Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway
Abstract
:1. Introduction
2. TLR4 Signaling Pathways
3. TLR4 and NF-κB in the Development of Inflammatory Bowel Disease
4. Polyphenols Alleviate Intestinal Inflammation via Modulating the TLR4/NF-κB Signaling Pathway
4.1. Flavonoids
4.1.1. Flavones
Apigenin
Luteolin
Baicalein
4.1.2. Flavonols
Quercetin
Kaempferol
Rutin
Myricetin and Myricetin-3-O-b-D-Lactose Sodium Salt
4.1.3. Flavanones
Hesperidin
Naringenin
4.1.4. Flavanols
Epigallocatechin-3-Gallate (EGCG)
4.1.5. Isoflavones
Genistein
4.1.6. Anthocyanins
Cyanidin-3-glucoside (C3G)
Malvidin 3-glucoside (MV3G)
Pelargonidin and Pelargonidin-3-O-glucoside (P3G)
4.2. Phenolic Acids
4.2.1. Caffeic Acid and Caffeic acid Phenethyl Ester (CAPE)
4.2.2. Chlorogenic Acid (CGA)
4.2.3. Ellagic Acid (EA)
4.3. Stilbenes
Resveratrol
4.4. Other Polyphenols
4.4.1. Curcumin
4.4.2. Emodin/Rhein
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mascaraque, C.; Aranda, C.; Ocón, B.; Monte, M.J.; Suárez, M.D.; Zarzuelo, A.; Marín, J.J.G.; Martínez-Augustin, O.; de Medina, F.S. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 2014, 90, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Huang, X.; Lin, X.; Chan, T.F.; Lai, K.P.; Li, R. Analyzing the synergistic adverse effects of BPA and its substitute, BHPF, on ulcerative colitis through comparative metabolomics. Chemosphere 2022, 287, 132160. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Yuan, W.; Park, S.M. Association between IL-10 rs3024505 and susceptibility to inflammatory bowel disease: A systematic review and meta-analysis. Cytokine 2022, 149, 155721. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.G.; Yao, H.; Wei, Q.; Mao, X.-M.; Jiang, T.; Xiang, J.; Dila, N. Expression and activity of the TLR4/NF-κB signaling pathway in mouse intestine following administration of a short-term high-fat diet. Exp. Ther. Med. 2013, 6, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and intestinal inflammatory diseases: Molecular mechanisms of protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, X.R.; Liu, S.S.; Zhang, Y.F.; Zhang, D.K. Toll-like receptors and inflammatory bowel disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.W.; Qi, S.Z.; Xue, X.F.; Al Naggar, Y.; Wu, L.M.; Wang, K. Understanding the gastrointestinal protective effects of polyphenols using foodomics-based approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary phytochemicals: Natural swords combating inflammation and oxidation-mediated degenerative diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Peng, L.; Li, W.Y.; Dai, T.Y.; Nie, L.; Xie, J.; Ai, Y.; Li, L.F.; Tian, Y.; Sheng, J. Polyphenol extract of moringa oleifera leaves alleviates colonic inflammation in dextran sulfate sodium-treated mice. Evid.-Based Complementary Altern. 2020, 2020, 6295402. [Google Scholar] [CrossRef]
- Ding, S.; Chi, M.; Scull, B.; Rigby, R.; Schwerbrock, N.; Magness, S.; Jobin, C.; Lund, P. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, S.; Falcao-Pires, I.; Balligand, J.; Bauersachs, J.; Brutsaert, D.; Ciccarelli, M.; Dawson, D.; de Windt, L.J.; Giacca, M.; Hamdani, N.; et al. The innate immune system in chronic cardiomyopathy: A European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur. J. Heart Fail. 2018, 20, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immun. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. Toll-like receptors in innate immunity. Adv. Immunol. 2001, 78, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Gohda, J.; Matsumura, T.; Inoue, J. Cutting edge: TNFR-associated factor (TRIF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a6049. [Google Scholar] [CrossRef] [PubMed]
- Guven-Maiorov, E.; Keskin, O.; Gursoy, A.; Nussinov, R. A structural view of negative regulation of the toll-like receptor-mediated inflammatory pathway. Biophys. J. 2015, 109, 1214–1226. [Google Scholar] [CrossRef] [Green Version]
- Keating, S.E.; Maloney, G.M.; Moran, E.M.; Bowie, A.G. IRAK-2 participates in multiple toll-like receptor signaling pathways to NFκB via activation of TRAF6 ubiquitination. J. Biol. Chem. 2007, 282, 33435–33443. [Google Scholar] [CrossRef] [Green Version]
