Diterpenoid Caesalmin C Delays Aβ-Induced Paralysis Symptoms via the DAF-16 Pathway in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
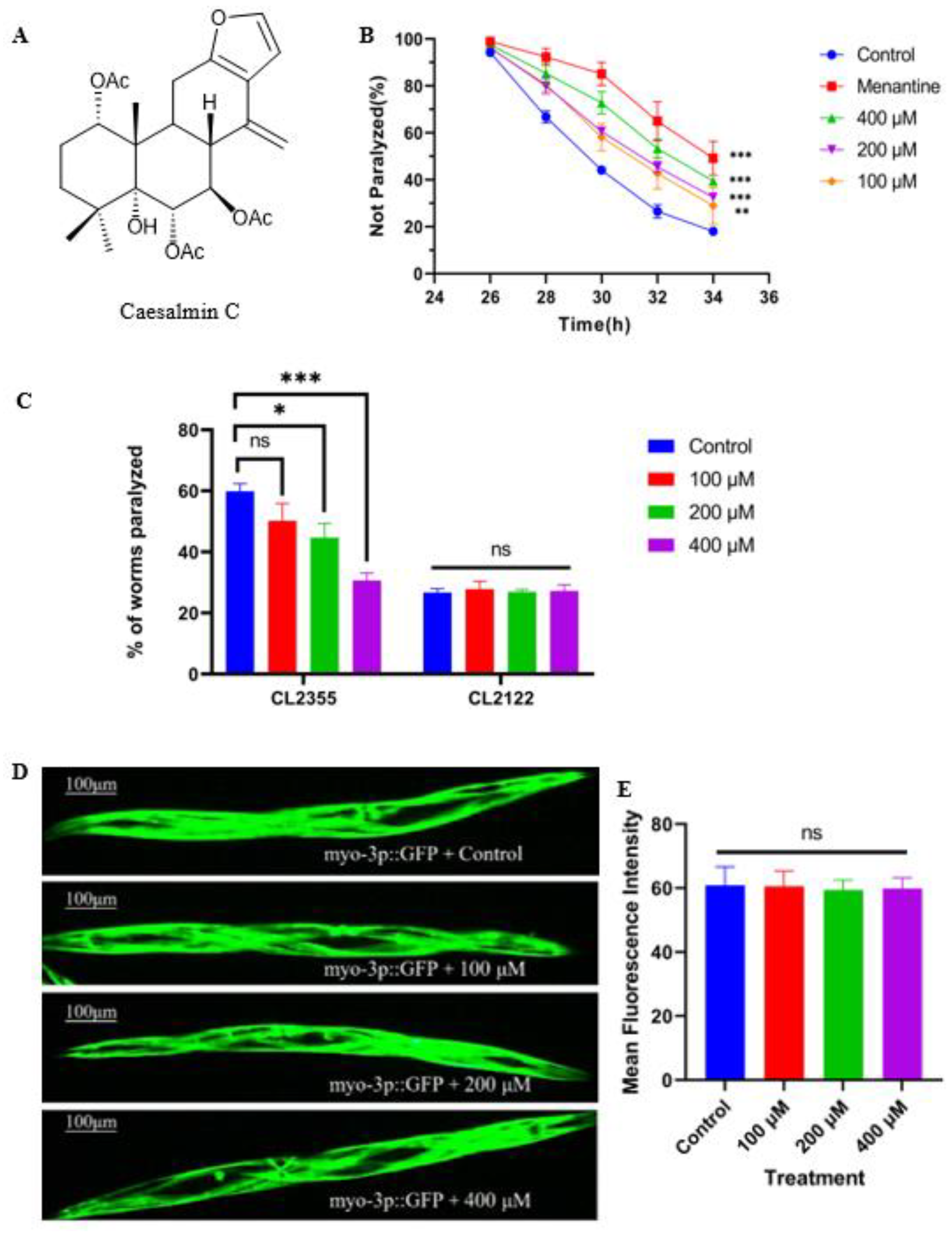
2.1. Caesalmin C Alleviated the Symptoms of Aβ Toxicity-Induced Paralysis
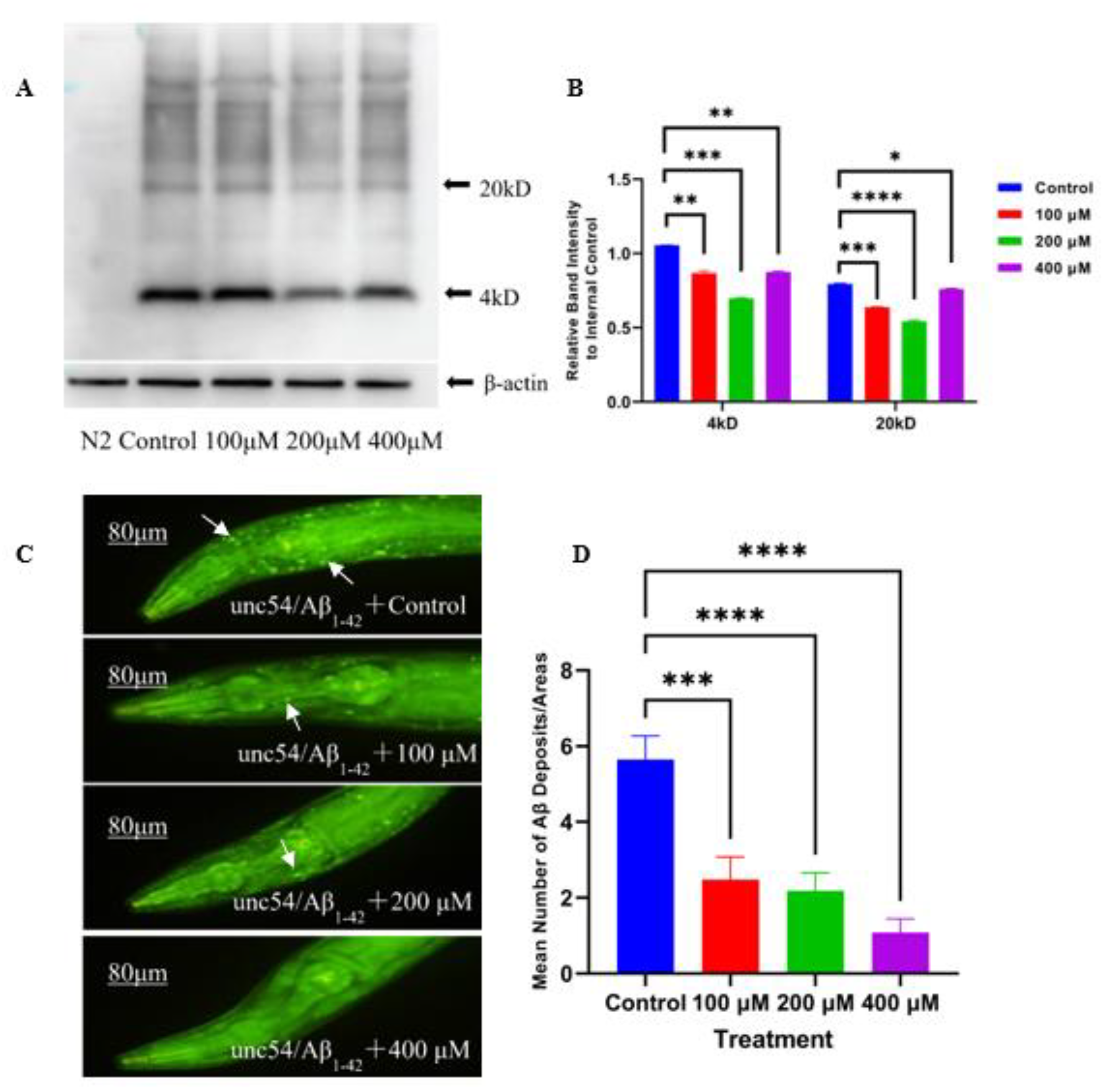
2.2. Caesalmin C Inhibited Aβ Aggregation
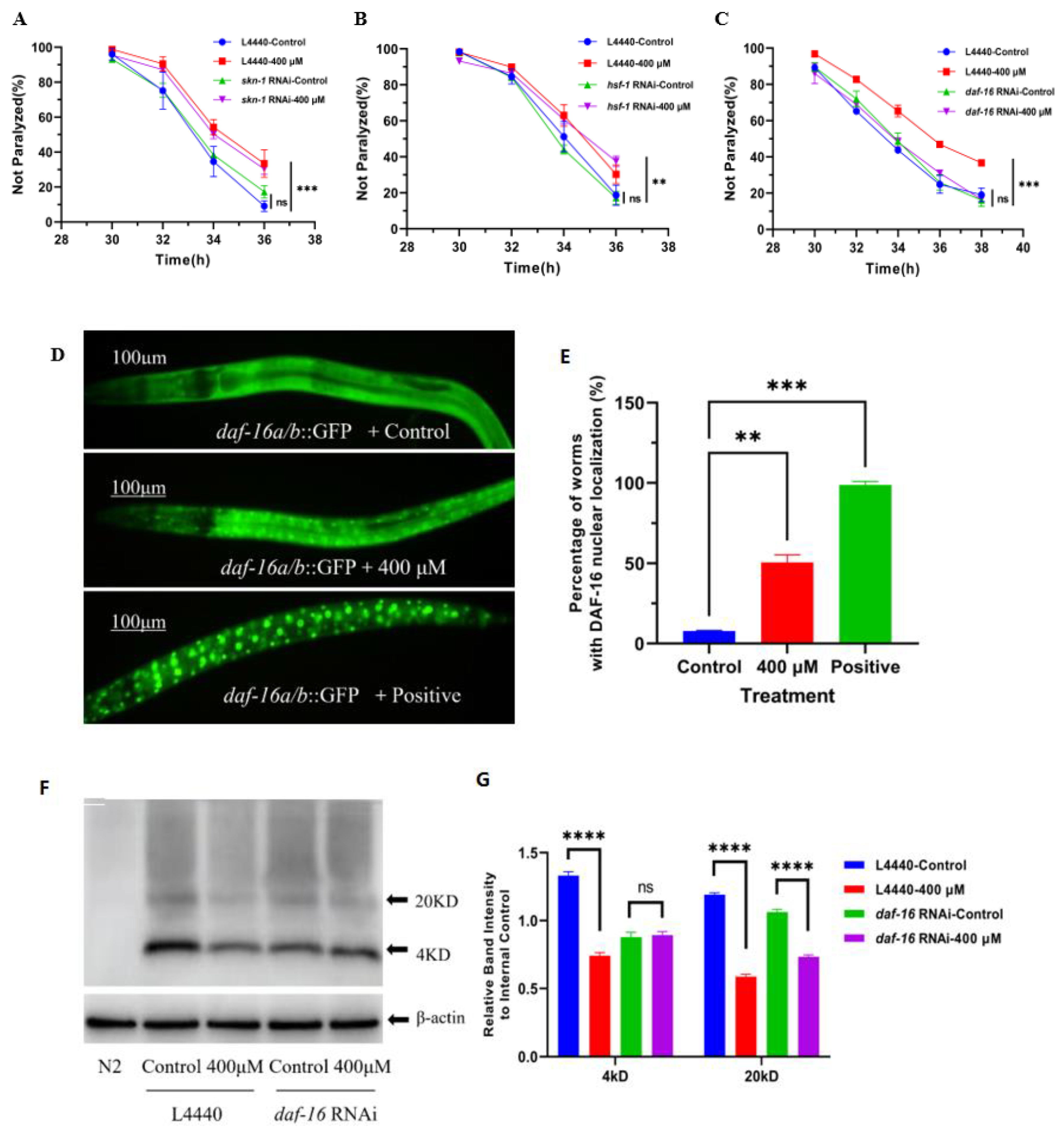
2.3. DAF-16 Signaling Pathway Played an Important Role in the Alleviation of Nematode Paralysis by Caesalmin C
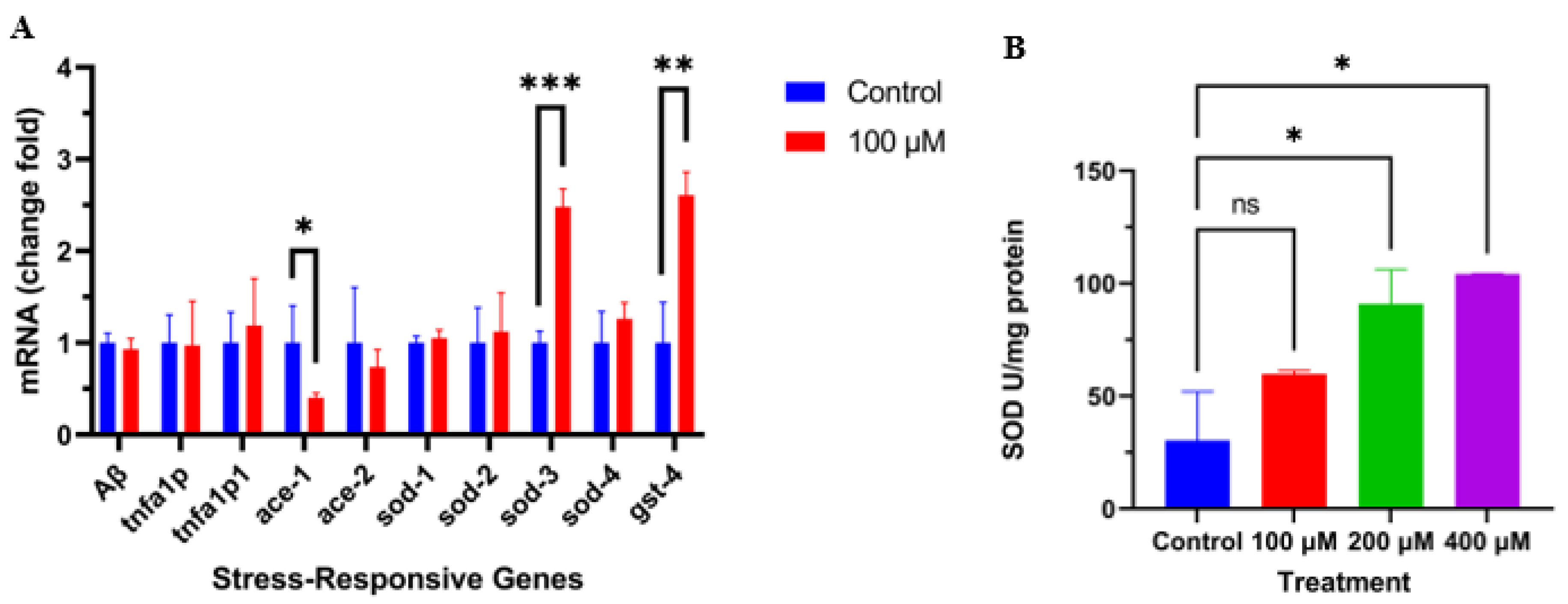
2.4. Caesalmin C Promoted the Expression of Sod-3 and Gst-4 in C. elegans
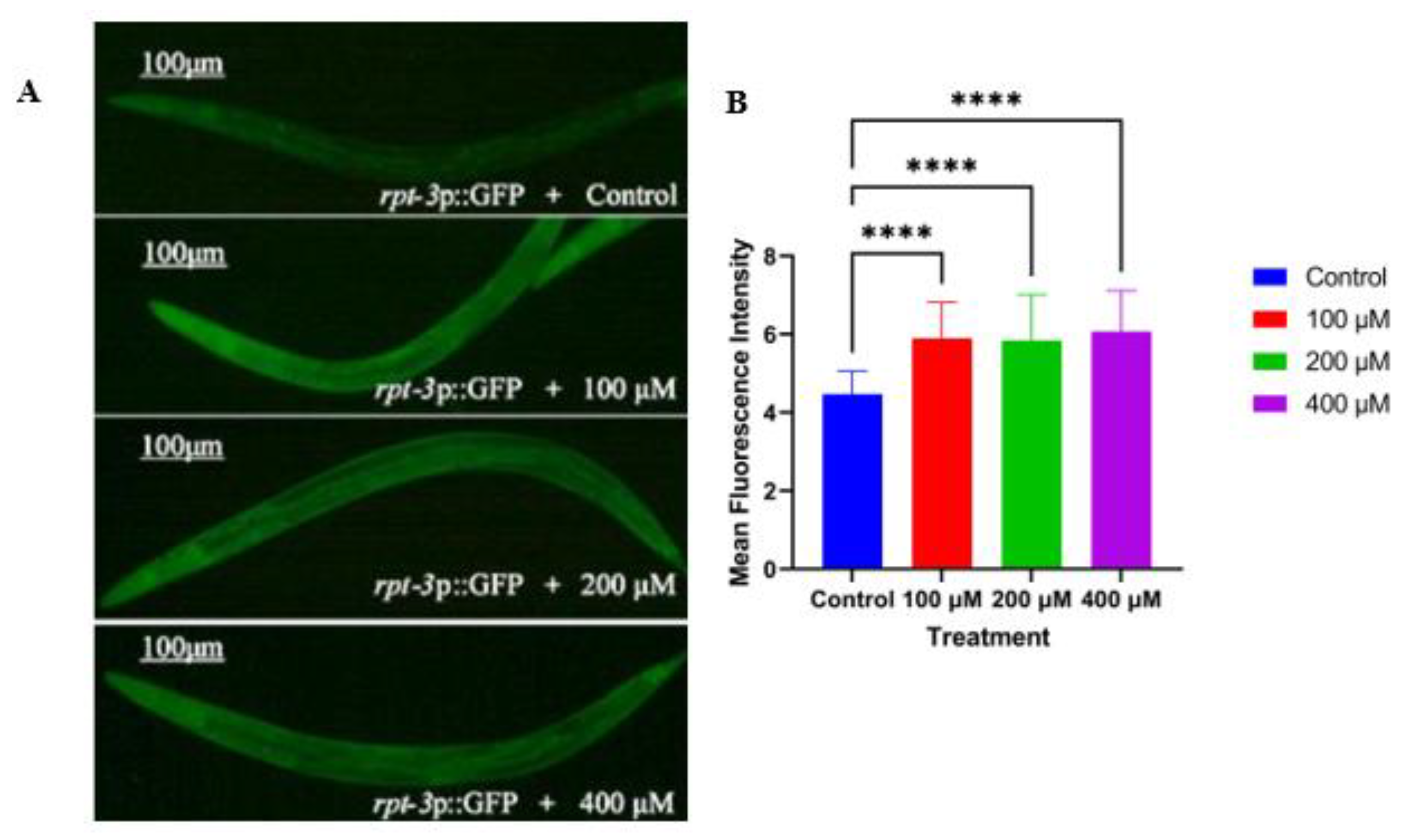
2.5. Caesalmin C Promotes the Expression of Rpt-3p::GFP in C. elegans
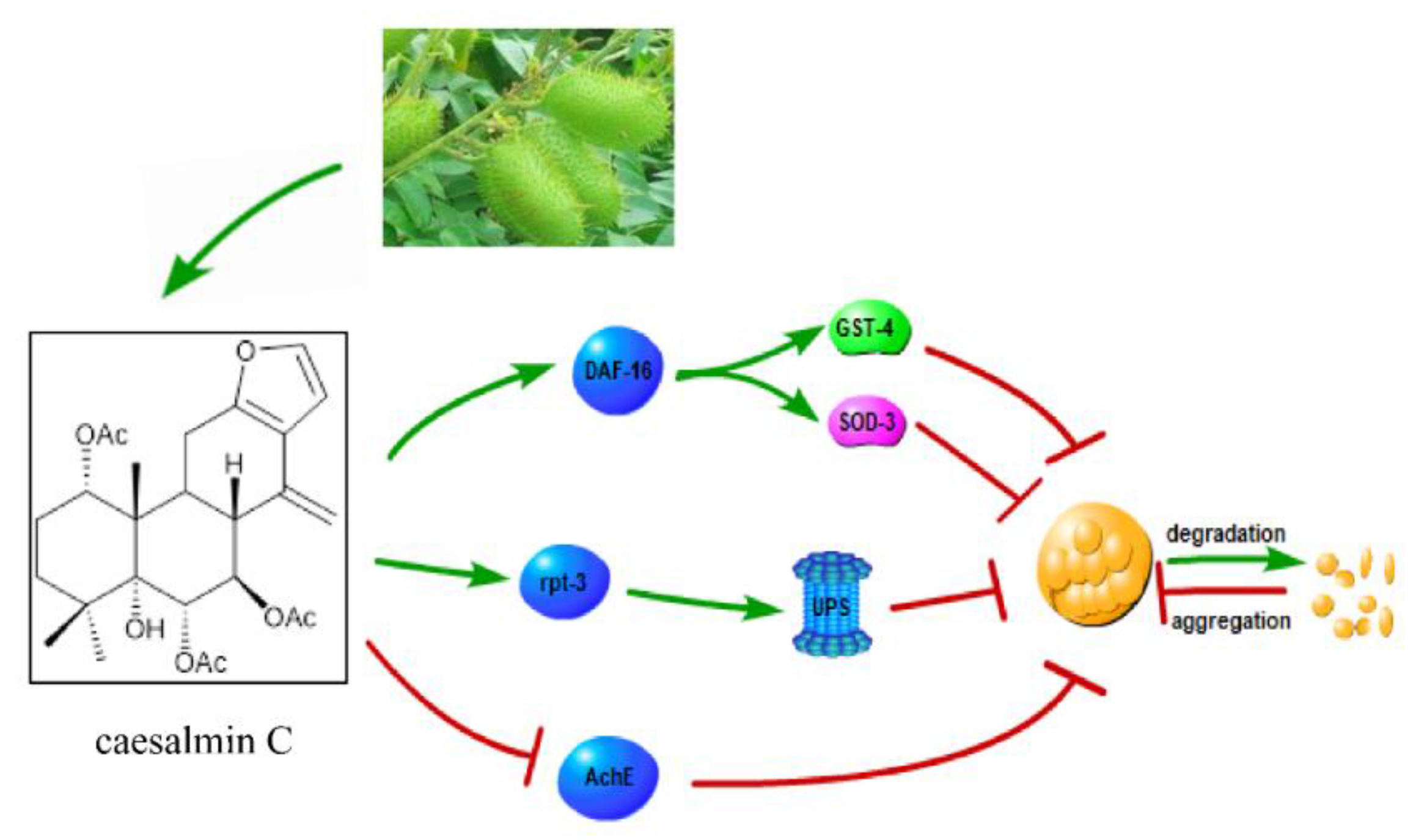
3. Discussion
4. Materials and Methods
4.1. Chemicals and Treatment
4.2. C. elegans Strains and Handling Conditions
4.3. Paralysis Assays
4.4. Exogenous Serotonin Sensitivity Assay
4.5. Effect of Caesalmin C on Exogenous Protein Expression in C. elegans
4.6. Fluorescence Staining of Aβ Deposits Assay
4.7. RNA Extraction and Quantitative Real-Time PCR Analysis
4.8. RNA Interference (RNAi) Assay
4.9. Subcellular DAF-16 Nuclear Localization Assay
4.10. Western Blot (WB) Assay
4.11. Assay of SOD Activity in C. elegans
4.12. Effect of Caesalmin C on Rpt-3::GFP Expression in C. elegans
