Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers
Abstract
:1. Introduction
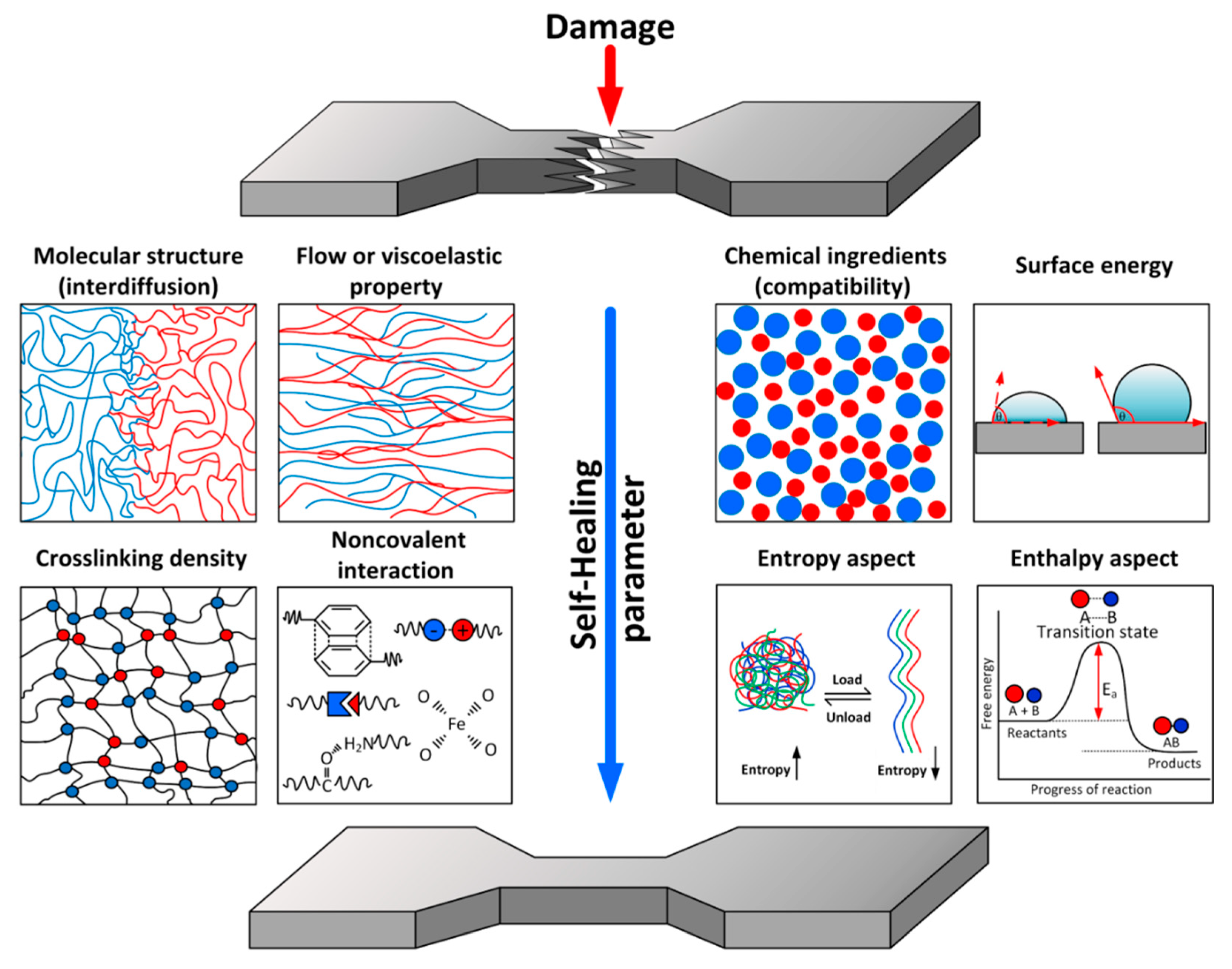
- (i)
- The molecular structure of the polymer (in terms of the rate of interdiffusion of polymer chains);
- (ii)
- The flow or viscoelastic properties of the polymer;
- (iii)
- The chemical ingredients or blended matrix in the polymer composite (in terms of compatible or incompatible materials);
- (iv)
- The surface energy of the polymer;
- (v)
- The crosslinking density (uncrosslinked or weakly crosslinked polymer);
- (vi)
- Non-covalent interaction (metal–ligand coordination, hydrogen bonding, π–π interaction, etc.);
- (vii)
- Entropy aspects (conformational entropy of polymer chains);
- (viii)
- Enthalpy aspects (chemical reaction in the polymer).
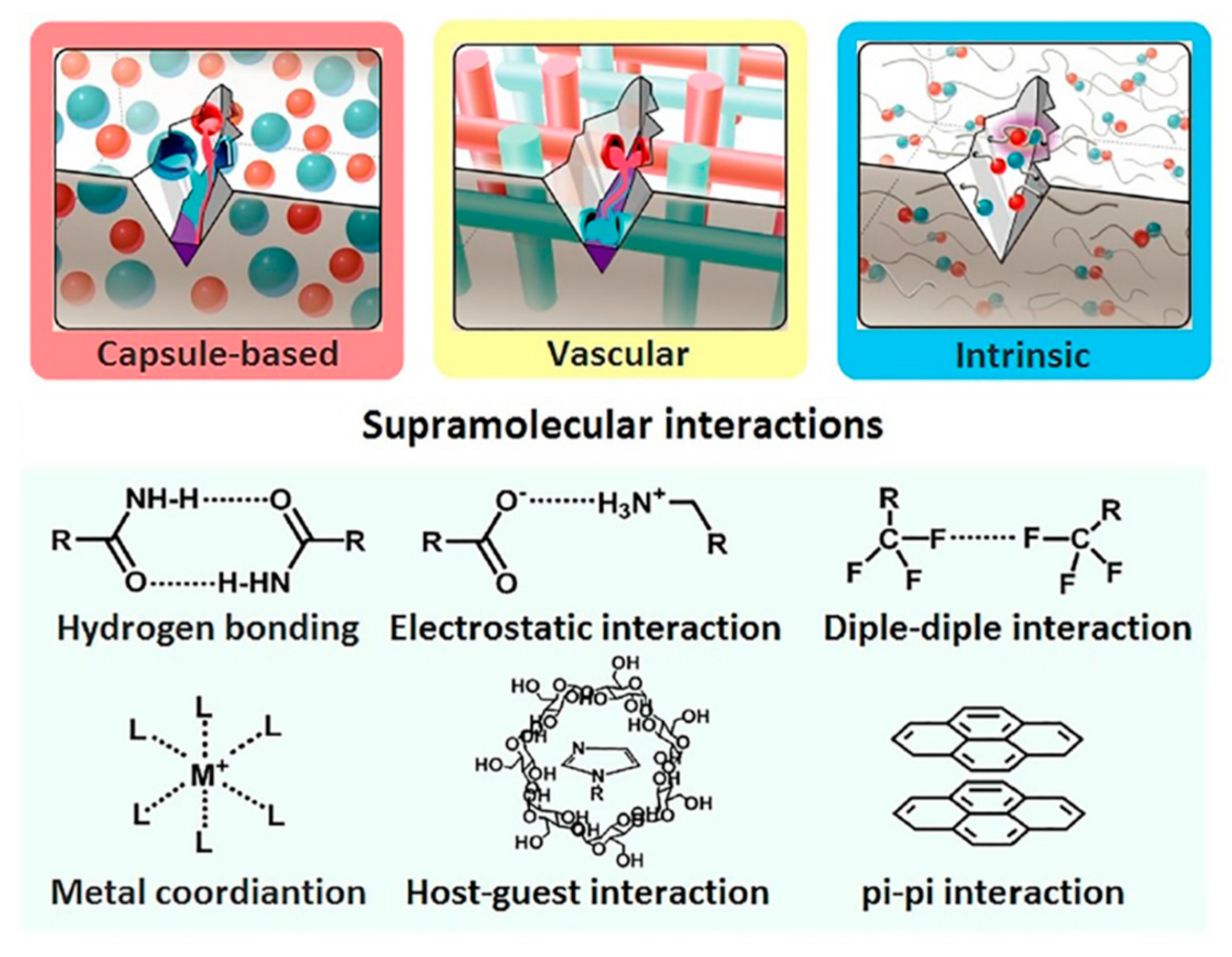
2. Metal–Ligand Coordination of Polymers
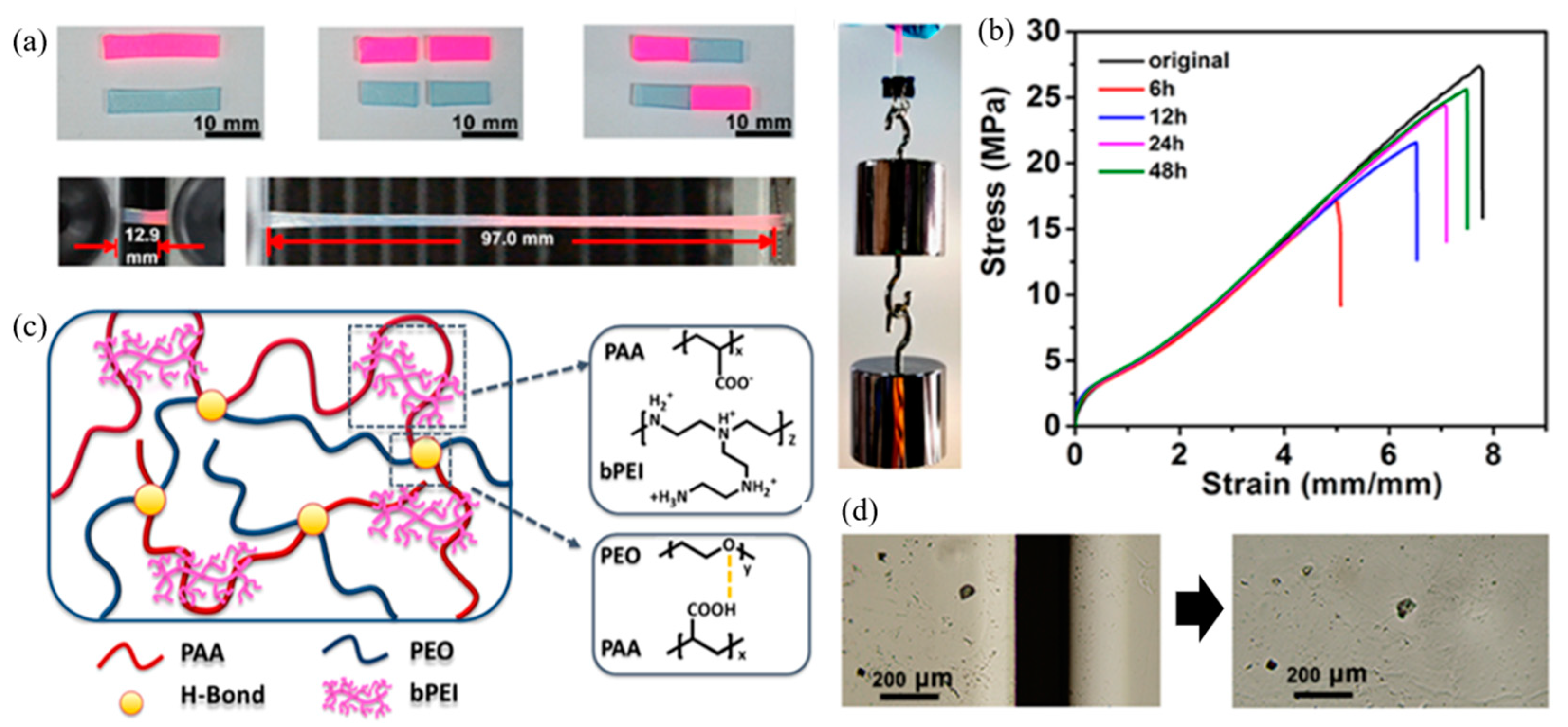
3. Hydrogen Bonding of Polymers
4. π–π Interaction of Polymers
5. Electrostatic Interaction of Polymers
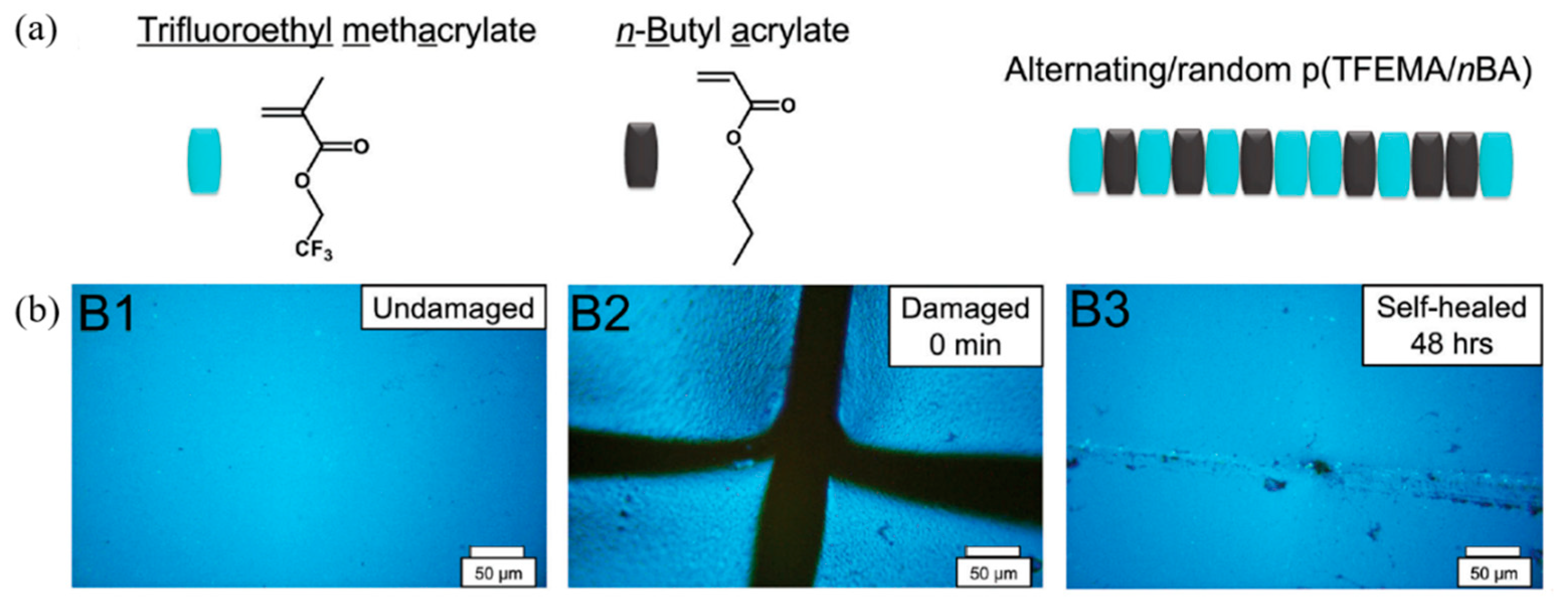
6. Dipole–Dipole Interaction of Polymers
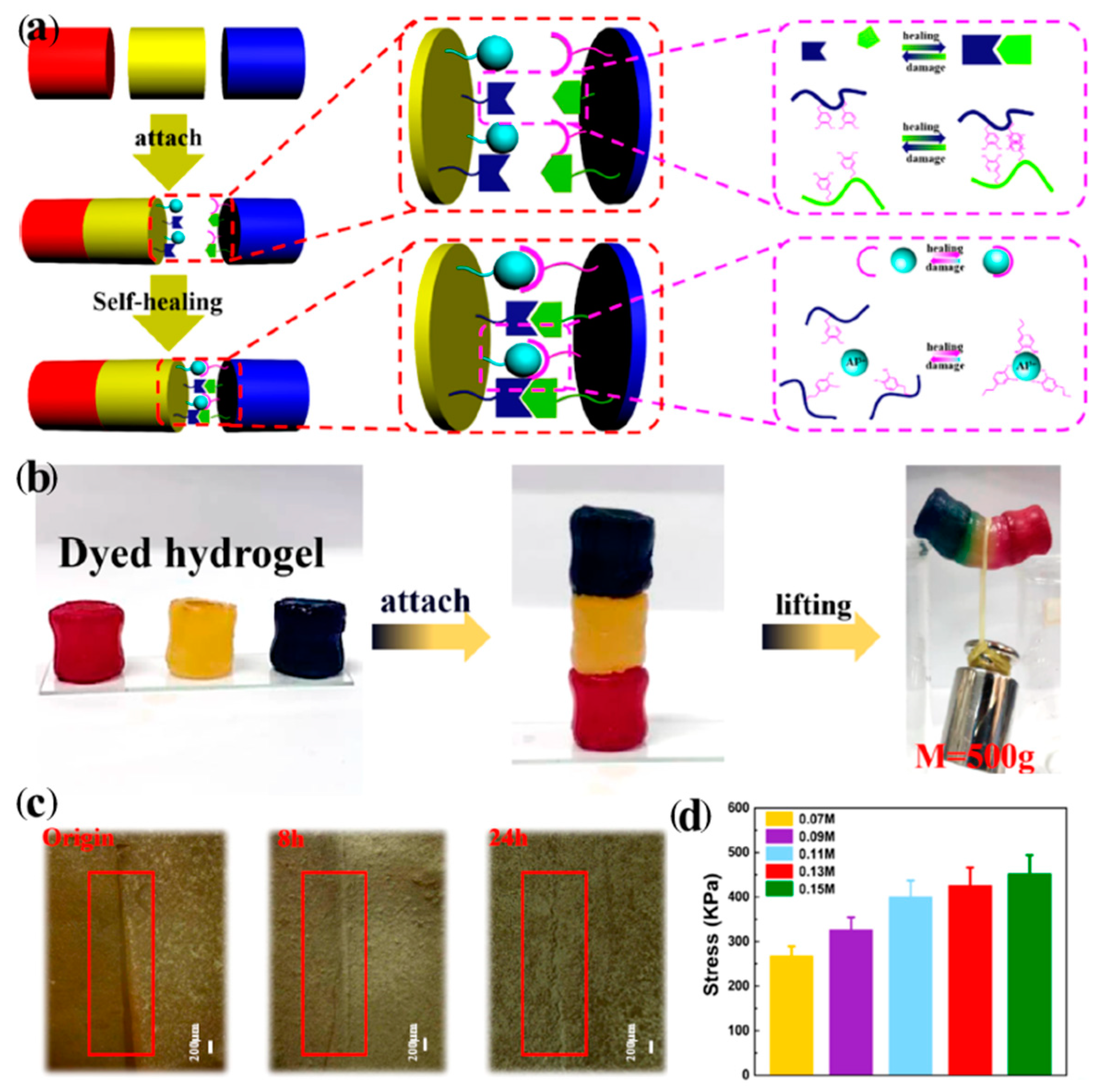
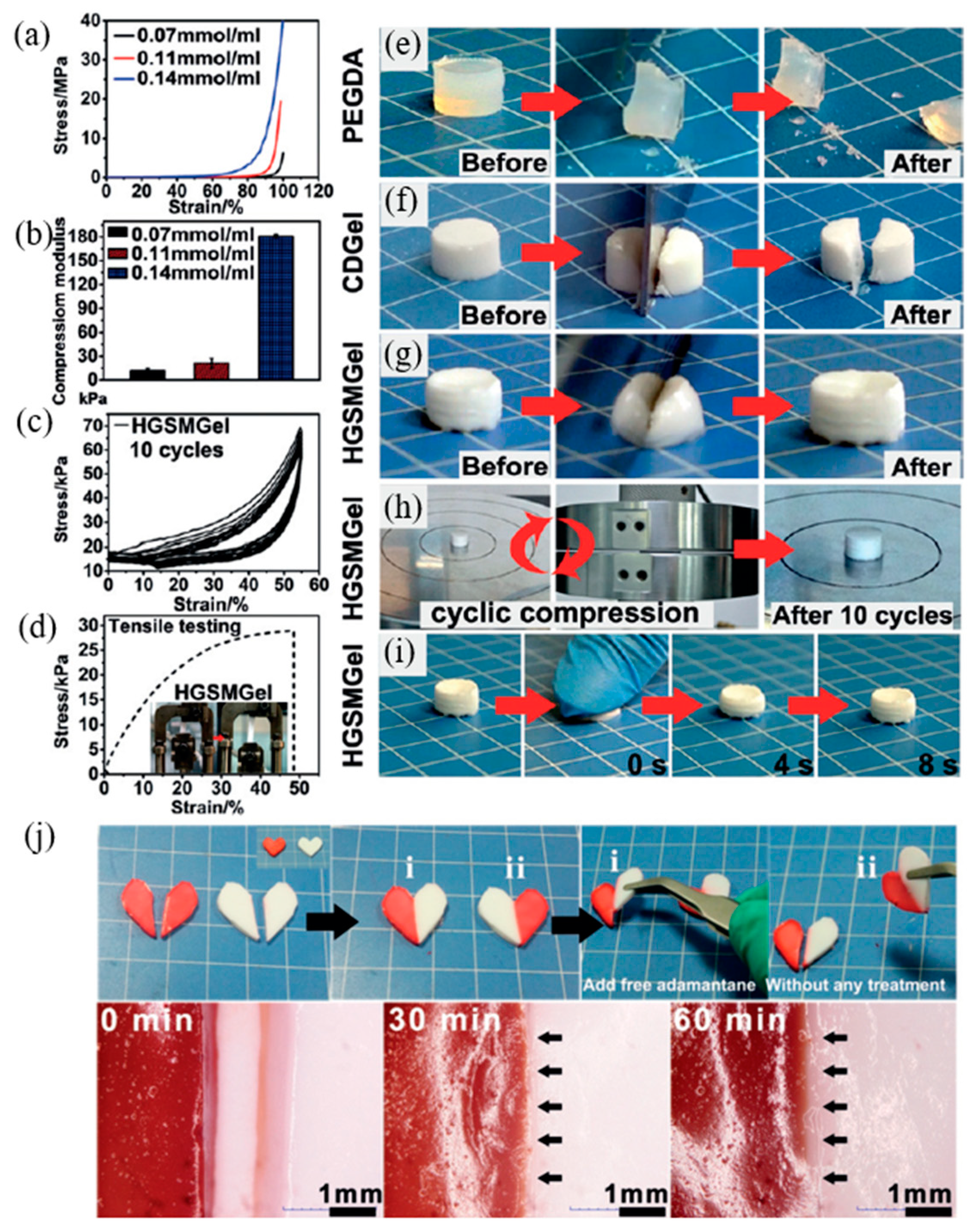
7. Host–Guest Interaction of Polymers
8. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinhoudt, D.N. Supramolecular Chemistry and Heterocycles. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–2. [Google Scholar]
- Gokel, G.W. Introduction and Overview of Supramolecular Receptor Types. In Comprehensive Supramolecular Chemistry II, 2nd ed.; Atwood, J.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–10. [Google Scholar]
- James, T.D. Specialty Grand Challenges in Supramolecular Chemistry. Front. Chem. 2017, 5, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuther, J.F.; Scanga, R.A.; Shahrokhinia, A.; Biswas, P. Self-healing materials utilizing supramolecular interactions. In Self-Healing Polymer-Based Systems, 1st ed.; Thomas, S., Surendran, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 293–367. [Google Scholar]
- Henderson, W.R.; Liu, G.; Abboud, K.A.; Castellano, R.K. Tuning Supramolecular Polymer Assembly through Stereoelectronic Interactions. J. Am. Chem. Soc. 2021, 143, 12688–12698. [Google Scholar] [CrossRef]
- Liu, B.; Rocca, D.; Yan, H.; Pan, D. Beyond Conformational Control: Effects of Noncovalent Interactions on Molecular Electronic Properties of Conjugated Polymers. JACS Au 2021, 1, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.O.; Caruso, M.M.; Schelkopf, S.R.; Sottos, N.R.; White, S.R.; Moore, J.S. Adhesion Promotion via Noncovalent Interactions in Self-Healing Polymers. ACS Appl. Mater. Interfaces 2011, 3, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Columbus, I.; Eren, N.; Elitsur, R.; Davidovich-Pinhas, M.; Shenhar, R. Branched Supramolecular Copolymers: Inducing Branching in Bisurea-Based Monomers Using Multi-Sulfonate Molecules. Macromolecules 2022, 55, 472–487. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host–Guest Interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Datta, S.; Takahashi, S.; Yagai, S. Nanoengineering of Curved Supramolecular Polymers: Toward Single-Chain Mesoscale Materials. Acc. Mater. Res. 2022, 3, 259–271. [Google Scholar] [CrossRef]
- Menger, F.M. Supramolecular Chemistry and Self-Assembly. Proc. Natl. Acad. Sci. USA 2002, 99, 4818–4822. [Google Scholar] [CrossRef] [Green Version]
- Lehn, J.-M. Toward Complex Matter: Supramolecular Chemistry and Self-Organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [Green Version]
- Turro, N.J. Molecular Structure as a Blueprint for Supramolecular Structure Chemistry in Confined Spaces. Proc. Natl. Acad. Sci. USA 2005, 102, 10766–10770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrenti, A.; Leira-Iglesias, J.; Markvoort, A.J.; de Greef, T.F.A.; Hermans, T.M. Non-equilibrium Supramolecular Polymerization. Chem. Soc. Rev. 2017, 46, 5476–5490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired Fabrication of High Strength Hydrogels from Non-Covalent Interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
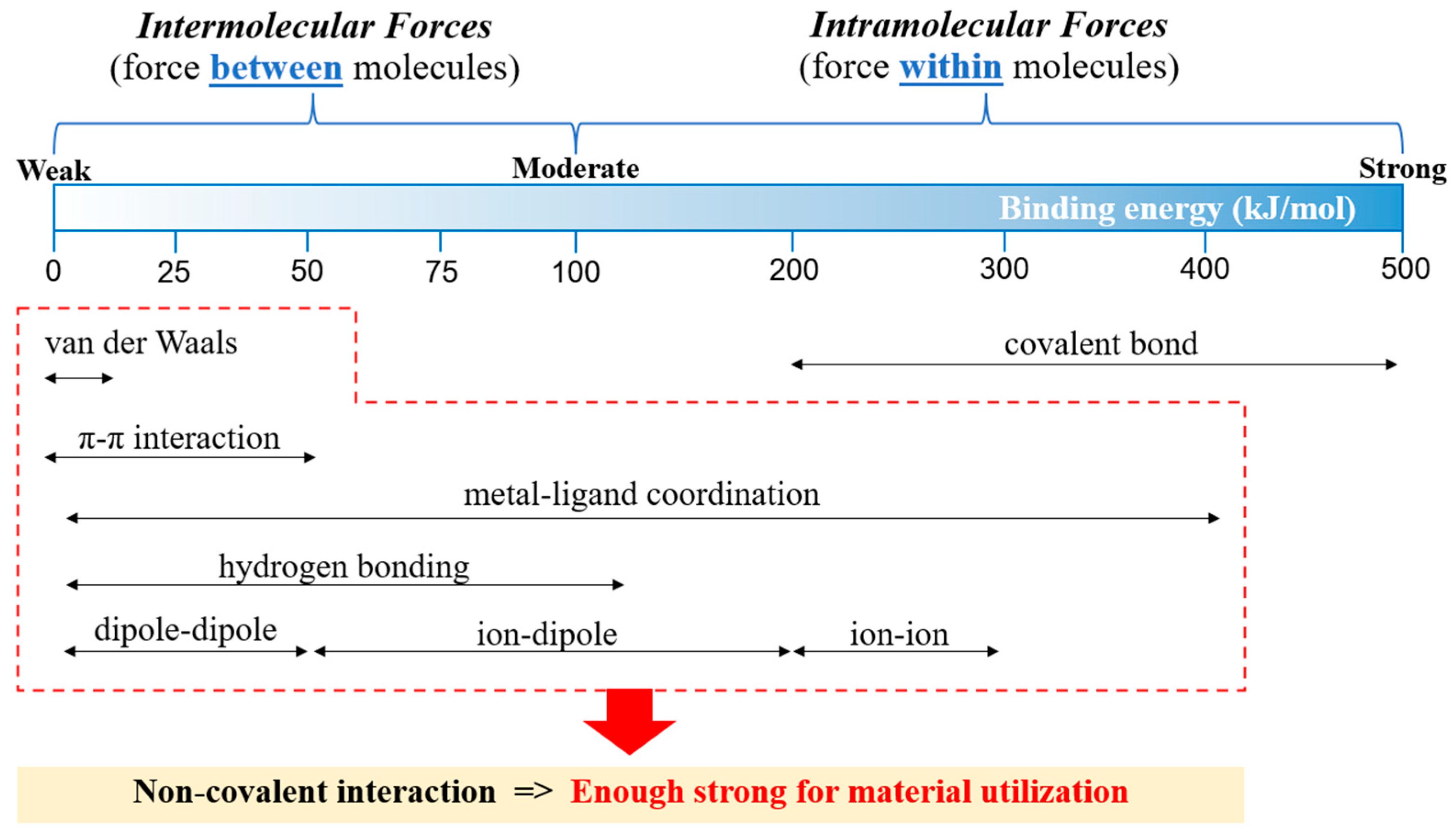
- Cooper, M.M.; Williams, L.C.; Underwood, S.M. Student Understanding of Intermolecular Forces: A Multimodal Study. J. Chem. Educ. 2015, 92, 1288–1298. [Google Scholar] [CrossRef]
- Raczyński, K.; Pihut, A.; Panek, J.J.; Jezierska, A. Competition of Intra- and Intermolecular Forces in Anthraquinone and Its Selected Derivatives. Molecules 2021, 26, 3448. [Google Scholar] [CrossRef]
- Huler, E.; Warshel, A. Incorporation of Inter- and Intramolecular Forces in The Calculation of Crystal Packing and Lattice Vibrations. Acta Cryst. 1974, 30B, 1822–1826. [Google Scholar] [CrossRef]
- Moy, V.T.; Florin, E.-L.; Gaub, H.E. Intermolecular Forces and Energies Between Ligands and Receptors. Science 1994, 266, 257–259. [Google Scholar]
- Yi, X.; He, J.; Wang, X.; Zhang, Y.; Tan, G.; Zhou, Z.; Chen, J.; Chen, D.; Wang, R.; Tian, W.; et al. Tunable Mechanical, Antibacterial, and Cytocompatible Hydrogels Based on a Functionalized Dual Network of Metal Coordination Bonds and Covalent Crosslinking. ACS Appl. Mater. Interfaces 2018, 10, 6190–6198. [Google Scholar] [CrossRef]
