Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications
Abstract
:1. Introduction
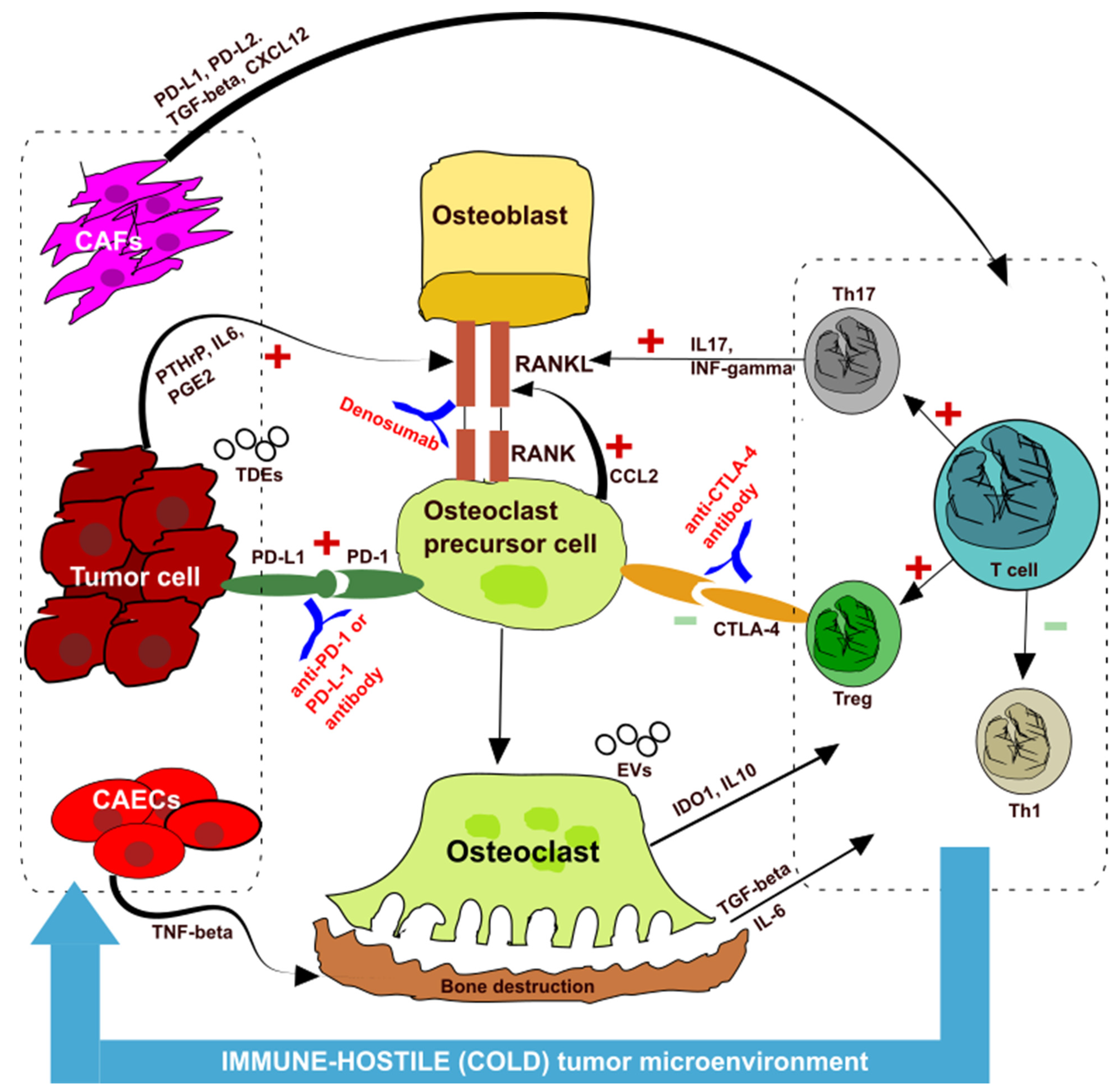
2. Bone Metastasis and Microenvironment
3. Bone Metastasis in NSCLC Treated with Immune Checkpoint Inhibitors
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistica, 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasello, G.; Pavan, A.; Attili, I.; Bortolami, A.; Menis, J.; Conte, P.; Guarneri, V. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat. Rev. 2020, 87, 102031. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Salati, M.; Di Pietro, F.R.; Strigari, L.; Cerbelli, B.; Zizzari, I.G.; Giusti, R.; Mazzotta, M.; Mazzuca, F.; Roberto, M.; et al. A normogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J. Transl. Med. 2019, 17, 99. [Google Scholar] [CrossRef] [Green Version]
- Tamiya, M.; Tamiya, A.; Inoue, T.; Kimura, M.; Kunimasa, K.; Nakahama, K.; Taniguchi, Y.; Shiroyama, T.; Isa, S.-I.; Nishino, K.; et al. Metastatic site as predictor of nivolumab efficacy in patient with advanced non-small cell lung cancer: A retrospective multicenter trial. PLoS ONE 2018, 13, e0192227. [Google Scholar] [CrossRef]
- Schmid, S.; Diem, S.; Li, Q.; Krapf, M.; Flatz, L.; Leschka, S.; Desbiolles, L.; Klingbiel, D.; Jochum, W.; Früh, M. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol. Immunother. 2018, 67, 1825–1832. [Google Scholar] [CrossRef]
- Santini, D.; Barni, S.; Intagliata, S.; Falcone, A.; Ferraù, F.; Galetta, D.; Moscetti, L.; La Verde, N.; Ibrahim, T.; Petrelli, F.; et al. Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci. Rep. 2015, 5, 18670. [Google Scholar] [CrossRef] [Green Version]
- Kuchuk, M.; Addison, C.L.; Clemons, M.; Kuchuk, I.; Wheatley-Price, P. Incidence and Consequences of Bone Metastases in Lung Cancer Patients. J. Bone Oncol. 2013, 2, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2011, 27, 165–176. [Google Scholar] [CrossRef]
- Shi, S.; Wang, H.; Liu, X.; Xiao, J. Prediction of overall survival of non-small cell lung cancer with metastasis: An analysis of the Surveillance, Epidemiology and End Results (SEER) database. Transl. Cancer Res. 2021, 10, 5191–5203. [Google Scholar] [CrossRef]
- Zhao, E.; Xu, H.; Wang, L.; Kryczek, I.; Wu, K.; Hu, Y.; Wang, G.; Zou, W. Bone marrow and the control of immunity. Cell. Mol. Immunol. 2012, 9, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kähkönen, T.E.; Halleen, J.M.; Bernoulli, J. Osteoimmuno-oncology: Therapeutic opportunities targeting immune cells in bone metastasis. Cells 2021, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Zhao, S.; Miah, A.; Wei, L.; Patel, S.; Johns, A.; Grogan, M.; Bertino, E.M.; He, K.; Shields, P.G.; et al. Bone metastases, skeletal-related events, and survival in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. J. Natl. Compr. Cancer Netw. 2021, 19, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.M.; da Silva, G.T.; Bergmann, A.; Costa, G.J.; Zamboni, M.M.; Santos Thuler, L.C. Impact of Different Patterns of Metastasis in Non-Small-Cell Lung Cancer Patients. Future Oncol. 