Eco-Sustainable Silk Fibroin/Pomegranate Peel Extract Film as an Innovative Green Material for Skin Repair
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Pomegranate Peel Extracts (EPP)
2.2. Preparation of SF-EPP Films
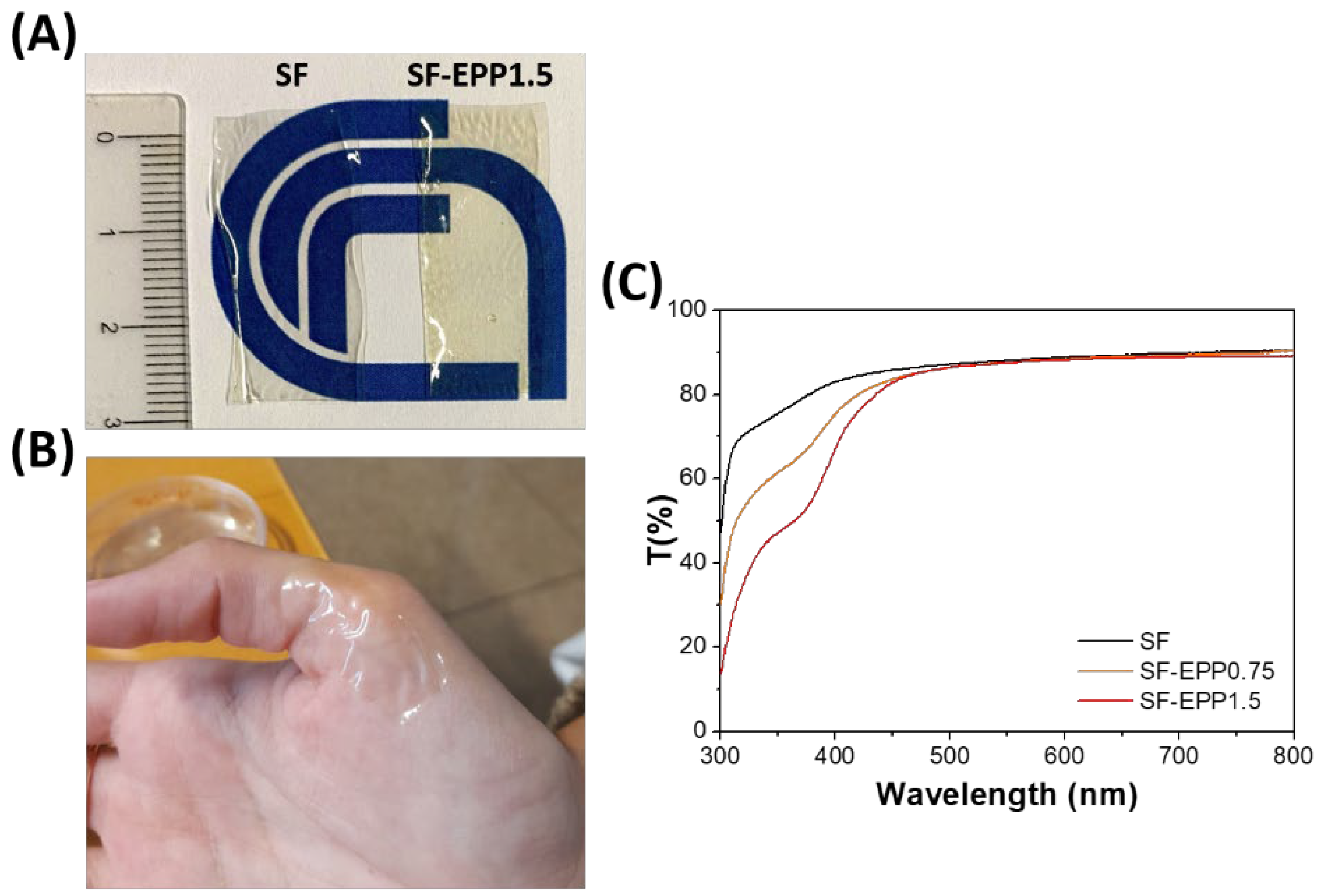
2.3. Optical and Structural Characterization of SF-EPP Films
2.4. Mechanical Properties of SF-EPP Films
2.5. Stability and Biodegradability of SF-EPP
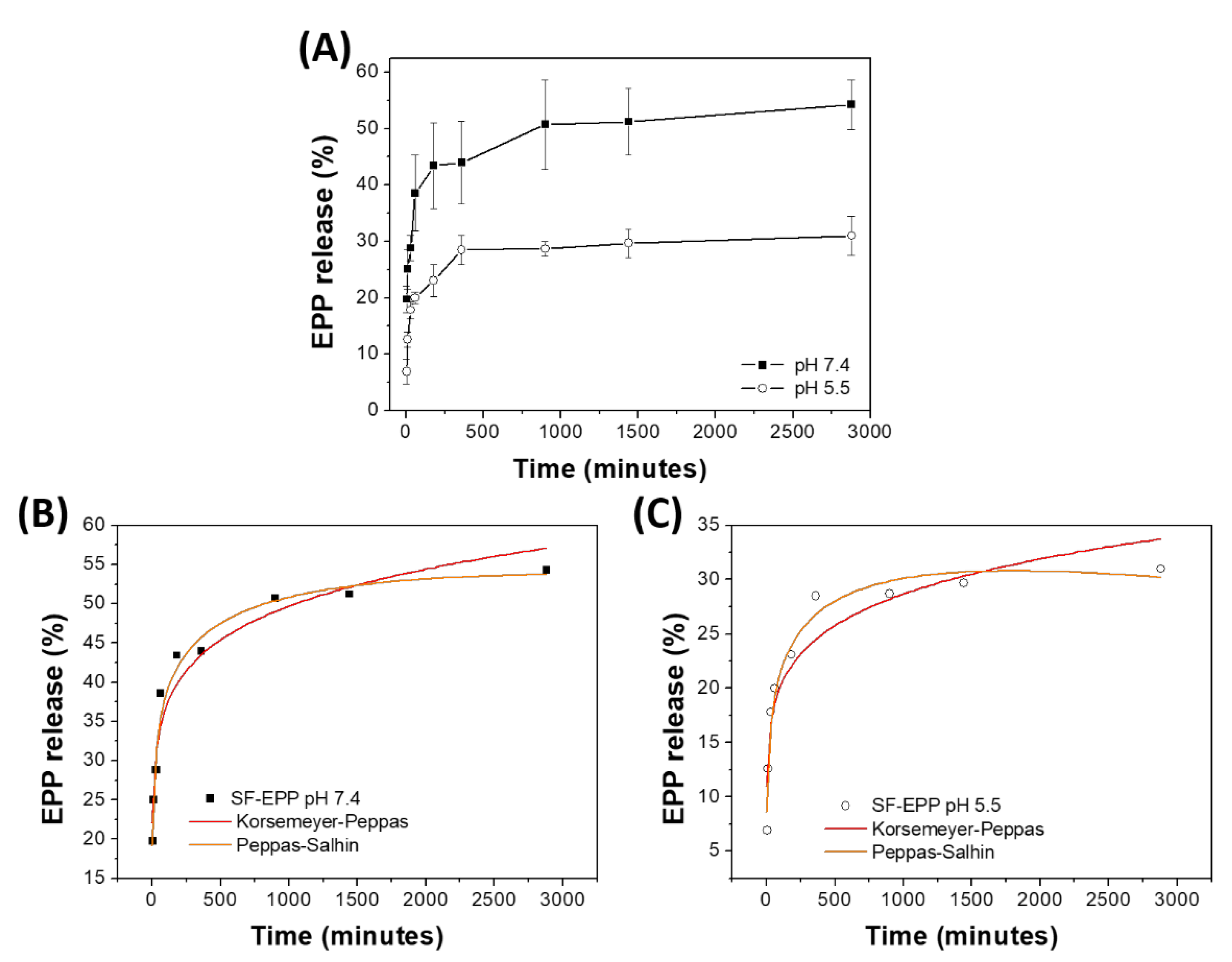
2.6. Release of Antioxidants from SF-EPP Film
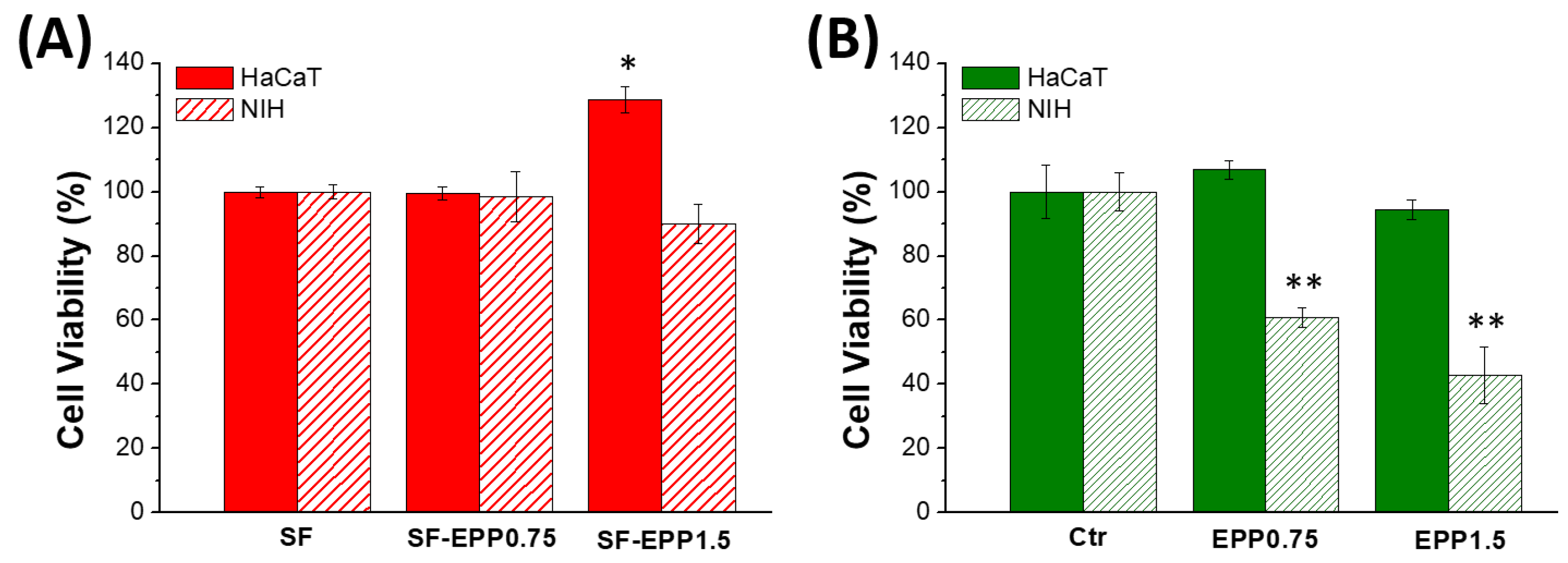
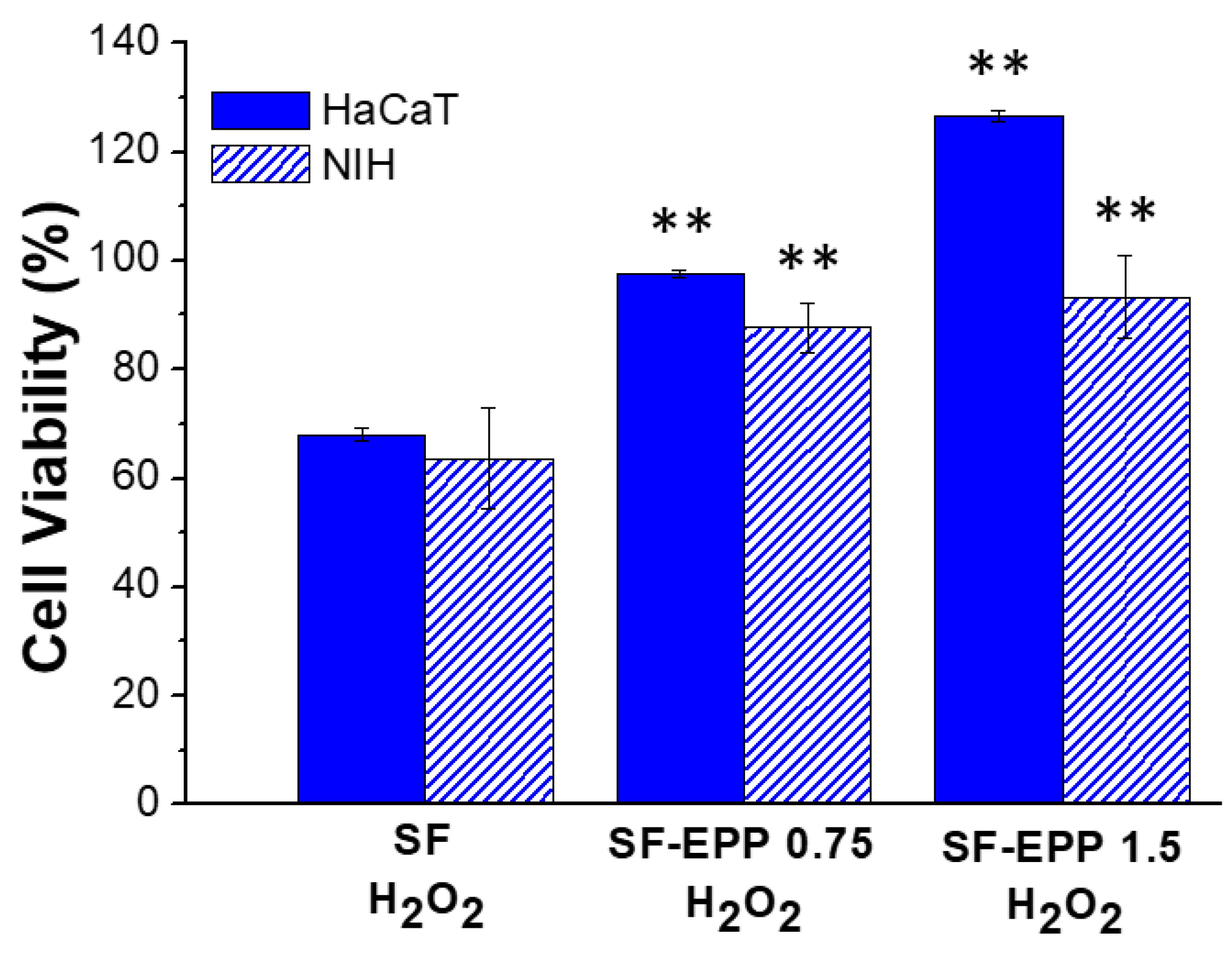
2.7. Cell Viability and ROS Inhibition
3. Materials and Methods
3.1. Pomegranate Peel Powder Preparation
3.2. Extraction of Antioxidants from Pomegranate Peel
3.3. HPLC-DAD-MS Analyses of EPP
3.4. Antioxidant Activity of EPP
3.5. Preparation of SF-EPP Films
3.6. Optical, Structural and Morphological Characterization of SF-EPP Films
3.7. SF and SF-EPP PBS Stability and Biodegradability
3.8. Mechanical Characterization of SF-EPP Films
3.9. Drug Release
3.10. Cell Culture
3.11. Cell Viability via Resazurin Reduction Assay and MTT Assay
3.12. Cellular Treatment
3.13. Measurement of Reactive Oxygen Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nørreslet, L.B.; Ebbehøj, N.E.; Ellekilde Bonde, J.P.; Thomsen, S.F.; Agner, T. The impact of atopic dermatitis on work life—A systematic review. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 23–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy: Perspective Article. