Conventional Amino Acid PET Radiotracers in the Evaluation of Glioma Recurrence also in Comparison with MRI. Diagnostics 2022, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.-W.; Zilles, K.; Coenen, H.H.; Langen, K.-J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Zacher, A.; Kaulich, K.; Stepanow, S.; Wolter, M.; Köhrer, K.; Felsberg, J.; Malzkorn, B.; Reifenberger, G. Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol. 2017, 27, 146–159. [Google Scholar] [CrossRef]
- Werner, J.M.; Stoffels, G.; Lichtenstein, T.; Borggrefe, J.; Lohmann, P.; Ceccon, G.; Shah, N.J.; Fink, G.R.; Langen, K.J.; Kabbasch, C.; et al. Differentiation of treatment-related changes from tumor progression: A direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Lai, C.Y.; Tian, L.; Schisterman, E.F. Exact confidence interval estimation for the Youden index and its corresponding optimal cut-point. Comput. Stat. Data Anal. 2012, 56, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Han, Y.; Chen, T.; Tu, X.M. Causal inference for Mann-Whitney-Wilcoxon rank sum and other nonparametric statistics. Stat. Med. 2014, 33, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Appin, C.L.; Brat, D.J. Molecular pathways in gliomagenesis and their relevance to neuropathologic diagnosis. Adv. Anat. Pathol. 2015, 22, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, L.; Hong, C.S.; Yang, C.; Zhuang, Z.; Heiss, J.D. New developments in the pathogenesis and therapeutic targeting of the IDH1 mutation in glioma. Int. J. Med. Sci. 2015, 12, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef]
- Turkalp, Z.; Karamchandani, J.; Das, S. IDH Mutation in Glioma: New Insights and Promises for the Future. JAMA Neurol. 2014, 71, 1319–1325. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Harmanci, A.S.; Erson-Omay, E.Z.; Li, J.; Coskun, S.; Simon, M.; Krischek, B.; Özduman, K.; Omay, S.B.; Sorensen, E.A.; et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat. Genet. 2016, 48, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.G.; Fine, H.A. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef]
- Kloosterhof, N.K.; Bralten, L.B.; Dubbink, H.J.; French, P.J.; van den Bent, M.J. Isocitrate dehydrogenase-1 mutations: A fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011, 12, 83–91. [Google Scholar] [CrossRef]