- Watuthanthrige, N.D.A.; Dunn, D.; Dolan, M.; Sparks, J.L.; Ye, Z.; Zanjani, M.B.; Konkolewicz, D. Tuning Dual-Dynamic Network Materials through Polymer Architectural Features. ACS Appl. Polym. Mater. 2022, 4, 1475–1486. [Google Scholar] [CrossRef]
- Lan, J.; Li, Y.; Yan, B.; Yin, C.; Ran, R.; Shi, L.-Y. Transparent Stretchable Dual-Network Ionogel with Temperature Tolerance for High-Performance Flexible Strain Sensors. ACS Appl. Mater. Interfaces 2020, 12, 37597–37606. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.; Qiu, C.; Xu, X.; Jin, Z. A Dual Cross-Linked Strategy to Construct Moldable Hydrogels with High Stretchability, Good Self-Recovery, and Self-Healing Capability. J. Agric. Food Chem. 2019, 67, 3966–3980. [Google Scholar] [CrossRef] [PubMed]
- Sriring, M.; Nimpaiboon, A.; Kumarn, S.; Sirisinha, C.; Sakdapipanich, J.; Toki, S. Viscoelastic and mechanical properties of large- and small-particle natural rubber before and after vulcanization. Polym. Test. 2018, 70, 127–134. [Google Scholar] [CrossRef]
- Promhuad, K.; Smitthipong, W. Effect of Stabilizer States (Solid vs Liquid) on Properties of Stabilized Natural Rubbers. Polymers 2020, 12, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suethao, S.; Phongphanphanee, S.; Wong-ekkabut, J.; Smitthipong, W. The Relationship between the Morphology and Elasticity of Natural Rubber Foam Based on the Concentration of the Chemical Blowing Agent. Polymers 2021, 13, 1091. [Google Scholar] [CrossRef]
- Suethao, S.; Ponloa, W.; Phongphanphanee, S.; Wong-ekkabut, J.; Smitthipong, W. Current challenges in thermodynamic aspects of rubber foam. Sci. Rep. 2021, 11, 6097. [Google Scholar] [CrossRef]
- Prasopdee, T.; Smitthipong, W. Effect of Fillers on the Recovery of Rubber Foam: From Theory to Applications. Polymers 2020, 12, 2745. [Google Scholar]
- Phuhiangpa, N.; Ponloa, W.; Phongphanphanee, S.; Smitthipong, W. Performance of Nano- and Microcalcium Carbonate in Uncrosslinked Natural Rubber Composites: New Results of Structure-Properties Relationship. Polymers 2020, 12, 2002. [Google Scholar] [CrossRef]
- Liu, C.; Huang, S.; Hou, J.; Zhang, W.; Wang, J.; Yang, H.; Zhang, J. Natural Rubber Latex Reinforced by Graphene Oxide/Zwitterionic Chitin Nanocrystal Hybrids for High-Performance Elastomers without Sulfur Vulcanization. ACS Sustain. Chem. Eng. 2021, 9, 6470–6478. [Google Scholar] [CrossRef]
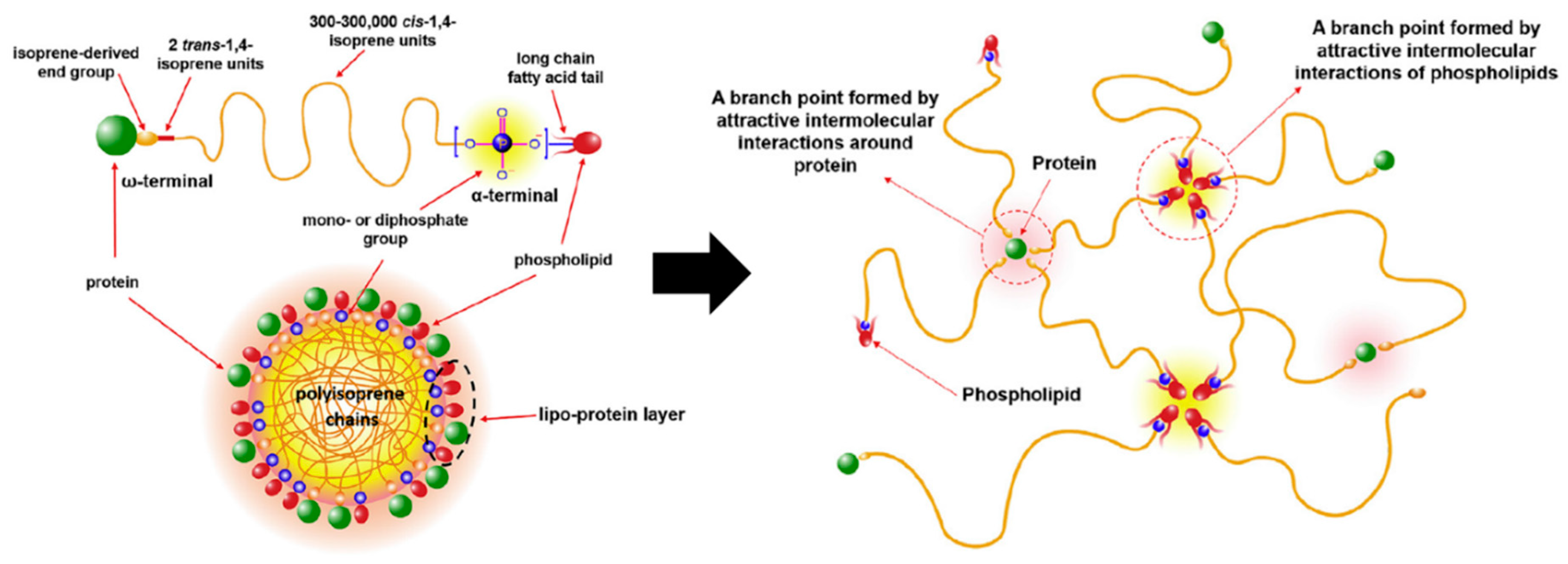
- Mekkriengkrai, D.; T.Sakdapipanich, J.; Tanaka, Y. Structural Characterization of Terminal Groups in Natural Rubber: Origin of Nitrogenous Groups. Rubber Chem. Technol. 2006, 79, 366–379. [Google Scholar] [CrossRef]
- Tarachiwin, L.; Sakdapipanich, J.; Ute, K.; Kitayama, T.; Tanaka, Y. Structural Characterization of α-Terminal Group of Natural Rubber. 2. Decomposition of Branch-Points by Phospholipase and Chemical Treatments. Biomacromolecules 2005, 6, 1858–1863. [Google Scholar] [CrossRef]
- Tangpakdee, J.; Tanaka, Y. Purification of Natural Rubber. J. Nat. Rubber Res. 1997, 12, 112–119. [Google Scholar]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C.; Ma, Y.; Song, J.; Vancso, J.G. Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 157–166. [Google Scholar] [CrossRef]
- De Gennes, P.G. Reptation of a Polymer Chain in the Presence of Fixed Obstacles. J. Chem. Phys. 1971, 55, 572–579. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Hopmann, C.; Twardowski, B.; Bakir, C. Limitations of Reptation Theory for Modeling the Stress-Dependent Rheological Behavior of Polyethylene Terephthalate Above the Glass-Transition Temperature. Polym. Eng. Sci. 2020, 60, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Banks, H.T.; Medhin, N.G.; Pinter, G.A. Nonlinear reptation in molecular based hysteresis models for polymers. Q. Appl. Math. 2004, 62, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.F. Dynamical Theory of Rubber Elasticity. Polym. J. 1985, 17, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Urayama, K. Network Topology-Mechanical Properties Relationships of Model Elastomers. Polym. J. 2008, 40, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Payungwong, N.; Tuampoemsab, S.; Rojruthai, P.; Sakdapipanich, J. The role of model fatty acid and protein on thermal aging and ozone resistance of peroxide vulcanized natural rubber. J. Rubber Res. 2021, 24, 543–553. [Google Scholar] [CrossRef]
- Li, G.; Meng, H. Overview of crack self-healing. In Recent Advances in Smart Self-Healing Polymers and Composites, 1st ed.; Li, G., Meng, H., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–19. [Google Scholar]
- Xu, J.H.; Ding, C.D.; Chen, P.; Tan, L.H.; Chen, C.B.; Fu, J.J. Intrinsic self-healing polymers for advanced lithium-based batteries: Advances and strategies. Appl. Phys. Rev. 2020, 7, 031304. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Liow, S.S.; Xue, K.; Zhang, X.; Li, Z.; Loh, X.J. Autonomous Chitosan-Based Self-Healing Hydrogel Formed through Noncovalent Interactions. ACS Appl. Polym. Mater. 2019, 1, 1769–1777. [Google Scholar] [CrossRef]