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K. Influences of Environmental Chemicals on Atopic Dermatitis. Toxicol. Res. 2015, 31, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Moseley, R.; Hilton, J.R.; Waddington, R.J.; Harding, K.G.; Stephens, P.; Thomas, D.W. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen. 2004, 12, 419–429. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative stress and atopic dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Antonescu (Mintaș), I.A.; Antonescu, A.; Miere (Groza), F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of Wound Healing Potential of Novel Hydrogel Based on Ocimum basilicum and Trifolium pratense Extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Miere (Groza), F.; Teușdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Zdrinca, M.; Ganea, M.; Pașc, P.; et al. Evaluation of In Vitro Wound-Healing Potential, Antioxidant Capacity, and Antimicrobial Activity of Stellaria media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A comprehensive review of natural products against atopic dermatitis: Flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Morganti, P. Circular Economy: A New Horizon for Bio- Nanocomposites from Waste Materials. Int. J. Biotechnol. Wellness Ind. 2016, 5, 121–127. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci. Technol. 2021, 114, 410–423. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; Vergaro, V.; De Castro, F.; Biondo, F.; Suranna, G.P.; Papadia, P.; Fanizzi, F.P.; Rongai, D.; Ciccarella, G. Enhanced Bioactivity of Pomegranate Peel Extract following Controlled Release from CaCO3 Nanocrystals. Bioinorg. Chem. Appl. 2022, 2022, 6341298. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, A.L.; Cedola, A.; Conte, A.; Del Nobile, M.A. Juice and by-products from pomegranate to enrich pancake: Characterisation and shelf-life evaluation. Int. J. Food Sci. Technol. 2021, 56, 2886–2894. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Natural and genetically engineered proteins for tissue engineering. Prog. Polym. Sci. 2012, 37, 1–17. [Google Scholar] [CrossRef]
- Lu, Q.; Ganesan, K.; Simionescu, D.T.; Vyavahare, N.R. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 2004, 25, 5227–5237. [Google Scholar] [CrossRef]
- Giannelli, M.; Barbalinardo, M.; Riminucci, A.; Belvedere, K.; Boccalon, E.; Sotgiu, G.; Corticelli, F.; Ruani, G.; Zamboni, R.; Aluigi, A.; et al. Magnetic keratin/hydrotalcites sponges as potential scaffolds for tissue regeneration. Appl. Clay Sci. 2021, 207, 106090. [Google Scholar] [CrossRef]
- Abbott, A.; Coburn, J.M. HepaRG Maturation in Silk Fibroin Scaffolds: Toward Developing a 3D In Vitro Liver Model. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Stahl, K.; Gomis-Perez, C.; Toffanin, S.; Sagnella, A.; Torp, R.; Kaplan, D.L.; Ruani, G.; Omenetto, F.G.; Zamboni, R.; et al. Biofunctional silk/neuron interfaces. Adv. Funct. Mater. 2012, 22, 1871–1884. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, Z.; Liu, Z.; Yi, Y.; Tsigkou, O.; Li, J.; Li, Y. Controllable release of vascular endothelial growth factor (VEGF) by wheel spinning alginate/silk fibroin fibers for wound healing. Mater. Des. 2021, 212, 110231. [Google Scholar] [CrossRef]