- Doyle, S.L.; O’Neill, L.A. Toll-like receptors: From the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef]
- Häcker, H.; Redecke, V.; Blagoev, B.; Kratchmarova, I.; Hsu, L.; Wang, G.G.; Kamps, M.P.; Raz, E.; Wagner, H.; Häcker, G.; et al. Specificity in toll-like receptor signaling through distinct effector functions of TRAF3 and TRAF6. Nature 2006, 439, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Sugiyama, M.; Yamamoto, M.; Watanabe, Y.; Kawai, T.; Takeda, K.; Akira, S.Z. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the toll-like receptor signaling. J. Immunol. 2003, 171, 4304–4310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Gastroenterology 1 Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- de Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Araki, T.; Yoshiyama, S.; Hiro, J.; Miki, C.; Kusunoki, M. The expression patterns of toll-like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg. Today 2006, 36, 287–290. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [Green Version]
- Terra, X.; Valls, J.; Xavier, V.; Mérrillon, J.; Arola, L.; Ardèvol, A.; Bladé, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvadó, M.; et al. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J. Agri. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef]
- Luo, H.; Guo, P.; Zhou, Q. Role of TLR4/NF-kappaB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS ONE 2012, 7, e46291. [Google Scholar] [CrossRef] [Green Version]
- Toumi, R.; Soufli, I.; Rafa, H.; Belkhelfa, M.; Biad, A.; Touil-Boukoffa, C. Probiotic bacteria Lactobacillus and Bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int. J. Immunopathol. Pharmacol. 2014, 27, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.P.; Xia, T.S.; Yu, X.P. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Terra, X.; Richart, C.; Ardèvol, A.; Pinent, M.; Blay, M. Omega-3 docosahexaenoic acid and procyanidins inhibit cyclo-oxygenase activity and attenuate NF-κB activation through a p105/p50 regulatory mechanism in macrophage inflammation. Biochem. J. 2012, 441, 653–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, J.H.; Chang, S.K. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in colorectal cancer cell lines related to microsatellite instability. Gastroenterology 2003, 124, A365–A366. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kim, K.; Nam, H.J.; Lee, D. Discovering health benefits of phytochemicals with integrated analysis of the molecular network, chemical properties and ethnopharmacological evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Salih, M.; Osman, W.; Garelnabi, E.; Osman, Z.; Osman, B.; Khalid, H.; Mohamed, M. Secondary metabolites as anti-inflammatory agents. J. Phytopharmacol. 2014, 3, 275–285. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1107. [Google Scholar] [CrossRef] [Green Version]
- Perez-Gregorio, R.; Simal-Gandara, J. A critical review of bioactive food components, and of their functional mechanisms, biological effects and health outcomes. Curr. Pharm. Des. 2017, 23, 2731–2741. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Medina, F.S.D. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Schink, A.; Neumann, J.; Leifke, A.L.; Ziegler, K.; Fröhlich-Nowoisky, J.; Cremer, C.; Thines, E.; Weber, B.; Pöschl, U.; Schuppan, D.; et al. Screening of herbal extracts for TLR2- and TLR4-dependent anti-inflammatory effects. PLoS ONE 2018, 13, e0203907. [Google Scholar] [CrossRef]
- Begum, N.; Rajendra, P.N.; Kanimozhi, G.; Agilan, B. Apigenin prevents gamma radiation-induced gastrointestinal damages by modulating inflammatory and apoptotic signalling mediators. Nat. Prod. Res. 2021, 36, 1631–1635. [Google Scholar] [CrossRef]