- Li, Z.; Yu, R.; Guo, B. Shape-Memory and Self-Healing Polymers Based on Dynamic Covalent Bonds and Dynamic Noncovalent Interactions: Synthesis, Mechanism, and Application. ACS Appl. Bio. Mater. 2021, 4, 5926–5943. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.A.; Gangadharan, S.; Patnaik, A. Design of Dual Hybrid Network Natural Rubber-SiO2 Elastomers with Tailored Mechanical and Self-Healing Properties. ACS Omega 2019, 4, 10939–10949. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Lu, X.; Zhou, J.; Qin, R.; Yang, Y. Dual Cross-Linked Self-Healing and Recyclable Epoxidized Natural Rubber Based on Multiple Reversible Effects. ACS Sustain. Chem. Eng. 2019, 7, 4443–4455. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Santana, M.H.; Verdejo, R.; López-Manchado, M.A. Design of Rubber Composites with Autonomous Self-Healing Capability. ACS Omega 2020, 5, 1902–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Santana, M.H. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Campanella, A.; Döhler, D.; Binder, W.H. Self-Healing in Supramolecular Polymers. Macromol. Rapid Commun. 2018, 39, 1700739. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Walker, B.; Michaelides, A. Quantum nature of the hydrogen bond. Proc. Natl. Acad. Sci. USA 2011, 108, 6369–6373. [Google Scholar] [CrossRef] [Green Version]
- Sordo, F.; Mougnier, S.-J.; Loureiro, N.; Tournilhac, F.; Michaud, V. Design of Self-Healing Supramolecular Rubbers with a Tunable Number of Chemical Cross-Links. Macromolecules 2015, 48, 4394–4402. [Google Scholar] [CrossRef]
- Pal, A.; Mahapatra, R.D.; Dey, J. Understanding the Role of H-Bonding in Self-Aggregation in Organic Liquids by Fatty Acid Amphiphiles with a Hydrocarbon Tail Containing Different H-Bonding Linker Groups. Langmuir 2014, 30, 13791–13798. [Google Scholar] [CrossRef]
- Fedorova, I.V.; Krestyaninov, M.A.; Safanova, L.P. Ab Initio Study of Structural Features and H-Bonding in Alkylammonium-Based Protic Ionic Liquids. J. Phys. Chem. A. 2017, 121, 7675–7683. [Google Scholar] [CrossRef]
- Deng, G.; Wong, W.-T.; Huang, M.; Wu, R.; Lai, W.-F. Self-Healing Properties of Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 223–245. [Google Scholar]
- Onsager, L. Electrostatic Interaction of Molecules. J. Phys. Chem. 1939, 43, 189–196. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.; Greer, J.M.; Kreuels, K.; Anderson, D.G.; Langer, R. Exploiting Electrostatic Interactions in Polymer—Nanoparticle Hydrogels. ACS Macro Lett. 2015, 4, 848–852. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Sukhishvili, S.A. Competition of Hydrogen-Bonding and Electrostatic Interactions within Hybrid Polymer Multilayers. Langmuir 2004, 20, 10712–10717. [Google Scholar] [CrossRef]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E. The Concept of Self-Assembling and the Interactions Involved. In Controlled Drug Delivery; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–20. [Google Scholar]
- Das, A.; Lin, S.; Theato, P. Supramolecularly Cross-Linked Nanogel by Merocyanine Pendent Copolymer. ACS Macro Lett. 2017, 6, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-C.; Hao, Q.; Wang, C.-S. Improved Polarizable Dipole–Dipole Interaction Model for Hydrogen Bonding, Stacking, T-Shaped, and X–H·π Interactions. J. Chem. Theory Comput. 2017, 13, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Zoppo, M.D.; Tommasini, M.; Zerbi, G. The Effect of Intermolecular Dipole–Dipole Interaction on Raman Spectra of Polyconjugated Molecules: Density Functional Theory Simulations and Mathematical Models. J. Phys. Chem. B 2008, 112, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R. Coordination Polymers and Metal–Organic Frameworks: Materials by Design. Phil. Trans. R. Soc. A 2016, 375, 20160032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noro, A.; Matsushima, S.; He, X.; Hayashi, M.; Matsushita, Y. Thermoreversible Supramolecular Polymer Gels via Metal–Ligand Coordination in an Ionic Liquid. Macromolecules 2013, 46, 8304–8310. [Google Scholar] [CrossRef]
- Sanoja, G.E.; Schauser, N.S.; Bartels, J.M.; Evans, C.M.; Helgeson, M.E.; Seshadri, R.; Segalman, R.A. Ion Transport in Dynamic Polymer Networks Based on Metal–Ligand Coordination: Effect of Cross-Linker Concentration. Macromolecules 2018, 51, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.-L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.-C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable Self-Healing Polymeric Dielectrics Cross-Linked Through Metal–Ligand Coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef]
- Li, X.; Cai, Z.; Jiang, L.-P.; He, Z.; Zhu, J.-J. Metal–Ligand Coordination Nanomaterials for Biomedical Imaging. Bioconjugate Chem. 2020, 31, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, E.; Decher, G. Layer-by-Layer Assembly of Multifunctional Hybrid Materials and Nanoscale Devices. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 159–185. [Google Scholar]
- Wu, S. Surface and Interfacial Tensions of Polymer Melts. II. Poly (methyl methacrylate), Poly (n-butyl methacrylate), and Polystyrene. J. Phys. Chem. 1970, 74, 632–638. [Google Scholar] [CrossRef]
- Zhan, W.; Wei, T.; Yu, Q.; Chen, H. Fabrication of Supramolecular Bioactive Surfaces via β-Cyclodextrin-Based Host–Guest Interactions. ACS Appl. Mater. Interfaces 2018, 10, 36585–36601. [Google Scholar] [CrossRef] [PubMed]
- Market Revenue of Self-Healing Materials in the United States from 2020 to 2025. Available online: https://www.statista.com/statistics/1043558/us-market-value-of-self-healing-materials-by-product/ (accessed on 1 March 2022).