- Liu, J.; Huang, R.; Li, G.; Kaplan, D.L.; Zheng, Z.; Wang, X. Generation of Nano-pores in Silk Fibroin Films Using Silk Nanoparticles for Full-Thickness Wound Healing. Biomacromolecules 2021, 22, 546–556. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rivero, H.C.; Pérez Hernández, M.d.C.; Pagán, A.; Montalbán, M.G.; Víllora, G.; Cénis, J.L. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int. J. Pharm. 2017, 518, 11–19. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef]
- Fan, L.; Wang, H.; Zhang, K.; Cai, Z.; He, C.; Sheng, X.; Mo, X. Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RSC Adv. 2012, 2, 4110–4119. [Google Scholar] [CrossRef]
- Giannelli, M.; Lacivita, V.; Posati, T.; Aluigi, A.; Conte, A.; Zamboni, R.; Alessandro, M.; Nobile, D. Silk Fibroin and Pomegranate By-Products to Develop Sustainable Active Pad for Food Packaging Applications. Foods 2021, 10, 2921. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Kraszni, M.; Marosi, A.; Larive, C.K. NMR assignments and the acid–base characterization of the pomegranate ellagitannin punicalagin in the acidic pH-range. Anal. Bioanal. Chem. 2013, 405, 5807–5816. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.; Lu, Q.; Zhang, X.; Kluge, J.A.; Uppal, N.; Omenetto, F.; Kaplan, D.L. Insoluble and Flexible Silk Films Containing Glycerol. Biomacromolecules 2010, 11, 143–150. [Google Scholar] [CrossRef]