- Ai, X.; Qin, Y.; Liu, H.; Cui, Z.; Li, M.; Yang, J.; Zhong, W.; Liu, Y.; Chen, S.; Sun, T.; et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB signaling. Oncotarget 2017, 8, 100216–100226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.W.; Che, S.Y.; Ruan, Z.; Song, L.Q.; Tang, R.X.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Yue, Y.Z.; Wang, X.H.; Li, H.; Yan, S. Luteolin relieved DSS-induced colitis in mice via HMGB1-TLR-NF-κB signaling pathway. Inflammation 2021, 44, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Yamamoto, K.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of luteolin: Role of the aglycone in NF-κB inactivation in macrophages co-cultured with intestinal epithelial cells. Biofactors 2013, 39, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.P.; Yu, Z.L.; Deng, C.; Zhang, J.J.; Ren, G.Y.; Sun, A.; Mani, S.; Wang, Z.T.; Dou, W. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci. Rep. 2017, 7, 16374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Deng, X.; Lei, L.; Zheng, Y.; Ai, J.; Chen, L.; Xiong, H.; Mei, Z.; Cheng, Y.; Ren, Y. The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front. Pharmacol. 2019, 10, 01466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Li, B.R.; Zhang, Q.; Zhao, X.H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef]
- Das, T.; Mukherjee, S.; Chaudhuri, K. Effect of quercetin on Vibrio cholerae induced nuclear factor-κB activation and interleukin-8 expression in intestinal epithelial cells. Microbes Infect. 2012, 14, 690–695. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Hui, X.; Huang, C.L.; Fan, J.J.; Mei, Q.X.; Lu, Y.Y.; Lou, L.H.; Wang, X.P.; Yue, Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar] [CrossRef]
- Fuentes, J.; Brunser, O.; Atala, E.; Herranz, J.; de Camargo, A.C.; Zbinden-Foncea, H.; Speisky, H. Protection against indomethacin-induced loss of intestinal epithelial barrier function by a quercetin oxidation metabolite present in onion peel: In vitro and in vivo studies. J. Nutr. Biochem. 2022, 100, 108886. [Google Scholar] [CrossRef]
- Bian, Y.F.; Liu, P.; Zhong, J.; Hu, Y.S.; Fan, Y.S.; Zhuang, S.; Liu, Z.J. Kaempferol inhibits multiple pathways involved in the secretion of inflammatory mediators from LPS-induced rat intestinal microvascular endothelial cells. Mol. Med. Rep. 2019, 19, 1958–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Y.F.; Lei, J.Q.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.X.; Liao, C.Y.; He, Y.; Liu, Z.J.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; López-Posadas, R.; Monte, M.J.; Romero-Calvo, I.; Daddaoua, A.; González, M.; Martínez-Plata, E.; Suárez, M.D.; González, R.; Marín, J.J.G.; et al. The small intestinal mucosa acts as a rutin reservoir to extend flavonoid anti-inflammatory activity in experimental ileitis and colitis. J. Funct. Foods 2015, 13, 117–125. [Google Scholar] [CrossRef]
- Li, E.Y.; Wang, T.; Zhou, R.; Zhou, Z.W.; Zhang, C.Y.; Wu, W.H.; He, K. Myricetin and myricetrin alleviate liver and colon damage in a chronic colitis mice model: Effects on tight junction and intestinal microbiota. J. Funct. Foods 2021, 87, 104790. [Google Scholar] [CrossRef]
- Zhou, X.L.; Yang, J.; Qu, X.J.; Meng, J.; Miao, R.R.; Cui, S.X. M10, a Myricetin-3-O-b-D-Lactose Sodium salt, prevents ulcerative colitis through inhibiting necroptosis in mice. Front. Pharm. 2020, 11, 557312. [Google Scholar] [CrossRef]
- Polat, F.R.; Karaboga, I.; Polat, M.S.; Erboga, Z.; Yilmaz, A.; Güzel, S. Effect of hesperetin on inflammatory and oxidative status in trinitrobenzene sulfonic acid-induced experimental colitis model. Cell Mol. Biol. 2018, 64, 58–65. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Li, Z.L.; Gao, M.; Yang, B.C.; Zhang, H.L.; Wang, K.K.; Liu, Z.L.; Xiao, X.Z.; Yang, M.S. Naringin attenuates MLC phosphorylation and NF-κB activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed. Pharmacother. 2018, 103, 50–58. [Google Scholar] [CrossRef]
- Ha, S.K.; Park, H.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef]
- Myung, D.; Park, Y.; Joo, S.; Myung, E.; Chung, C.; Park, H.; Kim, J.; Cho, S.; Lee, W.; Kim, H.; et al. Epigallocatechin-3-gallate inhibits the expression of adhesion molecules by blocking nuclear factor kappa B signaling in intestinal epithelial cells. Intest. Res. 2013, 11, 261. [Google Scholar] [CrossRef]