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, N.; Barbui, C.; Anstey, K.J.; Kivipelto, M.; Barbera, M.; Peters, R.; Zheng, L.; Kulmala, J.; Stephen, R.; Ferri, C.P.; et al. Reducing the Risk of Cognitive Decline and Dementia: WHO Recommendations. Front. Neurol. 2021, 12, 765584. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Faustino, C. Therapeutic Strategies Targeting Amyloid-β in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 418–452. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of Amyloid Beta-Peptide-Associated Oxidative Stress and Brain Protein Modifications in the Pathogenesis of Alzheimer’s Disease and Mild Cognitive Impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, T.J.; Klegeris, A. Novel Multi-Target Directed Ligand-Based Strategies for Reducing Neuroinflammation in Alzheimer’s Disease. Life Sci. 2018, 207, 314–322. [Google Scholar] [CrossRef]
- Huang, L.; Ho, P.; Chen, C.-H. Activation and Inhibition of the Proteasome by Betulinic Acid and Its Derivatives. FEBS Lett. 2007, 581, 4955–4959. [Google Scholar] [CrossRef] [Green Version]
- Kaundal, M.; Deshmukh, R.; Akhtar, M. Protective Effect of Betulinic Acid against Intracerebroventricular Streptozotocin Induced Cognitive Impairment and Neuronal Damage in Rats: Possible Neurotransmitters and Neuroinflammatory Mechanism. Pharmacol. Rep. PR 2018, 70, 540–548. [Google Scholar] [CrossRef]