- Posati, T.; Benfenati, V.; Sagnella, A.; Pistone, A.; Nocchetti, M.; Donnadio, A.; Ruani, G.; Zamboni, R.; Muccini, M. Innovative multifunctional silk fibroin and hydrotalcite nanocomposites: A synergic effect of the components. Biomacromolecules 2014, 15, 158–168. [Google Scholar] [CrossRef]
- Sagnella, A.; Pistone, A.; Bonetti, S.; Donnadio, A.; Saracino, E.; Nocchetti, M.; Dionigi, C.; Ruani, G.; Muccini, M.; Posati, T.; et al. Effect of different fabrication methods on the chemo-physical properties of silk fibroin films and on their interaction with neural cells. RSC Adv. 2016, 6, 9304–9314. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation Mechanism and Control of Silk Fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Kaplan, D.; Cebe, P. Dynamic Protein−Water Relationships during β-Sheet Formation. Macromolecules 2008, 41, 3939–3948. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Bonifacio, M.A.; Licini, C.; Bellissimo, A.; Pinto, L.; Baruzzi, F.; Mattioli-Belmonte, M.; De Giglio, E. Innovative Eco-Friendly Hydrogel Film for Berberine Delivery in Skin Applications. Molecules 2021, 26, 4901. [Google Scholar] [CrossRef] [PubMed]
- Dellali, M.; Iurciuc (Tincu), C.E.; Savin, C.L.; Spahis, N.; Djennad, M.; Popa, M. Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules 2021, 26, 2185. [Google Scholar] [CrossRef]
- Giuri, D.; Barbalinardo, M.; Sotgiu, G.; Zamboni, R.; Nocchetti, M.; Donnadio, A.; Corticelli, F.; Valle, F.; Gennari, C.G.M.; Selmin, F.; et al. Nano-hybrid electrospun non-woven mats made of wool keratin and hydrotalcites as potential bio-active wound dressings. Nanoscale 2019, 11, 6422–6430. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Tavanai, H.; Fathi, M. A study on the release kinetics and mechanisms of vanillin incorporated in almond gum/polyvinyl alcohol composite nanofibers in different aqueous food simulants and simulated saliva. Flavour Fragr. J. 2016, 31, 442–447. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Bihamta, M.; Hosseini, A.; Ghorbani, A.; Boroushaki, M.T. Protective effect of pomegranate seed oil against H2O2-induced oxidative stress in cardiomyocytes. Avicenna J. Phytomed. 2017, 7, 46–53. [Google Scholar]
- Rapa, S.F.; Waltenberger, B.; Di Paola, R.; Adesso, S.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Autore, G.; Cuzzocrea, S.; Stuppner, H.; et al. Plumericin prevents intestinal inflammation and oxidative stress in vitro and in vivo. FASEB J. 2020, 34, 1576–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]





| Sample | E (MPa) | TS (MPa) | ε (%) |
|---|---|---|---|
| SF | 1361.33 ± 39.61 | 46.91 ± 2.59 | 6.64 ± 0.20 |
| SF-EPP 0.75 | 1393.54 ± 14.83 | 43.54 ± 0.65 | 5.95 ± 0.14 |
| SF-EPP 1.5 | 1428.28 ± 59.90 | 52.17 ± 4.47 | 5.88 ± 0.16 |
| Sample | E (MPa) | TS (MPa) | ε (%) |
|---|---|---|---|
| SF | 119.2 ± 6.5 | 14.37 ± 7.01 | 211.1 ± 12.8 |
| SF-EPP 0.75 | 157.8 ± 6.5 | 19.27 ± 0.24 | 276.9 ± 0.6 |
| SF-EPP 1.5 | 200.6 ± 8.5 | 14.34 ± 0.54 | 229.2 ± 7.3 |
| Sample | Korsmeyer–Peppas Qt = KKPtn | Peppas–Sahlin Qt = k1t0.5 + k2t1.0 | |||||
|---|---|---|---|---|---|---|---|
| KKP | n | R2 | k1 | k2 | R2 | |k1|/|k2| | |
| SF-EPP pH 7.4 | 20 ± 2 | 0.13 ± 0.01 | 0.944 | 18 ± 2 | −1.56 ± 0.28 | 0.973 | 12 |