- El Saghir, N.S.; Keating, N.L.; Carlson, R.W.; Khoury, K.E.; Fallowfield, L. Tumor boards: Optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e461–e466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.J. 18F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mihovilovic, M.I.; Kertels, O.; Hänscheid, H.; Löhr, M.; Monoranu, C.M.; Kleinlein, I.; Samnick, S.; Kessler, A.F.; Linsenmann, T.; Ernestus, R.I.; et al. O-(2-(18F)fluoroethyl)-L-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J. Neurol. Neurosurg. Psychiatry 2019, 90, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Kebir, S.; Fimmers, R.; Galldiks, N.; Schäfer, N.; Mack, F.; Schaub, C.; Stuplich, M.; Niessen, M.; Tzaridis, T.; Simon, M.; et al. Late Pseudoprogression in Glioblastoma: Diagnostic Value of Dynamic O-(2-[18F]fluoroethyl)-L-Tyrosine PET. Clin. Cancer Res. 2016, 22, 2190–2196. [Google Scholar] [CrossRef] [Green Version]
- Kertels, O.; Mihovilovic, M.I.; Linsenmann, T.; Kessler, A.F.; Tran-Gia, J.; Kircher, M.; Brumberg, J.; Monoranu, C.M.; Samnick, S.; Ernestus, R.I.; et al. Clinical Utility of Different Approaches for Detection of Late Pseudoprogression in Glioblastoma With O-(2-[18F]Fluoroethyl)-L-Tyrosine PET. Clin. Nucl. Med. 2019, 44, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Zorrilla-Veloz, R.I.; Hu, J.; Guan, B.; Ma, X. Diagnostic Accuracy of PET for Differentiating True Glioma Progression From Post Treatment-Related Changes: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 671867. [Google Scholar] [CrossRef]
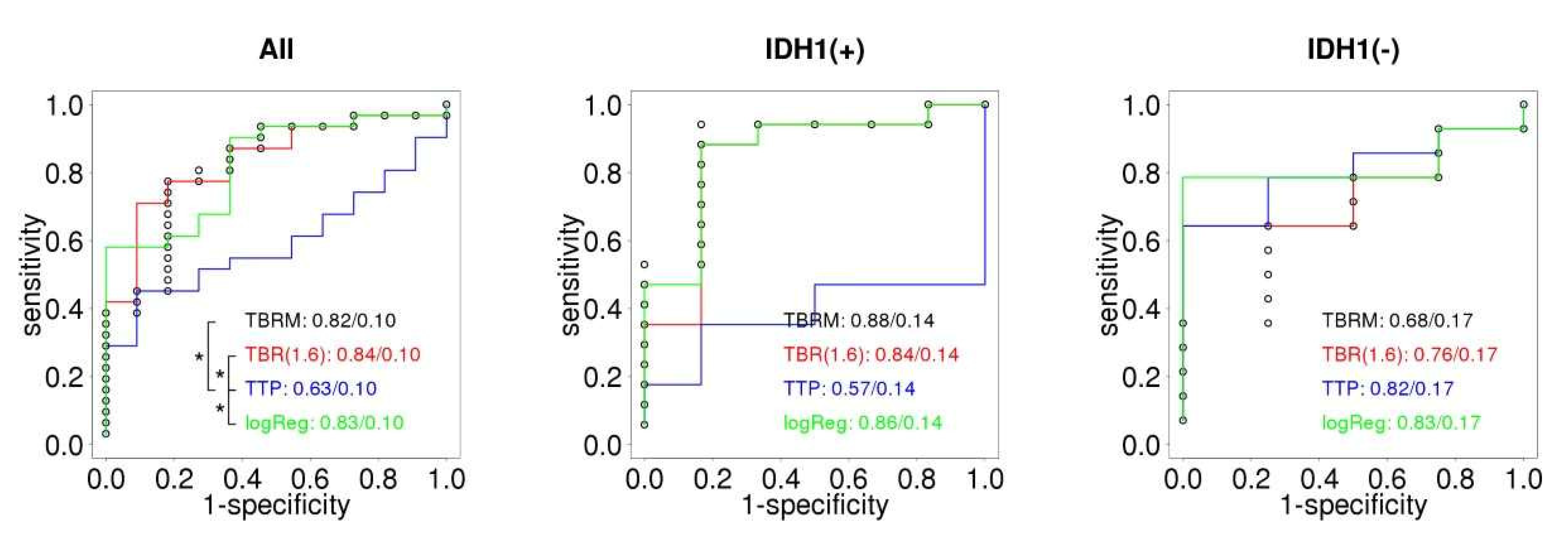
| All (N = 42) | IDHm (N = 23) | IDHwt (N = 18) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TBR | TTP | TBR | TTP | TBR | TTP | ||||||
| Max | Mean | (min) | LR | Max | Mean | (min) | LR | Max | Mean | (min) | LR |
| TP (count, mean, median, range) | |||||||||||
| N = 31 | N = 17 | N = 14 | |||||||||
| 4.1 | 2.2 | 26 | 0.8 | 4.2 | 2.2 | 30 | 0.8 | 4.0 | 2.1 | 22 | 0.8 |
| 4.0 | 2.1 | 32 | 4.1 | 2.1 | 40 | 3.8 | 2.2 | 14.5 | |||
| 1.1–8.0 | 0–3.2 | 5–40 | 0.1–1 | 2.1–6.4 | 1.7–3.2 | 7–40 | 0.3–1 | 1.1–8.0 | 0–3.1 | 7–40 | 0.4–1 |
| TRC (mean, median, range) | |||||||||||
| N = 11 | N = 6 | N = 4 | |||||||||
| 2.6 | 1.5 | 35 | 0.5 | 2.6 | 1.6 | 30 | 0.5 | 2.7 | 1.4 | 40 | 0.6 |
| 2.3 | 1.9 | 40 | 2.2 | 1.8 | 32 | 2.6 | 1.9 | 40 | |||