- Joo, S.; Song, Y.; Park, Y.; Myung, E.; Chung, C.; Park, K.; Cho, S.; Lee, W.; Kim, H.; Rew, J.; et al. Epigallocatechin-3-gallate inhibits LPS-induced NF-κB and MAPK signaling pathways in bone marrow-derived macrophages. Gut Liver 2012, 6, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Kou, J.; Wu, Y.J.; Wang, M.M.; Zhou, X.M.; Yang, Y.; Wu, Z.L. Dietary genistein supplementation improves intestinal mucosal barrier function in Escherichia coli O78-challenged broilers. J. Nutr. Biochem. 2020, 77, 108267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, J.; Zhao, J.; Chen, Y.Z. Genistein improves inflammatory response and colonic function through NF-κB signal in DSS-induced colonic injury. Oncotarget 2017, 8, 61385–61392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, E.; Sung, N.; Yang, M.; Lee, B.; Song, D.; Park, J.; Kim, J.; Jang, B.; Choi, D.; Park, S.; et al. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-κB and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food Chem. Toxicol. 2014, 74, 255–264. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signaling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busà, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside modulates the in vitro inflammatory crosstalk between intestinal epithelial and endothelial cells. Mediat. Inflamm. 2017, 2017, 3454023. [Google Scholar] [CrossRef]
- Tan, C.; Wang, M.Y.; Kong, Y.W.; Wan, M.Z.; Deng, H.T.; Tong, Y.Q.; Lyu, C.M.; Meng, X.J. Anti-inflammatory and intestinal microbiota modulation properties of high hydrostatic pressure treated cyanidin-3-glucoside and blueberry pectin complexes on dextran sodium sulfate-induced ulcerative colitis mice. Food Funct. 2022, 13, 4384. [Google Scholar] [CrossRef]
- Huang, W.Y.; Liu, Y.M.; Wang, J.; Wang, X.; Li, C.Y. Anti-inflammatory effect of the blueberry anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in endothelial cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.; Freitas, V.; Dinis, T.C.P.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e174116. [Google Scholar] [CrossRef] [Green Version]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagioli, M.; Carino, A.; Fiorucci, C.; Annunziato, G.; Marchianò, S.; Bordoni, M.; Roselli, R.; Giorgio, C.D.; Castiglione, F.; Ricci, P.; et al. The aryl hydrocarbon receptor (AhR) mediates the counter-regulatory effects of pelargonidins in models of inflammation and metabolic dysfunctions. Nutrients 2019, 11, 1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wu, X.Y.; Cao, S.Y.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.M.; Wang, S.L.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Luna-Vital, D.; de Mejia, E.G. Anthocyanins from colored maize ameliorated the inflammatory paracrine interplay between macrophages and adipocytes through regulation of NF-κB and JNK-dependent MAPK pathways. J. Funct. Foods 2019, 54, 175–186. [Google Scholar] [CrossRef]
- Duarte, L.J.; Chaves, V.C.; Nascimento, M.V.P.D.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simões, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65. [Google Scholar] [CrossRef]
- Jin, L.G.; Chu, J.J.; Pang, Q.F.; Zhang, F.Z.; Wu, G.; Zhou, L.Y.; Zhang, X.J.; Xing, C.G. Caffeic acid phenethyl ester attenuates ionize radiation-induced intestinal injury through modulation of oxidative stress, apoptosis and p38MAPK in rats. Environ. Toxicol. Pharmacol. 2015, 40, 156–163. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Hasanpourghadi, M.; Mohd Esa, N. Caffeic acid phenethyl ester attenuates dextran sulfate sodium-induced ulcerative colitis through modulation of NF-κB and cell adhesion molecules. Appl. Biochem. Biotechnol. 2022, 194, 1091–1104. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, Y.; Chen, D.; Yu, B.; He, J. Chlorogenic acid attenuates oxidative stress-induced intestinal epithelium injury by co-regulating the PI3K/Akt and IκBαNF-κB signaling. Antioxidants 2021, 10, 1915. [Google Scholar] [CrossRef]
- Yu, L.M.; Mao, L.Q.; Wu, C.Y.; Ye, W.; Wang, X. Chlorogenic acid improves intestinal barrier function by downregulating CD14 to inhibit the NF-κB signaling pathway. J. Funct. Foods 2021, 85, 104640. [Google Scholar] [CrossRef]
- Gao, W.Y.; Wang, C.H.; Yu, L.; Sheng, T.J.; Wu, Z.L.; Wang, X.Q.; Zhang, D.Q.; Lin, Y.F.; Gong, Y. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int. 2019, 2019, 6769789. [Google Scholar] [CrossRef] [PubMed]