2021, 17, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; D’Incà, F.; Gelibter, A.; Chiari, R.; Grossi, F.; Delmonte, A.; Passaro, A.; Signorelli, D.; Gelsomino, F.; Galetta, D.; et al. Bone Metastases and Immunotherapy in Patients with Advanced Non-Small Cell Lung Cancer. J. Immunother. Cancer 2019, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Brunetti, O.; Longo, V.; Calabrese, A.; Calbi, R.; Antonio, G.S.; Licchetta, A. Immune system and microenvironment: Rationale for targeted cancer therapies. Oncotarget 2020, 11, 480–487. [Google Scholar]
- Zou, L.; Barnett, B.; Safah, H.; LaRussa, V.F.; Evdemon-Hogan, M.; Mottram, P.; Wei, S.; David, O.; Curiel, T.J.; Zou, W. Bone morrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signal. Cancer Res. 2004, 64, 8451–8455. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumors. Nat. Rev. Immunol. 2021, 12, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, M.; Xu, C.; Li, B.; Chen, J.; Chen, J.; Wang, Z. Immune Checkpoint inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J. Immunol. Res. 2021, 2021, 8970173. [Google Scholar] [CrossRef]
- Ponzetti, M.; Rucci, N. Updates on osteoimmunology: What’s new on cross-talk between bone and immune system. Front. Endocrinol. (Lausanne) 2019, 10, 236. [Google Scholar] [CrossRef]
- Gdowski, A.S.; Ranjan, A.; Vishwanatha, J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trial. J. Exp. Clin. Cancer Res. 2017, 36, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Gu, Y.; Liao, Y.; Bang, S.; Donnelly, C.; Chen, O.; Tao, X.; Mirando, A.J.; Hilton, M.J.; Ji, R.-R. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Investig. 2020, 130, 3603–3620. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, M.; Zhang, D.; Sasaki, S.I. Emergence of cancer-associated fibroblast as independent cellular player in bone metastasis process. Cancer 2019, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K. Role of RANKL in cancer development and metastasis. J. Bone Miner. Metab. 2021, 39, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, P.; Jiao, J.; Wei, H.; Xu, W.; Zhou, P. Role of the RANKL-RANK Axis in Immunity—Implications for pathogenesis and Treatment of Bone Metastasis. Front. Immunol. 2022, 13, 824117. [Google Scholar] [CrossRef]
- Fujimura, T.; Kambayashi, Y.; Furudate, S.; Asano, M.; Kakizaki, A.; Aiba, S. Receptor activator of NF-kB ligand promotes the production of CCL17 from RANK+ M2 macrophages. J. Investig. Dermatol. 2015, 135, 2884–2887. [Google Scholar] [CrossRef] [Green Version]