- Xu, P.; Li, Z.; Wang, H.; Zhang, X.; Yang, Z. Triptolide Inhibited Cytotoxicity of Differentiated PC12 Cells Induced by Amyloid-Beta₂₅₋₃₅ via the Autophagy Pathway. PLoS ONE 2015, 10, e0142719. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Gao, W.; Wang, Q.; Zhang, L.; Li, Y.; Li, L.; Zhang, L. Cornel Iridoid Glycoside Inhibits Tau Hyperphosphorylation via Regulating Cross-Talk Between GSK-3β and PP2A Signaling. Front. Pharmacol. 2018, 9, 682. [Google Scholar] [CrossRef]
- Yoo, K.-Y.; Park, S.-Y. Terpenoids as Potential Anti-Alzheimer’s Disease Therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Chatterjee, K.; Ali, K.M.; De, D.; Bera, T.K.; Ghosh, D. Antihyperglycemic and Antioxidative Effects of the Hydro-Methanolic Extract of the Seeds of Caesalpinia Bonduc on Streptozotocin-Induced Diabetes in Male Albino Rats. Pharmacogn. Res. 2012, 4, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, M.; Qi, S.; Shen, X.; Wang, Y.; Jing, W.; Yang, Y.; Li, X.; Gao, H. New Cassane-Type Diterpenoids from Kernels of Caesalpinia Bonduc (Linn.) Roxb. and Their Inhibitory Activities on Phosphodiesterase (PDE) and Nuclear Factor-Kappa B (NF-ΚB) Expression. Bioorganic Chem. 2020, 96, 103573. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.M.; Islam, R.; Khatun, H.; Parvin, S.; Islam, E.; Islam, S.A.; Mia, A.A. Antibacterial, Antidiarrhoeal, and Cytotoxic Activities of Methanol Extract and Its Fractions of Caesalpinia Bonducella (L.) Roxb Leaves. BMC Complement. Altern. Med. 2013, 13, 101. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.W.; Ma, S.C.; But, P.P.; Mak, T.C. New Antiviral Cassane Furanoditerpenes from Caesalpinia Minax. J. Nat. Prod. 2001, 64, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yuan, J.; Wu, S.; Huang, J.; Xu, X.; Wu, Z.; Gao, H. Anti-Inflammation Furanoditerpenoids from Caesalpinia Minax Hance. Phytochemistry 2015, 117, 325–331. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an Understanding of Amyloid-β Oligomers: Characterization, Toxicity Mechanisms, and Inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Epicatechin Modulates Stress-Resistance in C. Elegans via Insulin/IGF-1 Signaling Pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Dong, H.H. FoxO Integration of Insulin Signaling with Glucose and Lipid Metabolism. J. Endocrinol. 2017, 233, R67–R79. [Google Scholar] [CrossRef] [Green Version]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of Mitophagy and Mitochondrial Biogenesis during Ageing in C. Elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]
- Anckar, J.; Sistonen, L. Regulation of HSF1 Function in the Heat Stress Response: Implications in Aging and Disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Zhao, Y.; Ponnusamy, M.; Liu, Y. The Role of Ubiquitin Proteasomal System and Autophagy-Lysosome Pathway in Alzheimer’s Disease. Rev. Neurosci. 2017, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Hesp, K.; Smant, G.; Kammenga, J.E. Caenorhabditis Elegans DAF-16/FOXO Transcription Factor and Its Mammalian Homologs Associate with Age-Related Disease. Exp. Gerontol. 2015, 72, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatar, M.; Bartke, A.; Antebi, A. The Endocrine Regulation of Aging by Insulin-like Signals. Science 2003, 299, 1346–1351. [Google Scholar] [CrossRef]
- Accili, D.; Arden, K.C. FoxOs at the Crossroads of Cellular Metabolism, Differentiation, and Transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Webb, A.E. Neuronal Functions of FOXO/DAF-16. Nutr. Healthy Aging 2017, 4, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Honda, S. The Daf-2 Gene Network for Longevity Regulates Oxidative Stress Resistance and Mn-Superoxide Dismutase Gene Expression in Caenorhabditis Elegans. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 1385–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemva, J.; Schubert, M. Central Insulin and Insulin-like Growth Factor-1 Signaling: Implications for Diabetes Associated Dementia. Curr. Diabetes Rev. 2011, 7, 356–366. [Google Scholar] [CrossRef]