- Yipel, M.; Tekeli, İ.O.; Altinok-Yiïpel, F.; Coşkun, P.; Aslan, A.; Güvenç, M.; Beyazit, N.; Özsoy, Ş.Y. The protective effect of Boswellic acid and Ellagic acid loaded, colon targeted, and pH-sensitive N-succinyl chitosan in ulcerative colitis rat model. J. Drug Deliv. Sci. Technol. 2022, 68, 103023. [Google Scholar] [CrossRef]
- Marín, M.; María Giner, R.; Ríos, J.; Carmen Recio, M. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.P.; Li, F.F.; Pan, Z.G.; Liu, S.X.; Yu, H.; Wang, X.Y.; Bi, S.H.; Zhang, W.D. Resveratrol ameliorates subacute intestinal ischemia-reperfusion injury. J. Surg. Res. 2013, 185, 182–189. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitr. 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Gan, Z.D.; Wei, W.Y.; Li, Y.; Wu, J.M.; Zhao, Y.W.; Zhang, L.L.; Wang, T.; Zhong, X. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.W.; Gu, Y.; Yan, N.; Li, Y.F.; Sun, L.; Li, B. Curcumin functions as an anti-inflammatory and antioxidant agent on arsenic-induced hepatic and kidney injury by inhibiting MAPKs/NF-κB and activating Nrf2 pathways. Environ. Toxicol. 2021, 36, 2161–2173. [Google Scholar] [CrossRef]
- Baliga, M.S.; Joseph, N.; Venkataranganna, M.V.; Saxena, A.; Ponemone, V.; Fayad, R. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: Preclinical and clinical observations. Food Funct. 2012, 3, 1109–1117. [Google Scholar] [CrossRef]
- Jian, Y.T.; Mai, G.F.; Wang, J.D.; Zhang, Y.L.; Luo, R.C.; Fang, Y.X. Preventive and therapeutic effects of NF-kappa B inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J. Gastroenterol. 2005, 11, 1747–1752. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, R.Y.; Xiong, S.; Zhang, C.; Zhang, Y.K. Protective effects of curcumin against rat intestinal inflammation related motility disorders. Mol. Med. Rep. 2021, 23, 391. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhou, L.Y.; Gai, S.C.; Zhai, Y.M.; Gou, N.; Wang, X.C.; Zhang, X.Y.; Cui, M.X.; Wang, L.B.; Wang, S.W. Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts suppress LPS-induced pro-inflammatory responses through NF-κB, MAPK, and PI3K-Akt signaling pathways in alveolar epithelial type II cells. Phytother. Res. 2019, 33, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokra, S.L.; Kaushik, P.; Alam, M.M.; Zaman, M.S.; Ahmad, A.; Khan, S.A.; Husain, A. Quinoline based furanones and their nitrogen analogues: Docking, synthesis and biological evaluation. Saudi Pharm. J. 2016, 24, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J. Agri. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef]
- Mascaraque, C.; Gonzalez, R.; Suarez, M.D.; Zarzuelo, A.; Sanchez, D.M.F.; Martinez-Augustin, O. Intestinal anti-inflammatory activity of apigenin K in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. Br. J. Nutr. 2015, 113, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Huang, K.M. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol. 2013, 53, 376–383. [Google Scholar] [CrossRef]
- Mafuvadze, B.; Cook, M.; Xu, Z.; Besch-Williford, C.; Hyder, S. Effects of dietary apigenin on tumor latency, incidence and multiplicity in a medroxyprogesterone acetate-accelerated 7,12-Dimethylbenz(a)anthracene-induced breast cancer model. Nutr. Cancer 2013, 65, 1184–1191. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.S.; Yin, L.H.; Xu, L.N.; Peng, J.Y.; Zhou, H.; Kang, W. Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin in vitro. Chem. Biol. Interact. 2013, 206, 346–355. [Google Scholar] [CrossRef]