- Smitthipong, W.; Nardin, M.; Schultz, J.; Nipithakul, T.; Suchiva, K. Study of Tack Properties of Uncrosslinked Natural Rubber. J. Adhesion Sci. Technol. 2004, 18, 1449–1463. [Google Scholar] [CrossRef]
- Smitthipong, W.; Nardin, M.; Schultz, J.; Suchiva, K. Adhesion and Self-Adhesion of Rubbers, Crosslinked by Electron Beam Irradiation. Int. J. Adhes. Adhes. 2007, 27, 352–357. [Google Scholar] [CrossRef]
- Smitthipong, W.; Nardin, M.; Schultz, J.; Suchiva, K. Adhesion and Self-Adhesion of Immiscible Rubber Blends. Int. J. Adhes. Adhes. 2009, 29, 253–258. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, M.; Xu, C.; Wang, Y.; Wang, Q.; Liu, W. Sodium Alginate Crosslinked Oxidized Natural Rubber Supramolecular Network with Rapid Self-Healing at Room Temperature and Improved Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106601. [Google Scholar] [CrossRef]
- Hornat, C.C.; Urban, M.W. Entropy and Interfacial Energy Driven Self-Healable Polymers. Nat. Commun. 2020, 11, 1028–1036. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-Healing Polymeric Materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef]
- Voyiadjis, G.Z.; Shojaei, A.; Li, G. A Thermodynamic Consistent Damage and Healing Model for Self Healing Materials. Int. J. Plast. 2011, 27, 1025–1044. [Google Scholar] [CrossRef]
- Tan, Y.J.; Susanto, G.J.; Anwar, H.P.; Tee, B.C.K. Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater. 2021, 33, 2002800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Tian, Z.; Zhu, J.; Shi, Z.; Cui, Z.; Zhu, S. Enhancement Performance of Application Mussel-Biomimetic Adhesive Primer for Dentin Adhesives. RSC Adv. 2020, 10, 12035–12046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zuo, J.-L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2020, 32, 1903762. [Google Scholar] [CrossRef] [PubMed]
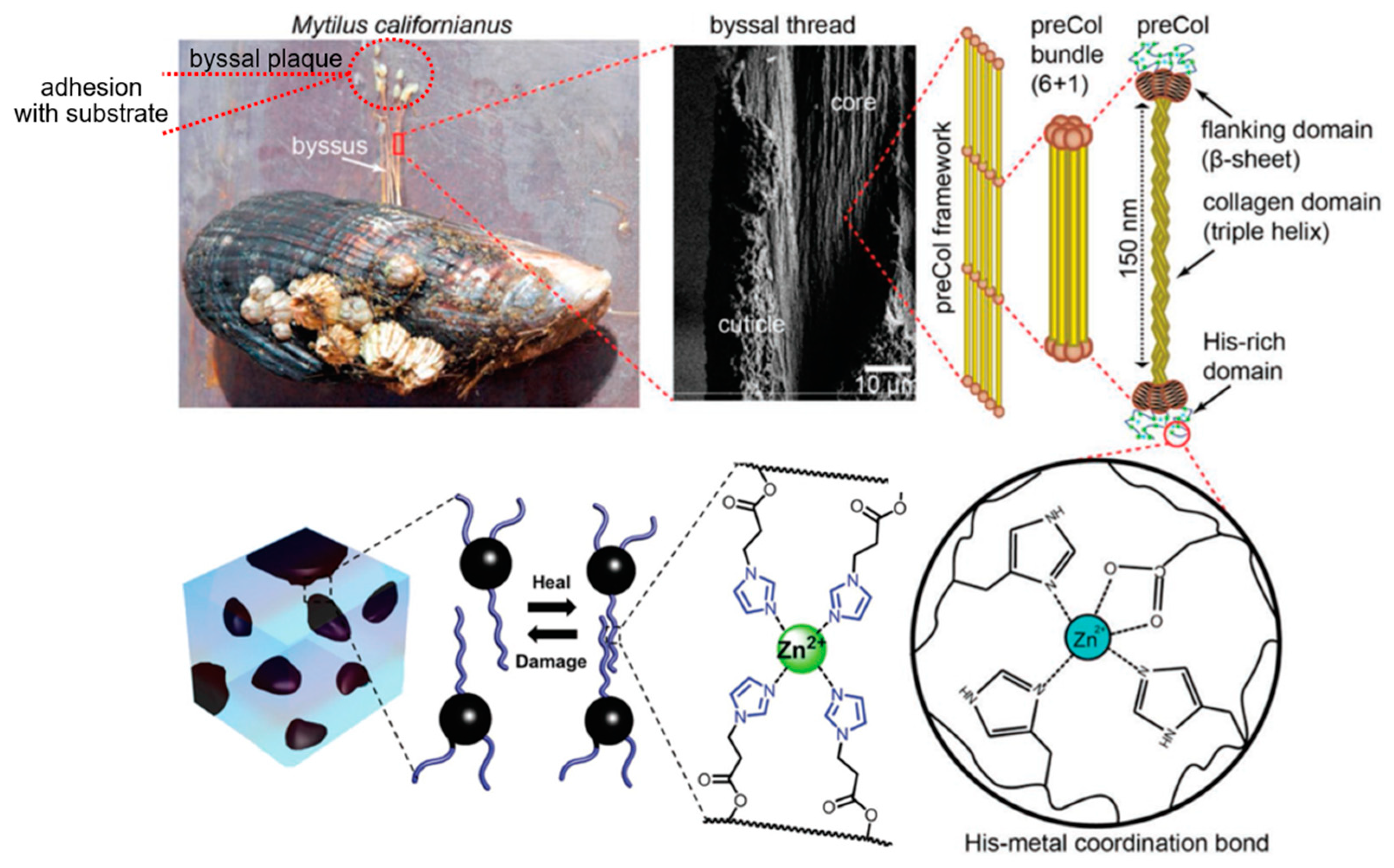
- Unraveling Secrets of the Mussel Foot Protein. Available online: https://dailynexus.com/2015-12-23/unraveling-secrets-of-the-mussel-foot-protein/ (accessed on 2 May 2022).
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-Inspired Hydrogels for Biomedical and Environmental Applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
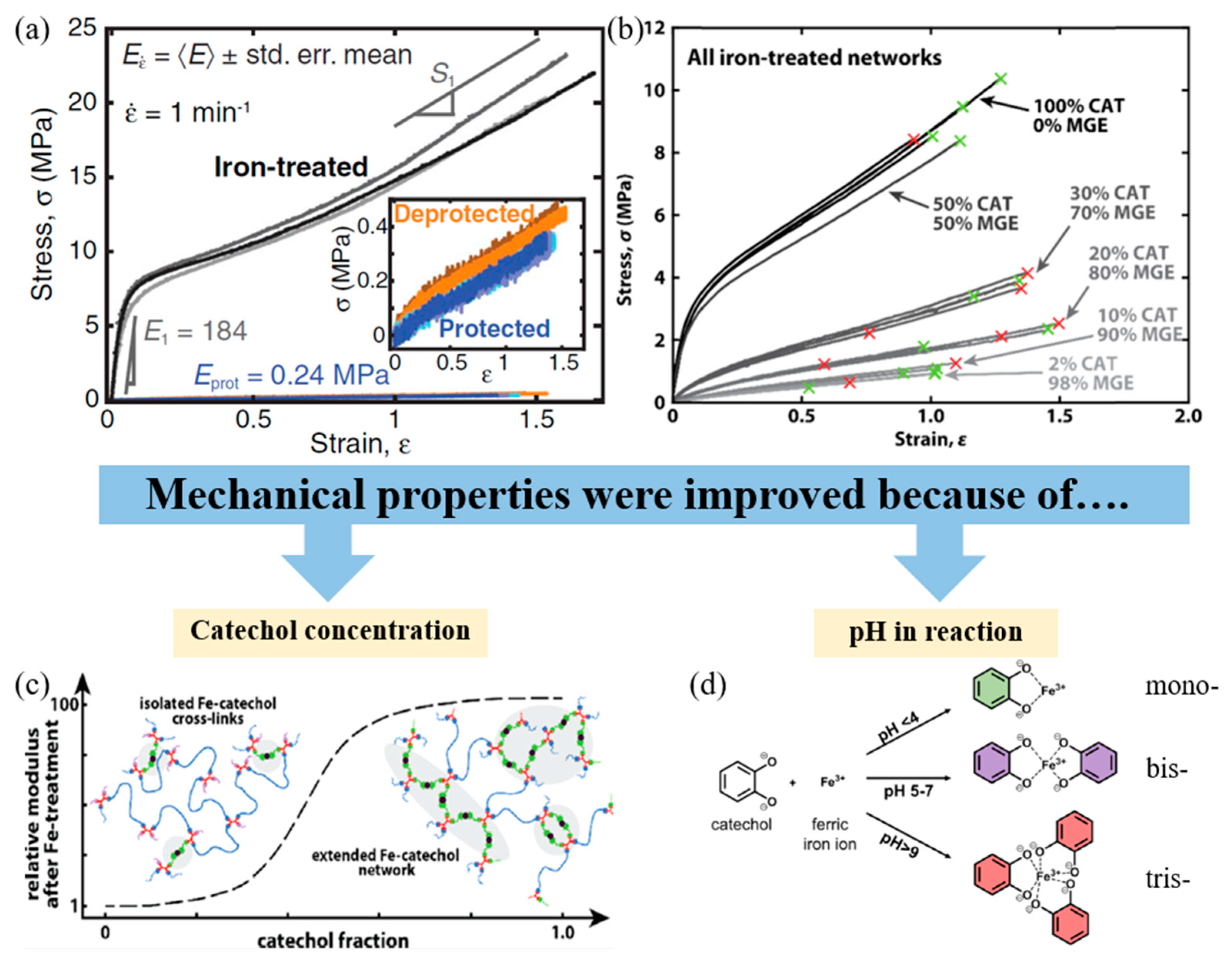
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening Elastomers Using Mussel-Inspired Iron-Catechol Complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [Green Version]
- Cristiani, T.R.; Filippidi, E.; Behrens, R.L.; Valentine, M.T.; Eisenbach, C.D. Tailoring the Toughness of Elastomers by Incorporating Ionic Cross-Linking. Macromolecules 2020, 53, 4099–4109. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-Induced Metal–Ligand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with Near-Covalent Elastic Moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, J.; de Bruijn, W.J.C.; Hageman, J.A.; Goos, P.; Velikov, K.P.; Vincken, J.-P. Revealing the Main Factors and Two-Way Interactions Contributing to Food Discolouration Caused by Iron-Catechol Complexation. Sci. Rep. 2020, 10, 8288. [Google Scholar] [CrossRef]
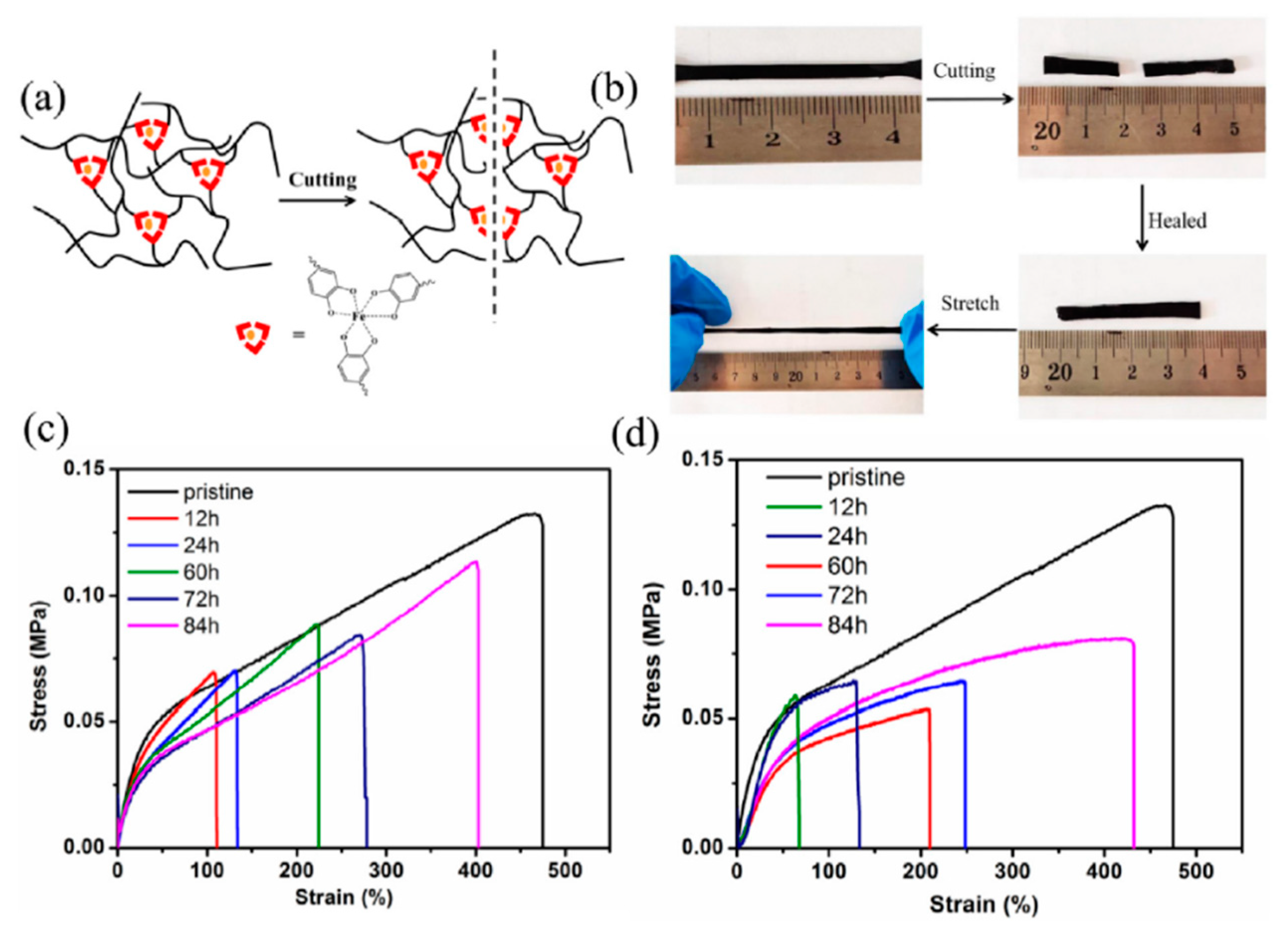
- Han, Y.; Wu, X.; Zhang, X.; Lu, C. Self-Healing, Highly Sensitive Electronic Sensors Enabled by Metal–Ligand Coordination and Hierarchical Structure Design. ACS Appl. Mater. Interfaces 2017, 9, 20106–20114. [Google Scholar] [CrossRef]
- Li, Z.; Shan, Y.; Wang, X.; Li, H.; Yang, K.; Cui, Y. Self-Healing Flexible Sensor Based on Metal–Ligand Coordination. Chem. Eng. J. 2020, 394, 124932. [Google Scholar] [CrossRef]