- Murphy, C.T. The Search for DAF-16/FOXO Transcriptional Targets: Approaches and Discoveries. Exp. Gerontol. 2006, 41, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.A.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct Inhibition of the Longevity-Promoting Factor SKN-1 by Insulin-like Signaling in C. Elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers Dis. JAD 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yu, X.; Li, C.; Ma, L.; Zhao, Z.; Guan, S.; Wang, L. Caffeic Acid Protects against Aβ Toxicity and Prolongs Lifespan in Caenorhabditis Elegans Models. Food Funct. 2021, 12, 1219–1231. [Google Scholar] [CrossRef]
- Suthammarak, W.; Somerlot, B.H.; Opheim, E.; Sedensky, M.; Morgan, P.G. Novel Interactions between Mitochondrial Superoxide Dismutases and the Electron Transport Chain. Aging Cell 2013, 12, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.; Maloney, A.J. Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet Lond. Engl. 1976, 2, 1403. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase Accelerates Assembly of Amyloid-Beta-Peptides into Alzheimer’s Fibrils: Possible Role of the Peripheral Site of the Enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A Structural Motif of Acetylcholinesterase That Promotes Amyloid Beta-Peptide Fibril Formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Fülöp, L. Protein Folding and Misfolding, Endoplasmic Reticulum Stress in Neurodegenerative Diseases: In Trace of Novel Drug Targets. Curr. Protein Pept. Sci. 2016, 17, 169–182. [Google Scholar] [CrossRef]