| SF-EPP pH 5.5 | 10 ± 1 | 0.15 ± 0.02 | 0.904 | 8 ± 1 | −0.48 ± 0.11 | 0.973 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalinardo, M.; Giannelli, M.; Forcini, L.; Luppi, B.; Donnadio, A.; Navacchia, M.L.; Ruani, G.; Sotgiu, G.; Aluigi, A.; Zamboni, R.; et al. Eco-Sustainable Silk Fibroin/Pomegranate Peel Extract Film as an Innovative Green Material for Skin Repair. Int. J. Mol. Sci. 2022, 23, 6805. https://doi.org/10.3390/ijms23126805
Barbalinardo M, Giannelli M, Forcini L, Luppi B, Donnadio A, Navacchia ML, Ruani G, Sotgiu G, Aluigi A, Zamboni R, et al. Eco-Sustainable Silk Fibroin/Pomegranate Peel Extract Film as an Innovative Green Material for Skin Repair. International Journal of Molecular Sciences. 2022; 23(12):6805. https://doi.org/10.3390/ijms23126805
Chicago/Turabian StyleBarbalinardo, Marianna, Marta Giannelli, Ludovica Forcini, Barbara Luppi, Anna Donnadio, Maria Luisa Navacchia, Giampiero Ruani, Giovanna Sotgiu, Annalisa Aluigi, Roberto Zamboni, and et al. 2022. "Eco-Sustainable Silk Fibroin/Pomegranate Peel Extract Film as an Innovative Green Material for Skin Repair" International Journal of Molecular Sciences 23, no. 12: 6805. https://doi.org/10.3390/ijms23126805
APA StyleBarbalinardo, M., Giannelli, M., Forcini, L., Luppi, B., Donnadio, A., Navacchia, M. L., Ruani, G., Sotgiu, G., Aluigi, A., Zamboni, R., & Posati, T. (2022). Eco-Sustainable Silk Fibroin/Pomegranate Peel Extract Film as an Innovative Green Material for Skin Repair. International Journal of Molecular Sciences, 23(12), 6805. https://doi.org/10.3390/ijms23126805