- Cao, L.; Gong, Z.; Liu, C.; Fan, J.; Chen, Y. Design and Fabrication of Mechanically Strong and Self-Healing Rubbers via Metal–Ligand Coordination Bonds as Dynamic Crosslinks. Compos. Sci. Technol. 2021, 207, 108750. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Zao, W.; Feng, H.; Hou, Y.; Huo, A. High-Performance Nitrile Butadiene Rubber Composites with Good Mechanical Properties, Tunable Elasticity, and Robust Shape Memory Behaviors. Ind. Eng. Chem. Res. 2020, 59, 15936–15947. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Zhang, J.; Ni, R.; Xu, L.; He, T.; Lin, X.; Li, X.; Qiu, H.; Yin, S.; et al. Self-Healing Heterometallic Supramolecular Polymers Constructed by Hierarchical Assembly of Triply Orthogonal Interactions with Tunable Photo-physical Properties. J. Am. Chem. Soc. 2019, 141, 17909–17917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, J.; Tian, X.; Wei, J.; Li, Q.; Wang, Y. Self-healing metallo-supramolecular polymers showing luminescence off/on switching based on lanthanide ions and terpyridine moieties. Chem. Eng. J. 2022, 434, 134806. [Google Scholar] [CrossRef]
- Li, F.; Xia, H. Dopamine-Functionalized Poly(vinyl alcohol) Elastomer with Melt Processability and Self-Healing Properties. J. Appl. Polym. Sci. 2017, 134, 45072. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Sun, J.; Yuan, J.; Yang, Z.; Gao, C.; Wu, Y. A Type of Hydrogen Bond Cross-Linked Silicone Rubber with the Thermal-Induced Self-Healing Properties Based on the Nonisocyanate Reaction. Ind. Eng. Chem. Res. 2019, 58, 21452–21458. [Google Scholar] [CrossRef]
- Xu, C.; Nie, J.; Wu, W.; Zheng, Z.; Chen, Y. Self-Healable, Recyclable, and Strengthened Epoxidized Natural Rubber/Carboxymethyl Chitosan Biobased Composites with Hydrogen Bonding Supramolecular Hybrid Networks. ACS Sustain. Chem. Eng. 2019, 7, 15778–15789. [Google Scholar] [CrossRef]
- Chen, G.; Wen, S.; Yue, Z. Design of Robust Self-Healing Silicone Elastomers Based on Multiple H-Bonding and Dynamic Covalent Bond. Langmuir 2022, 38, 1194–1203. [Google Scholar] [CrossRef]
- Wheeler, S.E. Understanding Substituent Effects in Noncovalent Interactions Involving Aromatic Rings. Acc. Chem. Res. 2013, 46, 1029–1038. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Liu, J. π–π Stacking Interaction: A Nondestructive and Facile Means in Material Engineering for Bioapplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Burattini, S.; Colquhoun, H.M.; Fox, J.D.; Friedmann, D.; Greenland, B.W.; Harris, P.J.F.; Hayes, W.; Mackay, M.E.; Rowan, S.J. A Self-Repairing, Supramolecular Polymer System: Healability as a Consequence of Donor-Acceptor p-p Stacking Interactions. Chem. Commun. 2009, 44, 6717–6719. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π–π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
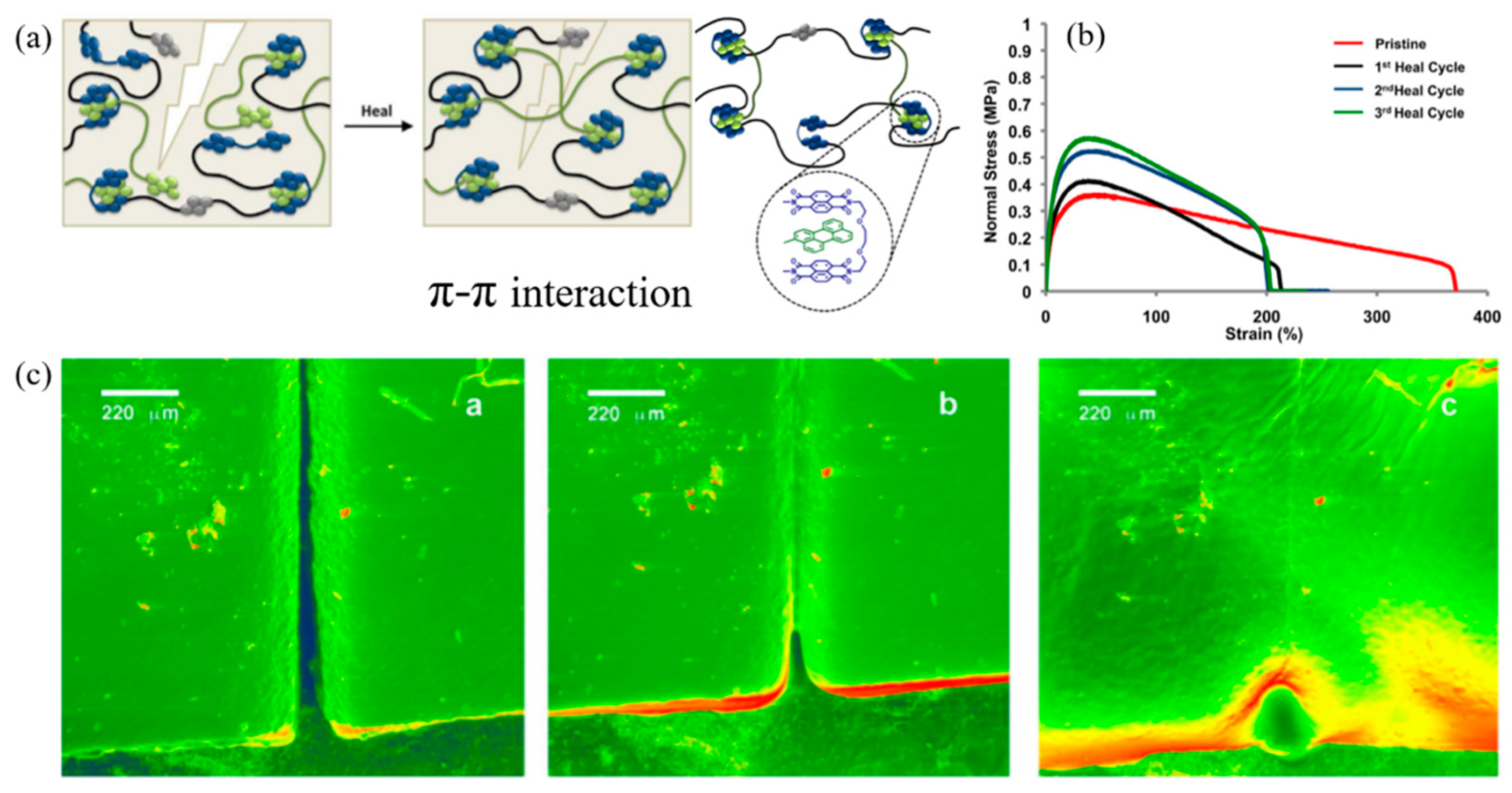
- Hart, L.R.; Nguyen, N.A.; Harries, J.L.; Mackay, M.E.; Colquhoun, H.M.; Hayes, W. Perylene as an Electron-Rich Moiety in Healable, Complementary π–π Stacked, Supramolecular Polymer Systems. Polymer 2015, 69, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Zhai, W.; Chen, C.; Lu, D.; Wang, J.; Zheng, W. Melt Blending In situ Enhances the Interaction between Polystyrene and Graphene through π–π Stacking. ACS Appl. Mater. Interfaces 2011, 3, 3103–3109. [Google Scholar] [CrossRef]
- Zajaczkowska, H.; Veith, L.; Waliszewski, W.; Bartkiewicz, M.A.; Borkowski, M.; Sleczkowski, P.; Ulanski, J.; Graczykowski, B.; Blow, P.W.M.; Pisula, W. Self-Aligned Bilayers for Flexible Free-Standing Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 59012–59022. [Google Scholar] [CrossRef]
- Guo, H.; Fang, X.; Zhang, L.; Sun, J. Facile Fabrication of Room-Temperature Self-Healing, Mechanically Robust, Highly Stretchable, and Tough Polymers Using Dual Dynamic Cross-Linked Polymer Complexes. ACS Appl. Mater. Interfaces 2019, 11, 33356–33363. [Google Scholar] [CrossRef]
- Deng, X.; Huang, B.; Wang, Q.; Wu, W.; Coates, P.; Sefat, F.; Lu, C.; Zhang, W.; Zhang, X. A Mussel-Inspired Antibacterial Hydrogel with High Cell Affinity, Toughness, Self-Healing, and Recycling Properties for Wound Healing. ACS Sustain. Chem. Eng. 2021, 9, 3070–3082. [Google Scholar] [CrossRef]