- Urabe, F.; Patil, K.; Ramm, G.A.; Ochiya, T.; Soekmadji, C. Extracellular vesicles in development of organ-specific metastasis. J. Extracell. Vesicles 2021, 10, e12125. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Huang, C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosoppresion and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.; Gooding, E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Osteoimmunology 2019, 8, 1593805. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J. The Potential Roles of Exosomal miR-214 in Bone Metastasis of Lung Adenocarcinoma. Front. Oncol. 2021, 10, 611054. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J., Jr.; McHugh, K.P.; Holliday, L.S. Characterization of regulatory extracellular vesicles from osteoclasts. J. Dent. Res. 2016, 95, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Pucci, M.; Giallombardo, M.; Di Bella, M.A.; Santarpia, M.; Reclusa, P.; Gil-Bazo, I.; Rolfo, C.; Alessandro, R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation though the activation of EGFR pathway. Sci. Rep. 2017, 7, 3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liede, A.; Hernandez, R.K.; Wade, S.W.; Bo, R.; Nussbaum, N.C.; Ahern, E.; Dougall, W.C.; Smyth, M.J. Observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology 2018, 7, e1480301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Chang, X.; Zhou, R.; Chen, Y.-D.; Ma, H.-C.; Xiao, Z.-Z.; Qu, X.; Liu, Y.-H.; Liu, L.-R.; Li, Y.; et al. Bone metastasis attenuates efficacy of immune checkpoint inhibitors and displays “cold” immune characteristics in Non-small cell lung cancer. Lung Cancer 2022, 166, 189–196. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Chen, S.; Zhou, F.; Zhao, J.; Zhao, W.; Su, C. Adverse impact of bone metastases on clinical outcomes of patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Thoracic Cancer 2020, 11, 2812–2819. [Google Scholar] [CrossRef]
- Qiang, H.; Lei, Y.; Shen, Y.; Li, J.; Zhong, H.; Zhong, R.; Zhang, X.; Chang, Q.; Lu, J.; Feng, H.; et al. Pembrolizumab monotherapy or combination therapy for bone metastases in advanced Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2022, 11, 87–99. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Foca, F.; Menis, J.; Stucci, L.S.; Artioli, F.; Guadalupi, V.; Forcignanò, M.R.C.; Fantini, M.; Recine, F.; Mercatali, L.; et al. Immune checkpoint inhibitors with or without bone-targeted therapy in NSCLC patients with bone metastasies and prognostic significance of neutrophil-to-lymphocyte ratio. Front. Immunol 2021, 12, 697298. [Google Scholar] [CrossRef]
- Asano, Y.; Yamamoto, N.; Demura, S.; Hayashi, K.; Takeuchi, A.; Kato, S.; Miwa, S.; Igarashi, K.; Higuchi, T.; Yonezawa, H.; et al. The therapeutic effect and clinical outcome of immune checkpoint inhibitors on bone metastasis in advanced non-small-cell lung cancer. Front. Oncol. 2022, 12, 871675. [Google Scholar] [CrossRef]