- Cordero, J.G.; García-Escudero, R.; Avila, J.; Gargini, R.; García-Escudero, V. Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxid. Med. Cell. Longev. 2018, 2018, 5010741. [Google Scholar] [CrossRef]
- Regitz, C.; Dußling, L.M.; Wenzel, U. Amyloid-Beta (Aβ₁₋₄₂)-Induced Paralysis in Caenorhabditis Elegans Is Inhibited by the Polyphenol Quercetin through Activation of Protein Degradation Pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef]
- Wang, X.; Yi, K.; Zhao, Y. Fucoidan Inhibits Amyloid-β-Induced Toxicity in Transgenic Caenorhabditis Elegans by Reducing the Accumulation of Amyloid-β and Decreasing the Production of Reactive Oxygen Species. Food Funct. 2018, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Reis, N.; Lee, Y.; Glickman, M.H.; Vierstra, R.D. Subunit Interaction Maps for the Regulatory Particle of the 26S Proteasome and the COP9 Signalosome. EMBO J. 2001, 20, 7096–7107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillette, T.G.; Kumar, B.; Thompson, D.; Slaughter, C.A.; DeMartino, G.N. Differential Roles of the COOH Termini of AAA Subunits of PA700 (19 S Regulator) in Asymmetric Assembly and Activation of the 26 S Proteasome. J. Biol. Chem. 2008, 283, 31813–31822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, J.S.; Sun, X.; Wally, O.S.D.; Zhang, K.; Ji, X.; Wang, Z.; Wang, Y.; Zidichouski, J.; Prithiviraj, B.; Zhang, J. Liuwei Dihuang (LWDH), a Traditional Chinese Medicinal Formula, Protects against β-Amyloid Toxicity in Transgenic Caenorhabditis Elegans. PLoS ONE 2012, 7, e43990. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Li, J.; Han, B.; Li, X.; Chen, J. Improvement of Oxidative Stress Tolerance in Saccharomyces Cerevisiae through Global Transcription Machinery Engineering. J. Ind. Microbiol. Biotechnol. 2014, 41, 869–878. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-P.; Bai, X.; Cui, W.-B.; Chen, X.-H.; Liu, X.; Zhi, D.-J.; Zhang, Z.-X.; Fei, D.-Q.; Wang, D.-S. Diterpenoid Caesalmin C Delays Aβ-Induced Paralysis Symptoms via the DAF-16 Pathway in Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 6871. https://doi.org/10.3390/ijms23126871
Zhang Z-P, Bai X, Cui W-B, Chen X-H, Liu X, Zhi D-J, Zhang Z-X, Fei D-Q, Wang D-S. Diterpenoid Caesalmin C Delays Aβ-Induced Paralysis Symptoms via the DAF-16 Pathway in Caenorhabditis elegans. International Journal of Molecular Sciences. 2022; 23(12):6871. https://doi.org/10.3390/ijms23126871
Chicago/Turabian StyleZhang, Zong-Ping, Xue Bai, Wen-Bo Cui, Xiao-Han Chen, Xu Liu, De-Juan Zhi, Zhan-Xin Zhang, Dong-Qing Fei, and Dong-Sheng Wang. 2022. "Diterpenoid Caesalmin C Delays Aβ-Induced Paralysis Symptoms via the DAF-16 Pathway in Caenorhabditis elegans" International Journal of Molecular Sciences 23, no. 12: 6871. https://doi.org/10.3390/ijms23126871