- Su, G.; Yin, S.; Guo, Y.; Zhao, F.; Guo, Q.; Zhang, X.; Zhou, T.; Yu, G. Balancing the Mechanical, Electronic, and Self-Healing Properties in Conductive Self-Healing Hydrogel for Wearable Sensor Applications. Mater. Horiz. 2021, 8, 1795–1804. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, H.; Allec, S.I.; Wong, B.M.; Nguyen, D.-S.; Wang, C. A Highly Stretchy, Transparent Elastomer with the Capability to Automatically Self-Heal Underwater. Adv. Mater. 2018, 30, 1804602. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Qin, B.; Chen, L.; Liu, Y.; Zhang, X.; Wang, C. Highly Transparent, Underwater Self-Healing, and Ionic Conductive Elastomer Based on Multivalent Ion–Dipole Interactions. Chem. Mater. 2020, 32, 6310–6317. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-Healable Fluorinated Copolymers Governed by Dipolar Interactions. Adv. Sci. 2021, 8, 2101399. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Chen, J.; Li, X.; Shi, J.; Wang, X.; Zhang, J.; Gao, D.; Zhai, Y.; Zhao, Y.; Weng, S.; et al. Investigation on the Dipole–Dipole Interactions between Tetramethylurea and Acetonitrile by Two-Dimensional Asynchronous Spectroscopy. J. Mol. Struct. 2014, 1069, 264–271. [Google Scholar] [CrossRef]
- Shimoaka, T.; Ukai, H.; Kurishima, K.; Takei, K.; Yamada, N.; Hasegawa, T. Molecular Aggregation of Perfluoroalkyl Groups Can Win the Hydrogen Bonding between Amides. J. Phys. Chem. C 2018, 122, 22018–22023. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Y.; Zhu, Y.; Hao, L.; Chen, Y.; An, G.; Wu, H.; Shi, X.; Mao, C. A Rapidly Self-Healing Host–Guest Supramolecular Hydrogel with High Mechanical Strength and Excellent Biocompatibility. Angew. Chem. Int. Ed. 2018, 57, 9008–9012. [Google Scholar] [CrossRef]
- Hou, J.-B.; Zhang, X.-Q.; Wu, D.; Feng, J.-F.; Ke, D.; Li, B.-J.; Zhang, S. Tough Self-Healing Elastomers Based on the Host–Guest Interaction of Polycyclodextrin. ACS Appl. Mater. Interfaces 2019, 11, 12105–12113. [Google Scholar] [CrossRef]
- Park, J.; Oh, Y.; Song, H.-W.; Choi, E.; Kim, H. Biobased Stimuli-Responsive Hydrogels That Comprise Supramolecular Interpenetrating Networks and Exhibit Programmed Behaviors. Chem. Mater. 2021, 33, 8124–8132. [Google Scholar] [CrossRef]




| Type of Product | Market Revenue (in USD Millions) | |||||
|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | |
| Fiber-reinforced composites | 53.50 | 62.70 | 73.57 | 81.93 | 92.80 | 101.99 |
| Concrete | 53.50 | 64.37 | 73.57 | 82.76 | 91.96 | 99.48 |
| Polymers | 39.29 | 56.85 | 63.54 | 74.40 | 81.09 | 89.45 |
| Coatings | 46.82 | 43.47 | 52.67 | 59.36 | 67.72 | 75.24 |
| Ceramic | 28.42 | 35.11 | 39.29 | 45.98 | 50.16 | 54.34 |
| Asphalt | 19.65 | 23.83 | 28.01 | 33.02 | 38.87 | 43.89 |
| Metals | 25.92 | 30.93 | 35.11 | 36.78 | 40.13 | 43.47 |
| Metal | Ligand | Characterization Methods | Ref. | ||
|---|---|---|---|---|---|
| FT-IR | Raman | UV/VIS | |||
| Fe3+ | Catechol | - | 512–627 cm−1 (catechol–iron bond vibrations) | - | [85] |
| Fe3+ | Dopa | - | 500–650 cm−1 (chelation of the Fe3+ by the oxygen of catechol) | - | [87] |
| Fe3+ | Dopa | - | 500–600 cm−1 (coordination between DOPA and Fe3+) | 550 nm (coordination is enhanced between Fe3+ and catechol groups) | [90] |
| Zn2+ | Acrylonitrile | 2280 cm−1 (restricted −CN in Zn2+−CN coordination) | - | - | [92] |
| Zn2+ | Terpyridine | - | - | 392 nm (Zn2+-terpyridine coordination complex) | [93] |
| Ln3+ | Terpyridine | 1587, 1571, and 1562 cm−1 (C=N of terpyridine, related to complexation of terpyridine and Ln3+) | - | 292 nm (π–π* transition of pyridine ring) and red-shifted to 324 nm (after the Ln3+ addition) | [94] |
| Materials | Characterization Methods | Ref. | |