- Márquez-Flores, Y.K.; Villegas, I.; Cárdeno, A.; Rosillo, M.Á.; Alarcón-de-la-Lastra, C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J. Nutr. Biochem. 2016, 30, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ganjare, A.B.; Nirmal, S.A.; Patil, A.N. Use of apigenin from Cordia dichotoma in the treatment of colitis. Fitoterapia 2011, 82, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Fornai, M.; Colucci, R.; Pellegrini, C.; Tirotta, E.; Benvenuti, L.; Segnani, C.; Ippolito, C.; Duranti, E.; Virdis, A.; et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS ONE 2018, 13, e195502. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; Souza, P.; Speca, S.; Somensi, L.B.; Mariano, L.N.B.; Cury, B.J.; Ferreira Dos Anjos, M.; Quintão, N.L.M.; Dubuqoy, L.; Desreumax, P.; et al. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 2020, 177, 2393–2408. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Yu, X.; Sun, H.Y.; Zhang, W.; Liu, G.; Zhu, L. Flos lonicerae flavonoids attenuate experimental ulcerative colitis in rats via suppression of NF-κB signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2481–2494. [Google Scholar] [CrossRef]
- Jiang, R.; Poschet, G.; Owen, R.; Celik, M.; Jansen, L.; Hell, R.; Hoffmeister, M.; Brenner, H.; Chang-Claude, J. Serum concentration of genistein, luteolin and colorectal cancer prognosis. Nutrients 2019, 11, 600. [Google Scholar] [CrossRef] [Green Version]
- Mizun, M.; Nishitani, Y. Luteolin ameliorates gut inflammation by inhibition of NF-κB activation in in vivo and in vitro inflammation models. Free Radic. Biol. Med. 2012, 53, S77. [Google Scholar] [CrossRef]
- Wang, M.F.; Dong, Y.P.; Wu, J.; Li, H.Y.; Zhang, Y.Y.; Fan, S.J.; Li, D.G. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci. 2020, 261, 118463. [Google Scholar] [CrossRef]
- Jang, H.S.; Lee, J.; Park, S.; Kim, J.S.; Shim, S.; Lee, S.B.; Han, S.; Myung, H.; Kim, H.; Jang, W.; et al. Baicalein mitigates radiation-induced enteritis by improving endothelial dysfunction. Front. Pharmacol. 2019, 10, 00892. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Vissenaekens, H.; Smagghe, G.; Criel, H.; Grootaert, C.; Raes, K.; Rajkovic, A.; Goeminne, G.; Boon, N.; De Schutter, K.; Van Camp, J. Intracellular quercetin accumulation and its impact on mitochondrial dysfunction in intestinal Caco-2 cells. Food Res. Int. 2021, 145, 11043. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Š.; Jonecová, Z.; Čurgali, K.; Maretta, M.; Šoltés, J.; Švaňa, M.; Kalpadikis, T.; Caprnda, M.; Adamek, M.; Rodrigo, L.; et al. Quercetin attenuates the ischemia reperfusion induced COX-2 and MPO expression in the small intestine mucosa. Biomed. Pharmacother. 2017, 95, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, J.; Wang, Y.; Zhang, Z.; Wang, F.; Tang, X. Uncovering the mechanism of Ge-Gen-Qin-Lian decoction for treating ulcerative colitis based on network pharmacology and molecular docking verification. Biosci. Rep. 2021, 41, BSR20203565. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Shah, Z.; Saeed, F.; Imran, A.; Arshad, M.; Bashir, A.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Lee, S.; Shin, J.; Han, H.; Lee, H.; Park, J.C.; Lee, K. Kaempferol 7-O-β -D-glucoside isolated from the leaves of Cudrania tricuspidata inhibits LPS-induced expression of pro-inflammatory mediators through inactivation of NF-κB, AP-1, and JAK-STAT in RAW 264.7 macrophages. Chem. Biol. Interact. 2018, 284, 101–111. [Google Scholar] [CrossRef]
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol is an anti-inflammatory compound with activity towards NF-kappaB pathway proteins. Anticancer. Res. 2015, 35, 2645–2650. [Google Scholar]
- Fan, J.; Zhao, X.H.; Li, T.J. Heat treatment of galangin and kaempferol inhibits their benefits to improve barrier function in rat intestinal epithelial cells. J. Nutr. Biochem. 2021, 87, 108517. [Google Scholar] [CrossRef]
- Jin, Y.H.; Zhai, Z.A.; Jia, H.; Lai, J.H.; Si, X.M.; Wu, Z.L. Kaempferol attenuates diquat-induced oxidative damage and apoptosis in intestinal porcine epithelial cells. Food Funct. 2021, 12, 6889–6899. [Google Scholar] [CrossRef]
- Tian, C.L.; Liu, X.; Chang, Y.; Wang, R.X.; Yang, M.; Liu, M.C. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Galvez, J.; Cruz, T.; Crespo, E.; Ocete, M.A.; Lorente, M.; Sánchez De Medina, F.; Zarzuelo, A. Rutoside as mucosal protective in acetic acid-induced rat colitis. Planta Med. 1997, 63, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Cruz, T.; Galvez, J.; Ocete, M.A.; Crespo, M.E.; Sánchez De Medina, F.; Zarzuelo, A. Oral administration of rutoside can ameliorate inflammatory bowel disease in rats. Life Sci. 1998, 62, 687–695. [Google Scholar] [CrossRef]