- Györi, D.S.; Mócsai, A. Osteoclast Signal Transduction During Bone Metastasis Formation. Front. Cell Dev. Biol. 2020, 8, 507. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Bozec, A.; Rauner, M.; Jakob, F.; Perner, S.; Pantel, K. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021, 18, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.J.; Tokuyama, H.; Mattarollo, S.R.; Hagi, T.; Suzuki, K.; Yokokawa, K.; Nieda, M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 2011, 105, 778–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, D.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, D.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef] [Green Version]
- Nagamine, I.; Yamaguchi, Y.; Ohara, M.; Ikeda, T.; Okada, M. Induction of gamma delta T cells using zoledronate plus interleukin-2 in patients with metastatic cancer. Hiroshima J. Med. Sci. 2009, 58, 37–44. [Google Scholar]
- Angela, Y.; Haferkamp, S.; Weishaupt, C.; Ugurel, F.; Becker, J.C.; Oberndörfer, F.; Alar, V.; Satzger, I.; Gutzmer, R. Combination of denosumab and immune checkpoint inhibition: Experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol. Immunother. 2019, 68, 1187–1194. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Shirai, K. Immune checkpoint inhibitor (anti-CTLA-4, anti-PD-1) therapy alone versus immune checkpoint inhibitor (anti-CTLA-4, anti-PD-1) therapy in combination with anti-RANKL denosumab in malignant melanoma: A retrospective analysis at a tertiary care center. Melanoma Res. 2018, 28, 341–347. [Google Scholar] [CrossRef]
| Bone Metastasis (BoM)+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | ICI Alone | CT-ICI | ICI (Mono or Combo) +BTT | ||||||
| ORR | PFS | OS | ORR | PFS | OS | ORR | PFS | OS | |
| Liede A [35] | NV | NV | NV | NV | NV | NV | ↑ | NR | ↑ |
| Zhu Y [36] | NR | ↓ | ↓ | NR | ↑ | ↑ | NR | ↑ | ↑ |
| Li X [37] | = | ↓ | ↓ | = | = | = | NR | = | = |
| Landi L [15] | ↓ | ↓ | ↓ | NV | NV | NV | NV | NV | NV |
| Qiang H [38] | NV | ↓ | ↓ | NV | ↑ | ↑ | ↑ | ↑ | = |
| Bongiovanni A [39] | ↓ | = | ↓ | NV | NV | NV | ↑ | ↑ * | ↑ |
| Asano Y [40] | ↓ | ↓ | ↓ | NV | NV | NV | ↑ | NR | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Conte, A.; De Carlo, E.; Bertoli, E.; Stanzione, B.; Revelant, A.; Bertola, M.; Spina, M.; Bearz, A. Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. Int. J. Mol. Sci. 2022, 23, 6832. https://doi.org/10.3390/ijms23126832
Del Conte A, De Carlo E, Bertoli E, Stanzione B, Revelant A, Bertola M, Spina M, Bearz A. Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. International Journal of Molecular Sciences. 2022; 23(12):6832. https://doi.org/10.3390/ijms23126832
Chicago/Turabian StyleDel Conte, Alessandro, Elisa De Carlo, Elisa Bertoli, Brigida Stanzione, Alberto Revelant, Manuela Bertola, Michele Spina, and Alessandra Bearz. 2022. "Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications" International Journal of Molecular Sciences 23, no. 12: 6832. https://doi.org/10.3390/ijms23126832
APA StyleDel Conte, A., De Carlo, E., Bertoli, E., Stanzione, B., Revelant, A., Bertola, M., Spina, M., & Bearz, A. (2022). Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. International Journal of Molecular Sciences, 23(12), 6832. https://doi.org/10.3390/ijms23126832