|---|---|---|---|
| FT-IR | 1H-NMR | ||
| Oxidized natural rubber (oNR), Sodium alginate (SA) | 3291 cm−1 is shifted to 3272 cm−1 (hydroxyl group) 1038 cm−1 is shifted to 1029 cm−1 (C-O stretching vibration of SA) | - | [76] |
| PVA, Dopamine | 3306 cm−1 (broad and strong of OH stretching) | - | [95] |
| α,ω-Aminopropyl-terminated poly (dimethylsiloxane) (A-PDMS), Ethylene carbonate (EC) | 1540 cm−1 (N−H bending) 1415 cm−1 (C−N stretching) 3420 cm−1 (broad and strong absorption bands of O−H) | 7.6 ppm (N−H signals) 3.5 ppm (hydroxyl resonance) | [96] |
| Epoxidized natural rubber (ENR), Carboxymethyl chitosan (CMCS) | 3425 cm−1 is shifted to 3388 cm−1 (hydroxyl group) | - | [97] |
| Aminopropyl-terminated polydimethylsiloxane (PDMS), 4,4′-methylenebis-(cyclohexyl isocyanate) (HMDI), 1,1′-Thiocarbonyldiimidazole (TCDI), Isophthalaldehyde (IPAL) | 3340–3310 cm−1 (N−H stretching) 1700 cm−1 (C−O vibrations) 1500 cm−1 (Amide II band) | - | [98] |
| Materials | Characterization Methods | Ref. | |
|---|---|---|---|
| FT-IR | UV/VIS | ||
| Polyurethane, Polyimide | - | Broad absorption at 525 nm (π–π* charge-transfer transition between the electron-rich pyrenyl and electron-poor diimide residues) | [102] |
| Perylene terminated polymer, Poly(diimide) | - | Broad absorption band at 611 nm (blended solutions of perylene terminated polymer /chain-folding polydiimide) | [103] |
| Polystyrene (PS), Graphene | 694, 749, 1386, and 1447 cm−1 (benzene ring of the PS segments), 2917 and 3020 cm−1 (methylene groups) | 269.8 nm (ring currents in graphene and PS) | [104] |
| Polystyrene (PS), 6,13-bis((triisopropy-lsilyl)ethynyl) (TIPS)-pentacene | - | 698 nm (TIPS-pentacene phase) | [105] |
| Materials | Characterization Methods | Ref. | ||
|---|---|---|---|---|
| FT-IR | UV/VIS | 1H-NMR | ||
| Poly (acrylic acid) (PAA), Poly(ethylene oxide) (PEO), oly(ethylenimine) (bPEI) | 1550 cm−1 (COO− groups of PAA) | - | - | [106] |
| Acrylamide and acrylic acid copolymer (PAM), Alginate-modified dopamine (Alg-DA), Aluminum ions (Al3+) | 2893 cm−1 (Stretching of C−H from −N+ (CH3)2−) 1215–1280 cm−1 (C−N stretching) | 280 nm (successful synthesis of Alg-DA) | 6.5–7.0 ppm (DA was successfully introduced to the alginate structure) | [107] |
| Polyaniline (PANI), Hydrophobic association poly(acrylic acid) (HAPAA) | 1483 and 1561 cm−1 (stretching vibrations of the benzenoid and quinoid ring of PANI) | - | - | [108] |
| Materials | Characterization Methods | Ref. |
|---|---|---|
| FT-IR | ||
| 1-Methylimidazole, Bis(2-bromoethyl) ether, EMITFSI, Lithium bis(trifluoromethanesulphonyl) imide (LiTFSI) | 1346 cm−1 shifted to 1351 cm−1 (S=O stretching band), 1051 cm−1 shifted to 1055 cm−1 (N−S stretching band) | [110] |
| 1,1,3,3-tetramethylurea (TMU), acetonitrile (CH3CN) and carbon tetrachloride (CCl4) | 1653 cm−1 (TMU and CD3CN interaction and slight shift of the carbonyl band of TMU around) | [112] |
| Amphiphilic compounds (N+C10-Azo-Gly-OC2Rfn: NAGFn) | 1148.9 cm−1 (the position of the νs(CF2) band) | [113] |
| Materials | Characterization Methods | Ref. | |
|---|---|---|---|
| FT-IR | 1H-NMR | ||
| Isocyanatoethyl acrylate modified b-cyclodextrin (b-CD-AOI2), 2-(2-(2-(2-(adamantyl-1-oxy)ethoxy)ethoxy)ethoxy)ethanol acrylate (A-TEG-Ad) | 1534 and 1635 cm−1 (stretching vibrations of the secondary amides) | 4.9 ppm (signal integral area ratio between C1-H of b-CD) 5.8–6.4 ppm (double bond, −CH=CH2) | [114] |
| Copolymerization of epichlorohydrin (EP) and β-cyclodextrin (CD) | - | 1.6–2.2 ppm (protons of Ad functional group), 3.0–4.0 ppm (internal proton of β-CD) | [115] |
| Azo-acrylamide, β-cyclodextrin polymer (bCDP) | - | 3.3–4 ppm (protons located in the cavity of the cyclodextrin units in bCDP), 6–8 ppm (The protons on sp2 carbons of azo-acrylamide) | [116] |