- Kwon, K.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. 2005, 69, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, S.X.; Sun, S.Y.; Shi, W.N.; Song, Z.Y.; Wang, S.Q.; Yu, X.F.; Gao, Z.H.; Qu, X.J. Chemoprevention of intestinal tumorigenesis by the natural dietary flavonoid myricetin in APCMin/+ mice. Oncotarget 2016, 13, 60446–60460. [Google Scholar] [CrossRef] [Green Version]
- Domitrović, R.; Rashed, K.; Cvijanovic, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Fan, J.; Li, T.J.; Zhao, X.H. Barrier-promoting efficiency of two bioactive flavonols quercetin and myricetin on rat intestinal epithelial (IEC-6) cells via suppressing Rho activation. RSC Adv. 2020, 10, 27249–27258. [Google Scholar] [CrossRef]
- Zhu, S.F.; Yang, C.; Zhang, L.; Wang, S.X.; Ma, M.X.; Zhao, J.C.; Song, Z.Y.; Wang, F.; Qu, X.J.; Li, F.; et al. Development of M10, myricetin-3-O-β-d-lactose sodium salt, a derivative of myricetin as a potent agent of anti-chronic colonic inflammation. Eur. J. Med. Chem. 2019, 174, 9–15. [Google Scholar] [CrossRef]
- Ferraz, C.; Carvalho, T.; Manchope, M.; Artero, N.; Rasquel-Oliveira, F.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic potential of flavonoids in pain and inflammation:mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Eun, S.H.; Woo, J.T.; Kim, D.H. Tangeretin inhibits IL-12 expression and NF-kappaB activation in dendritic cells and attenuates colitis in mice. Planta Med. 2017, 83, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Ren, J.N.; Gu, G.S.; Wang, G.F.; Gong, W.B.; Wu, X.W.; Ren, H.J.; Hong, Z.W.; Li, J.S. Hesperidin protects against intestinal inflammation by restoring intestinal barrier function and up-regulating treg cells. Mol. Nutr. Food Res. 2019, 63, e1800975. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Naringenin Regulates the Intestinal Tight Junction Barrier and Inflammation; Nova Science Publishers, Inc.: Haupage, NY, USA, 2015; pp. 137–149. [Google Scholar]
- Chaen, Y.; Yamamoto, Y.; Suzuki, T. Naringenin promotes recovery from colonic damage through suppression of epithelial tumor necrosis factor–α production and induction of M2-type macrophages in colitic mice. Nutr. Res. 2019, 64, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.I.; Rocha, J.; Vila-Real, H.; Figueira, M.E.; Mota-Filipe, H.; Sepodes, B.; Ribeiro, M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009, 42, 1010–1017. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Navarro-Perán, E.; Cabezas-Herrera, J.; Sánchez-del-Campo, L.; García-Cánovas, F.; Rodríguez-López, J.N. The anti-inflammatory and anti-cancer properties of epigallocatechin-3-gallate are mediated by folate cycle disruption, adenosine release and NF-κB suppression. Inflamm. Res. 2008, 57, 472–478. [Google Scholar] [CrossRef]
- Ortega-Santos, C.P.; Al-Nakkash, L.; Whisner, C.M. Exercise and/or genistein treatment impact gut microbiota and inflammation after 12 weeks on a high-fat, high-sugar diet in C57BL/6 mice. Nutrients 2020, 12, 3410. [Google Scholar] [CrossRef]
- Chen, Y.; Le, T.H.; Du, Q.M.; Zhao, Z.; Liu, Y.X.; Zou, J.J.; Hua, W.W.; Liu, C.; Zhu, Y.B. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int. Immunopharmacol. 2019, 71, 144–154. [Google Scholar] [CrossRef]
- Seibel, J.; Molzberger, A.F.; Hertrampf, T.; Laudenbach-Leschowski, U.; Diel, P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 2009, 48, 213–220. [Google Scholar] [CrossRef]
- Lv, Z.P.; Dai, H.J.; Wei, Q.W.; Jin, S.; Wang, J.; Wei, X.H.; Yuan, Y.W.; Yu, D.B.; Shi, F.X. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult. Sci. 2020, 99, 3411–3427. [Google Scholar] [CrossRef]
- Gan, Y.R.; Fu, Y.; Yang, L.P.; Chen, J.N.; Lei, H..; Liu, Q. Cyanidin-3-O-glucoside and cyanidin protect against intestinal barrier damage and 2,4,6-trinitrobenzenesulfonic acid-induced colitis. J. Med. Food 2020, 23, 90–99. [Google Scholar] [CrossRef]
- Ghattamaneni, N.K.R.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside from queen garnet plums and purple carrots attenuates DSS-induced inflammatory bowel disease in rats. J. Funct. Foods 2019, 56, 194–203. [Google Scholar] [CrossRef]