| 1.6–4.2 | 0–2.2 | 12–40 | 0.1–0.8 | 1.9–4.1 | 0–2.2 | 12–40 | 0–0.9 | 1.6–4.2 | 0–2.0 | 40–40 | 0.4–0.6 |
| Threshold (optimum, CI95%) | |||||||||||
| 3.03 | 2.04 | 32 | 0.79 | 3.03 | 1.96 | 32 | 0.66 | 2.9 | 2.09 | 40 | 0.65 |
| 2.6–3.4 | 1.8–2.3 | 28–36 | 0.7–0.9 | 2.6–3.4 | 1.7–2.3 | 27–37 | 0.6–0.8 | 2.2–3.6 | 1.6–2.6 | 36–40 | 0.6–0.8 |
| Sensitivity (%, value at optimum, CI95%) | |||||||||||
| 77 | 71 | 48 | 58 | 94 | 88 | 83 | 88 | 64 | 64 | 79 | 79 |
| 60–89 | 53–84 | 32–65 | 41–74 | 73–99 | 66–97 | 54–97 | 66–97 | 39–84 | 39–84 | 52–92 | 52–92 |
| Specificity (%, value at optimum, CI95%) | |||||||||||
| 82 | 91 | 91 | 100 | 83 | 83 | 53 | 83 | 75 | 100 | 100 | 100 |
| 52–92 | 62–98 | 62–98 | 74–100 | 44–97 | 44–97 | 31–74 | 44–97 | 30–95 | 51–100 | 51–100 | 51–100 |
| Accuracy (%, value at optimum, CI95%) | |||||||||||
| 79 | 76 | 60 | 69 | 91 | 87 | 61 | 87 | 67 | 72 | 83 | 83 |
| 64–88 | 61–87 | 44–73 | 54–81 | 73–98 | 68–95 | 41–78 | 68–95 | 44–84 | 49–88 | 61–94 | 61–94 |
| p-value | |||||||||||
| 0.001 | 0.001 | 0.18 | 0.002 | 0.004 | 0.01 | 0.61 | 0.01 | 0.33 | 0.14 | 0.05 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoblar Vidmar, M.; Doma, A.; Smrdel, U.; Zevnik, K.; Studen, A. The Value of FET PET/CT in Recurrent Glioma with a Different IDH Mutation Status: The Relationship between Imaging and Molecular Biomarkers. Int. J. Mol. Sci. 2022, 23, 6787. https://doi.org/10.3390/ijms23126787
Skoblar Vidmar M, Doma A, Smrdel U, Zevnik K, Studen A. The Value of FET PET/CT in Recurrent Glioma with a Different IDH Mutation Status: The Relationship between Imaging and Molecular Biomarkers. International Journal of Molecular Sciences. 2022; 23(12):6787. https://doi.org/10.3390/ijms23126787
Chicago/Turabian StyleSkoblar Vidmar, Marija, Andrej Doma, Uroš Smrdel, Katarina Zevnik, and Andrej Studen. 2022. "The Value of FET PET/CT in Recurrent Glioma with a Different IDH Mutation Status: The Relationship between Imaging and Molecular Biomarkers" International Journal of Molecular Sciences 23, no. 12: 6787. https://doi.org/10.3390/ijms23126787
APA StyleSkoblar Vidmar, M., Doma, A., Smrdel, U., Zevnik, K., & Studen, A. (2022). The Value of FET PET/CT in Recurrent Glioma with a Different IDH Mutation Status: The Relationship between Imaging and Molecular Biomarkers. International Journal of Molecular Sciences, 23(12), 6787. https://doi.org/10.3390/ijms23126787