| No. | Materials | Self-Healing Character | Preparation Method | Self-Healing Condition | Self-Healing Efficiency | Ref. |
|---|---|---|---|---|---|---|
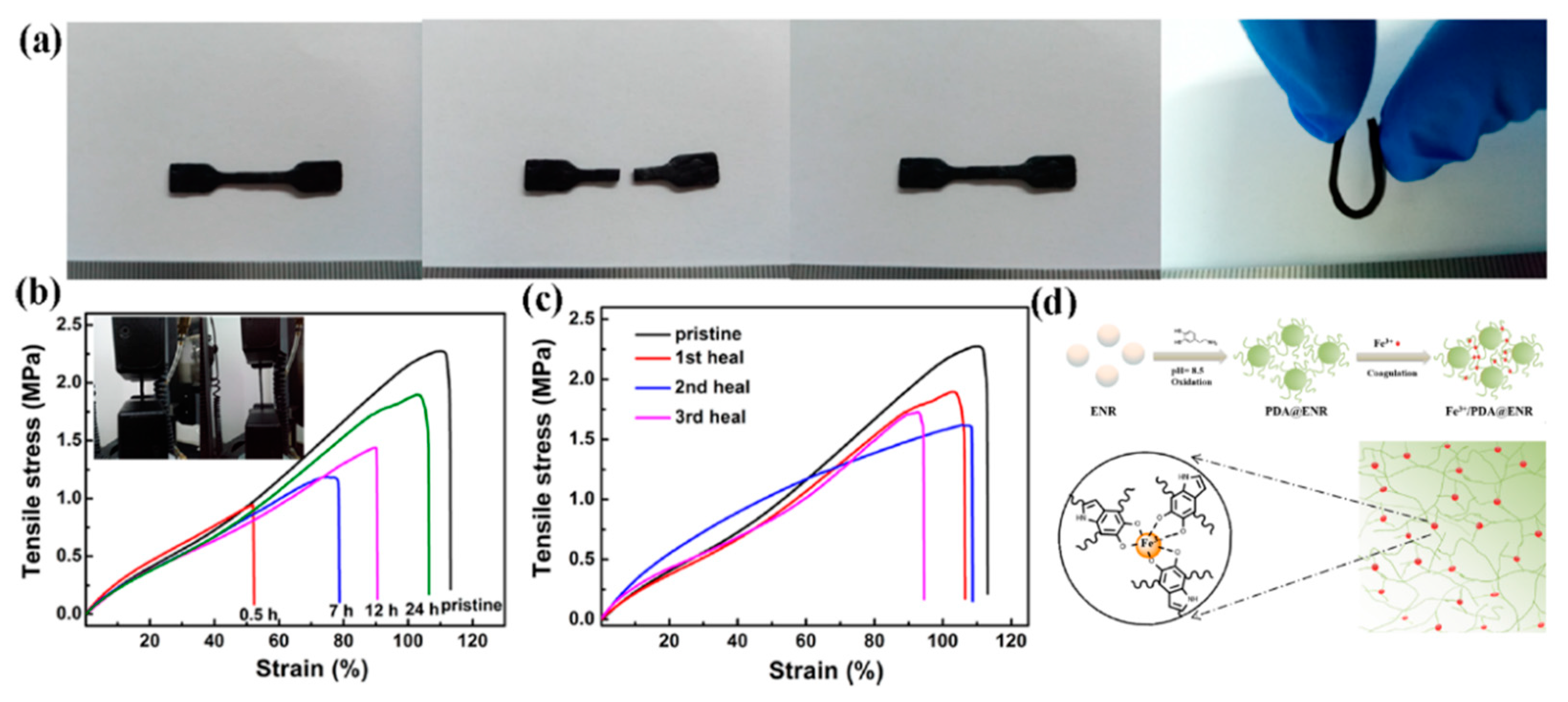
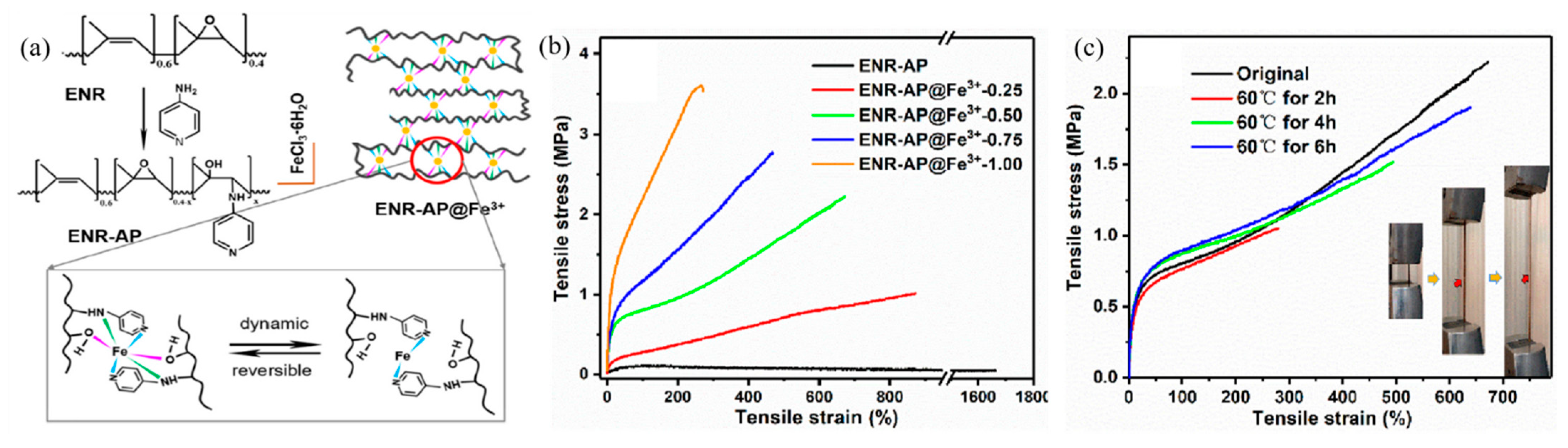
| 1 | ENR, Dopamine, Fe3+ | M-L coordination | Mixing and compression molding | 50 °C, 12 h | Elongation: 95.2 ± 15.9%, Tensile strength: 86.1 ± 5.2%. | [88] |
| 2 | Silicone rubber, Dopa, Fe3+ | M-L coordination | Mixing and pouring into a Petri dish | 120 °C, 84 h and pH = 9 underwater | Elongation: 84 ± 2% | [89] |
| 3 | ENR, Pyridine, Fe3+ | M-L coordination | Mixing and pouring and spray coating | 60 °C, 8 h | Tensile strength: 87% | [90] |
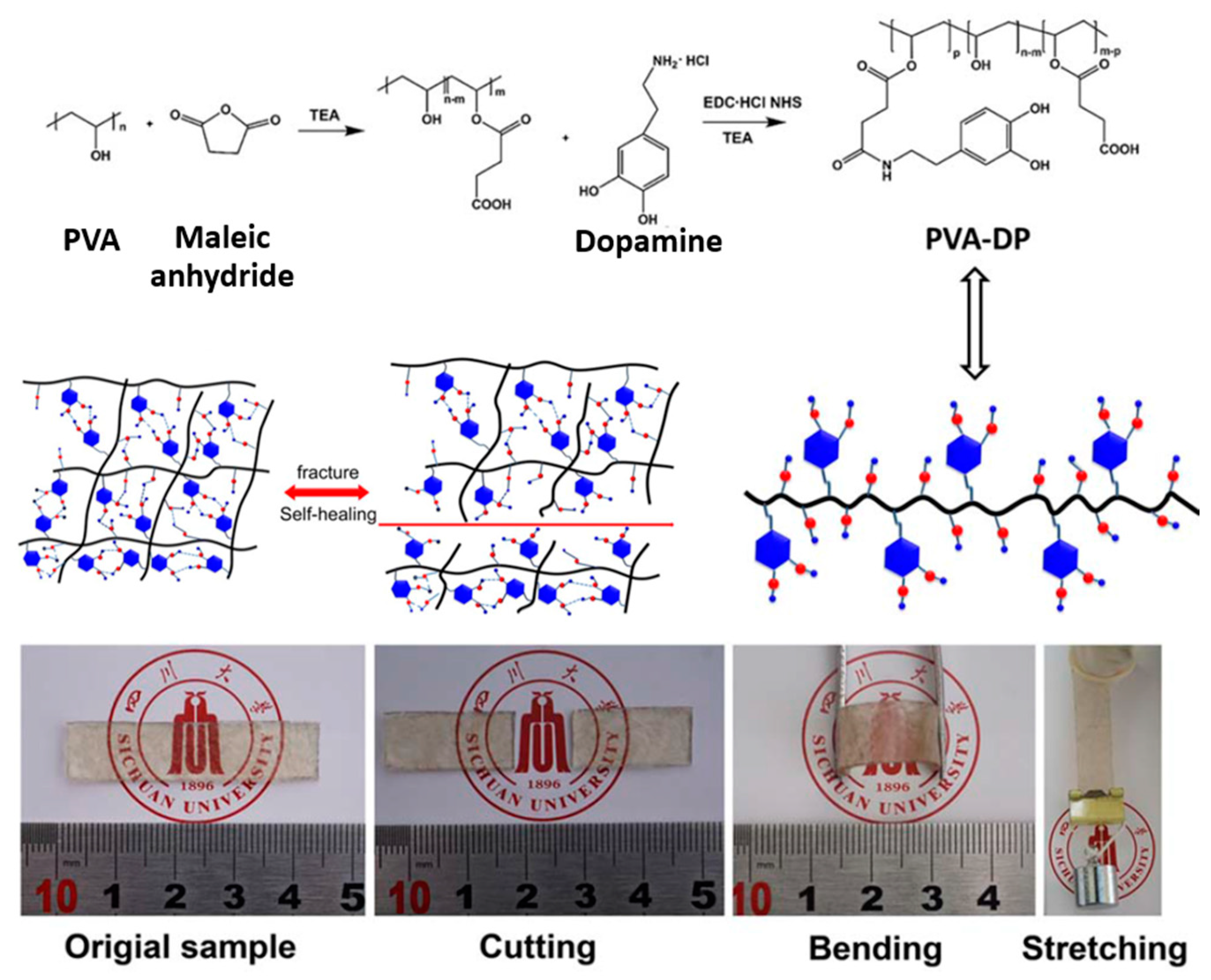
| 4 | PVA, Succinic anhydride, Dopamine | H-bonding | Melt processing | 25 °C, 24 h 25 °C, 48 h | Tensile strength: 10.87% Tensile strength: 17.39% | [95] |
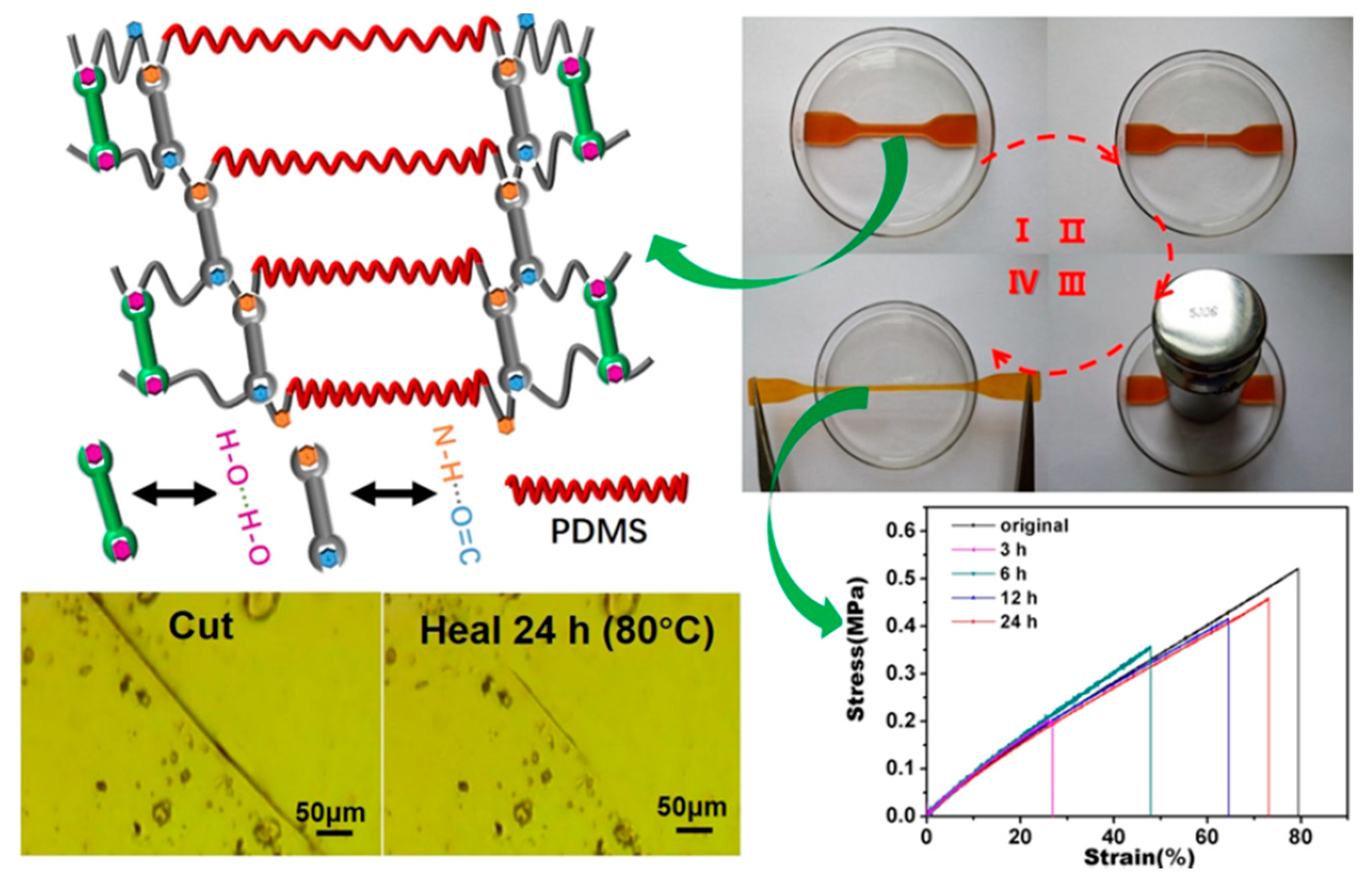
| 5 | Silicone rubber, Ethylene carbonate | H-bonding | Mixing and pouring into a mold | 80 °C, 24 h | Tensile strength: 88.5% | [96] |
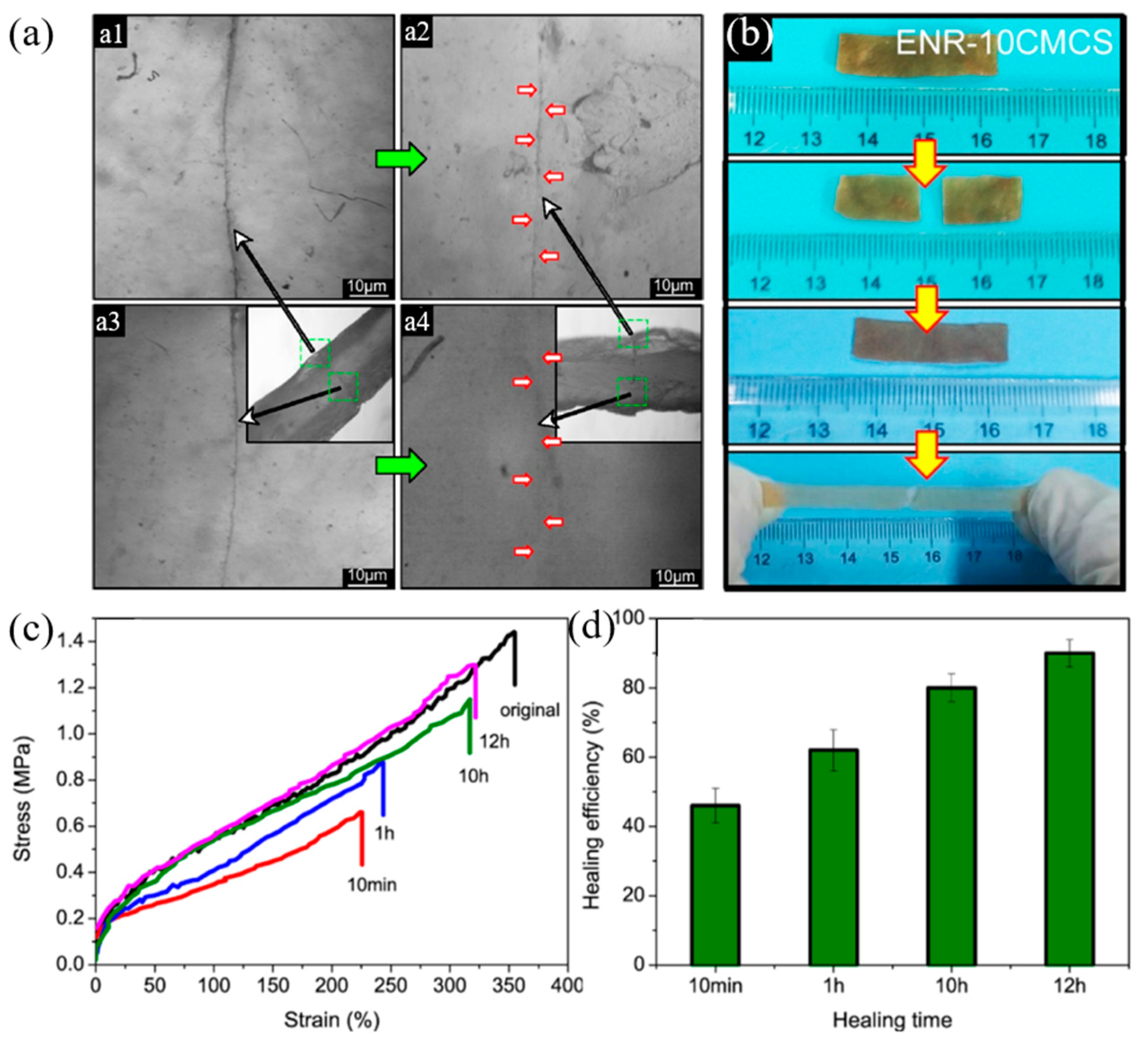
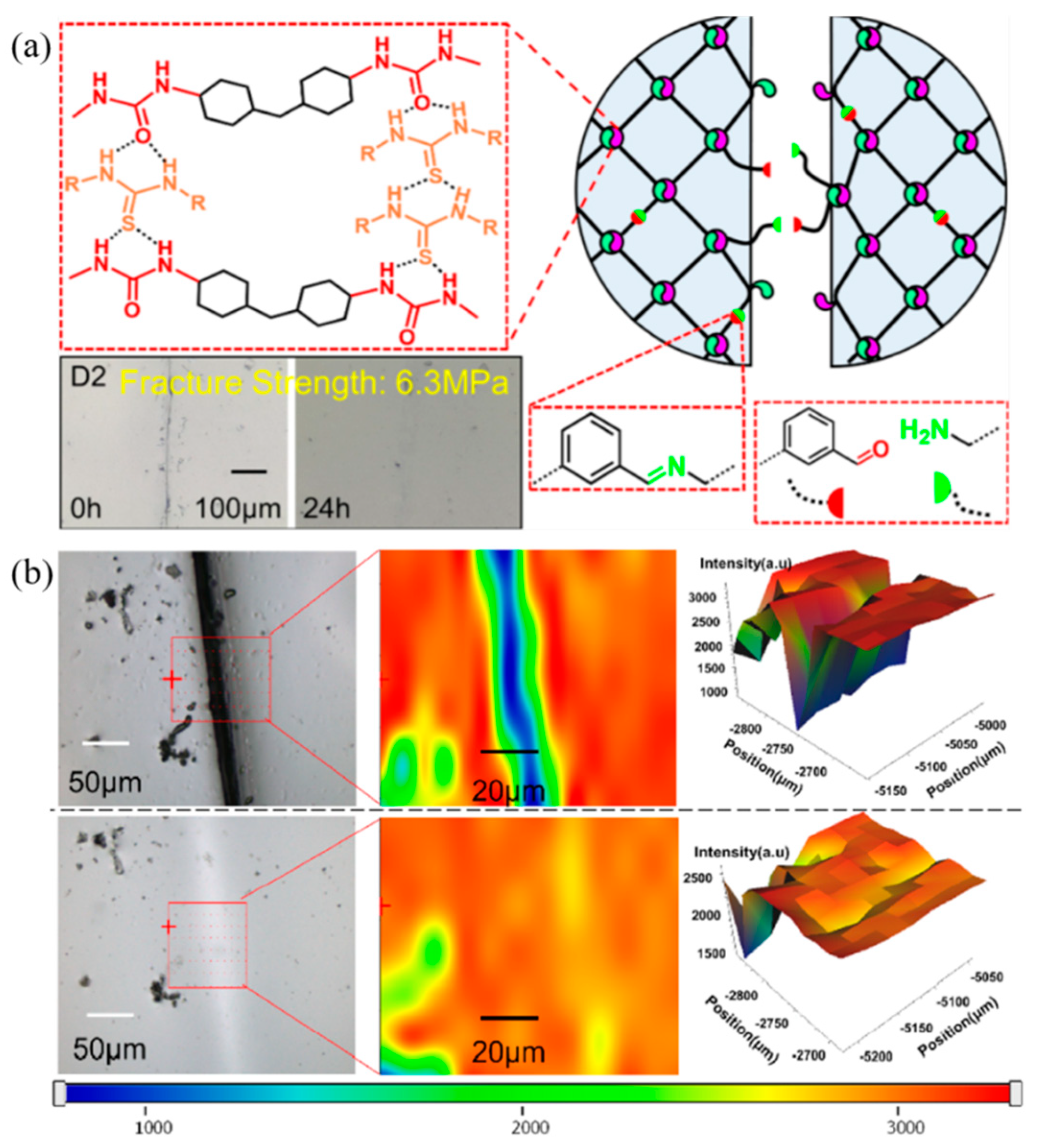
| 6 | ENR, Carboxymethyl chitosan | H-bonding | Solution mixing and pouring into a mold | RT, 12 h | Tensile strength: 90% | [97] |
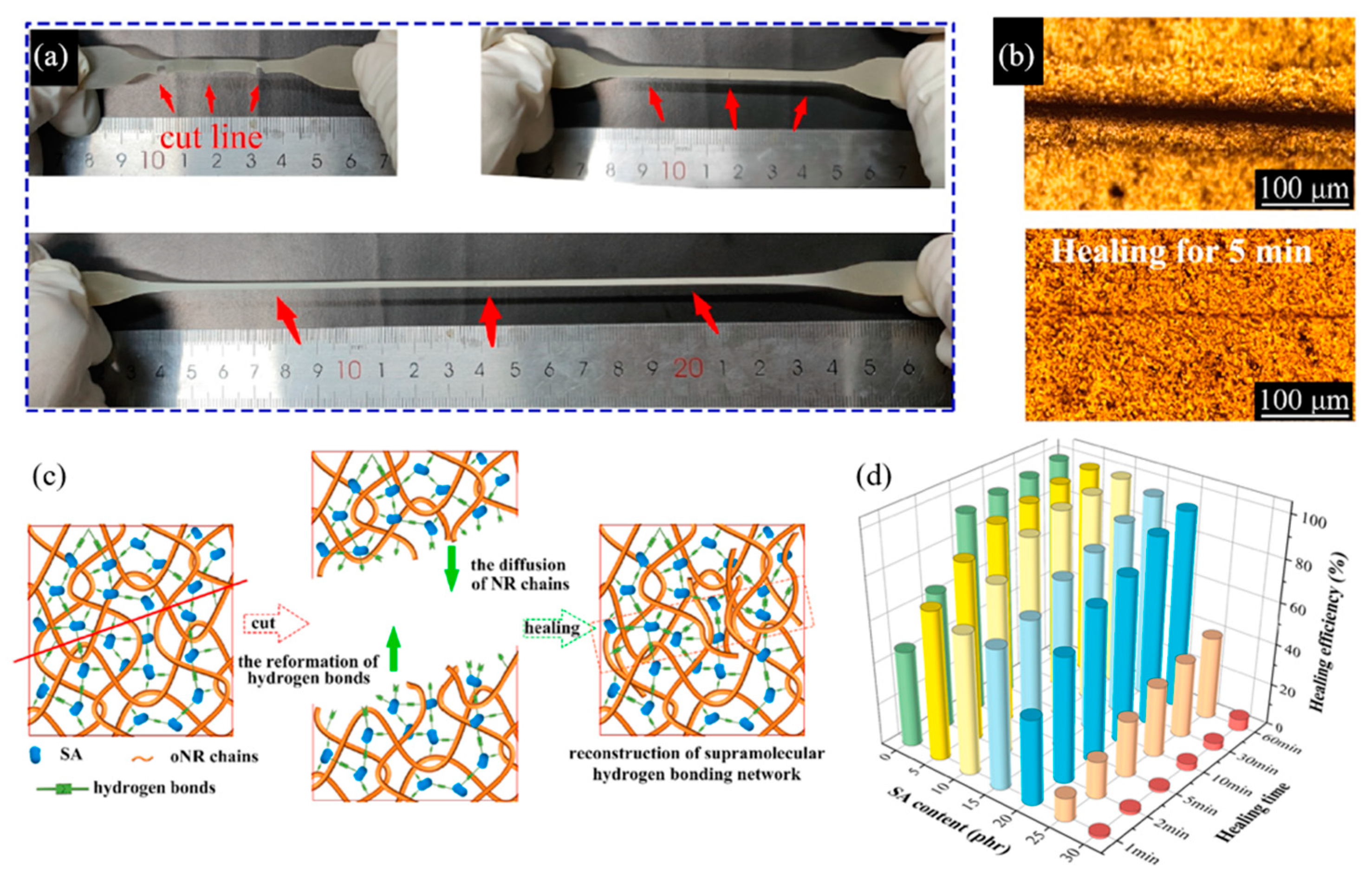
| 7 | Oxidized NR, Sodium alginate | H-bonding | Mixing and pouring into a mold | RT, 5 min | Elongation: 94% | [76] |
| 8 | Silicone elastomer, Thiourea | H-bonding | Mixing and pouring into a mold | RT, 24 h | Tensile strength: 94.9% | [98] |
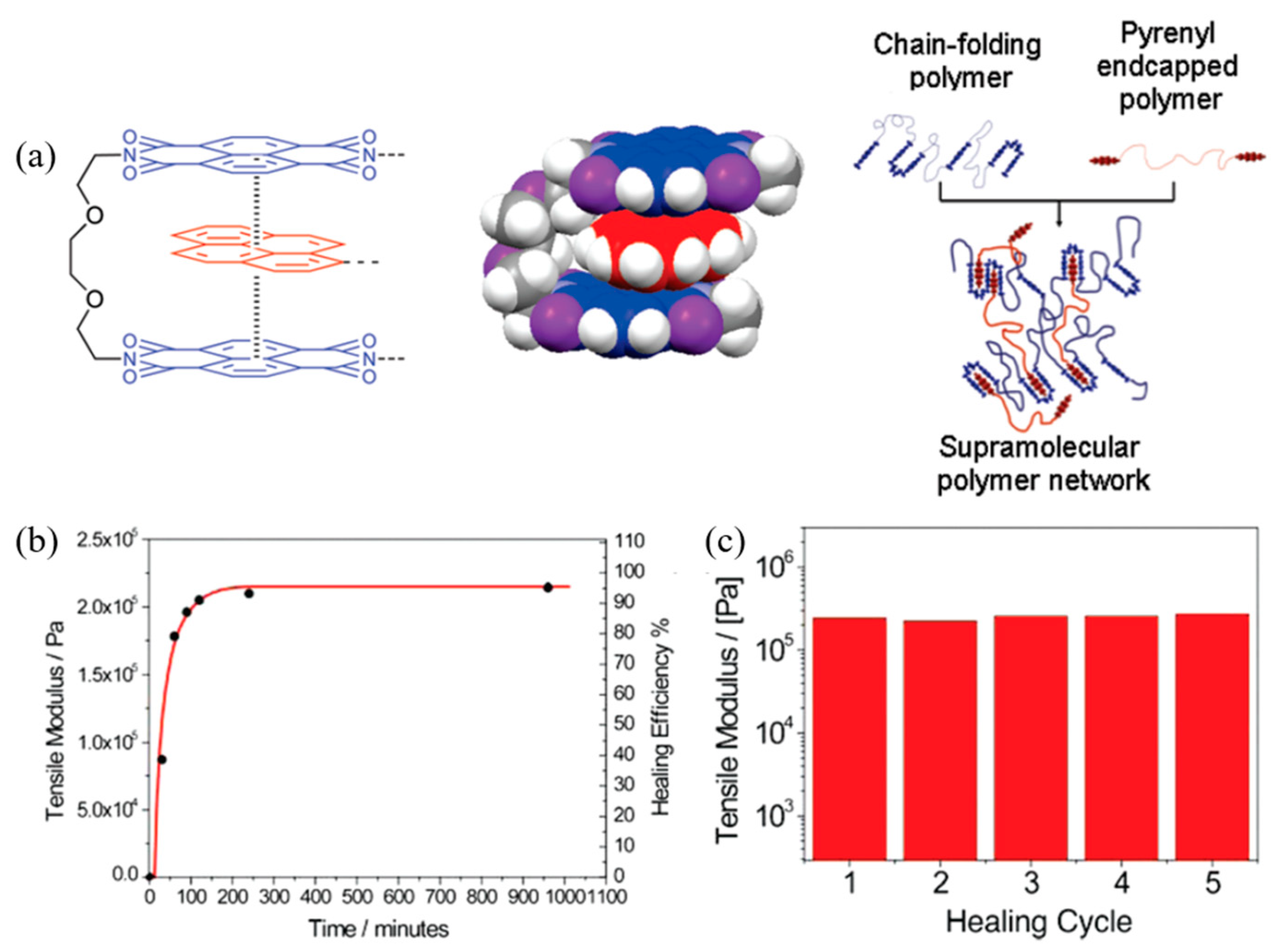
| 9 | Polyimide, Pyrenyl | π–π interaction | Mixing | 87 °C, 5 min | Tensile modulus: 100% | [101,102] |
| 10 | Poly(diimide), Perylene | π–π interaction | Mixing | 75 °C, 14 min or 125 °C, 30 min | Shear modulus: 100% | [103] |
| 11 | Poly(ethylenimine), Poly(acrylic acid), Poly(ethylene oxide) | Electrostatic interactions and H-bonding | Dropwise mixing and compression molding | RT, 48 h | Elongation: 96% | [106] |
| 12 | DA, Al3+ | Electrostatic interactions, M-L coordination, H-bonding | Mixing | RT, 24 h | Tensile strength: 91.83% | [107] |
| 13 | PA, PANI, HAPAA | Electrostatic interactions, H-bonding | Pre-infiltration method | RT, 72 h | Tensile strength: 70% Electrical: 92% | [108] |
| 14 | Fluorinated elastomer, Dibutyl phthalate | Dipole–dipole interactions | Mixing | RT, 24 h and underwater | Elongation: 30.61 ± 3.97% | [109] |
| 15 | Fluorinated elastomer, Ionic liquids | Dipole–dipole interactions | Mixing | 50 °C, 12 h | Elongation: 100% | [110] |
| 16 | Poly(isocyanatoethyl acrylate modified b-cyclodextrin), Acrylate compound | Host–guest interaction | Mixing | RT, 60 min | Tensile strength: 60% | [114] |
| 17 | Polycyclodextrin, Methacryl-1-adamantane ethylene glycol diester | Host–guest interaction | Mixing | RT, 24 h and moisture conditions | Tensile strength: 75% | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buaksuntear, K.; Limarun, P.; Suethao, S.; Smitthipong, W. Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers. Int. J. Mol. Sci. 2022, 23, 6902. https://doi.org/10.3390/ijms23136902
Buaksuntear K, Limarun P, Suethao S, Smitthipong W. Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers. International Journal of Molecular Sciences. 2022; 23(13):6902. https://doi.org/10.3390/ijms23136902
Chicago/Turabian StyleBuaksuntear, Kwanchai, Phakamat Limarun, Supitta Suethao, and Wirasak Smitthipong. 2022. "Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers" International Journal of Molecular Sciences 23, no. 13: 6902. https://doi.org/10.3390/ijms23136902
APA StyleBuaksuntear, K., Limarun, P., Suethao, S., & Smitthipong, W. (2022). Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers. International Journal of Molecular Sciences, 23(13), 6902. https://doi.org/10.3390/ijms23136902