- Tan, J.J.; Li, Y.L.; Hou, D.; Wu, S.S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.Y.; Zhu, Y.M.; Li, C.Y.; Sui, Z.Q.; Min, W.H. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxid. Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, T.T.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H. Malvidin- 3-Glucoside modulated gut microbial dysbiosis and global metabolome disrupted in a murine colitis model induced by dextran sulfate sodium. Mol. Nutr. Food Res. 2019, 63, 1900455. [Google Scholar] [CrossRef]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS ONE 2016, 11, e154817. [Google Scholar] [CrossRef] [Green Version]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [Green Version]
- El-Seedi, H.; El-Said, A.; Khalifa, S.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, Z.P.; Henderson, A.; Lee, K.; Hostetter, J.; Wannemuehler, M.; Hendrich, S. Increased CYP4B1 mRNA is associated with the inhibition of dextran sulfate sodium-induced colitis by caffeic acid in mice. Exp. Biol. Med. 2009, 234, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Xiang, C.G.; Liu, M.T.; Lu, Q.K.; Fan, C.; Lu, H.M.; Feng, C.L.; Yang, X.Q.; Li, H.; Tang, W. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell. Immunol. 2021, 365, 104364. [Google Scholar] [CrossRef]
- Tambuwala, M.M.; Kesharwani, P.; Shukla, R.; Thompson, P.D.; McCarron, P.A. Caffeic acid phenethyl ester (CAPE) reverses fibrosis caused by chronic colon inflammation in murine model of colitis. Pathol. Res. Pract. 2018, 214, 1909–1911. [Google Scholar] [CrossRef]
- Yildiz, Y.; Serter, M.; Ek, R.O.; Ergin, K.; Cecen, S.; Demir, E.M.; Yenisey, C. Protective effects of caffeic acid phenethyl ester on intestinal ischemia-reperfusion injury. Digest. Dis. Sci. 2009, 54, 738–744. [Google Scholar] [CrossRef] [PubMed]
- He, X.R.; Wang, J.H.; Li, M.X.; Hao, D.J.; Yang, Y.; Zhang, C.L.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Nan, T.G.; Zhan, Z.L.; Kang, L.P.; Yang, J.; Lai, C.J.S.; Yuan, Y.; Wang, B.M.; Huang, L.Q. A monoclonal antibody-based enzyme-linked immunosorbent assay for the determination of chlorogenic acid in honeysuckle. J. Pharmaceut. Biomed. 2018, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Shahid, A.; Majed, F.; Sultana, S. Protective effect of chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem. Biol. Interact. 2017, 272, 80–91. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.X.; Chang, J.; Wang, P.; Yin, Q.Q.; Liu, C.Q.; Li, M.L.; Song, A.D.; Zhu, Q.; Lu, F.S. Effect of chlorogenic acid on alleviating inflammation and apoptosis of IPEC-J2 cells induced by deoxyniyalenol. Ecotoxicol. Environ. Saf. 2020, 205, 111376. [Google Scholar] [CrossRef]
- Xue, Y.W.; Huang, F.; Tang, R.X.; Fan, Q.S.; Zhang, B.; Xu, Z.J.; Sun, X.M.; Ruan, Z. Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague–Dawley rats. Food Chem. Toxicol. 2019, 133, 110751. [Google Scholar] [CrossRef]
- Zhang, P.; Jiao, H.L.; Wang, C.L.; Lin, Y.B.; You, S.Y. Chlorogenic acid ameliorates colitis and alters colonic microbiota in a mouse model of dextran sulfate sodium-induced colitis. Front. Physiol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Vukelić, I.; Detel, D.; Pučar, L.B.; Potočnjak, I.; Buljević, S.; Domitrović, R. Chlorogenic acid ameliorates experimental colitis in mice by suppressing signaling pathways involved in inflammatory response and apoptosis. Food Chem. Toxicol. 2018, 121, 140–150. [Google Scholar] [CrossRef]
- Ruan, Z.; Mi, S.M.; Zhou, L.L.; Zhou, Y.; Li, J.; Liu, W.H.; Deng, Z.Y.; Yin, Y.L. Chlorogenic acid enhances intestinal barrier by decreasing MLCK expression and promoting dynamic distribution of tight junction proteins in colitic rats. J. Funct. Foods 2016, 26, 698–708. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010, 104, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez-Bastida, J.A.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.-T. Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J. Agric. Food Chem. 2012, 60, 8866–8876. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Banerjee, M.; Haribabu, B.; Jala, V.R. Urolithin A attenuates arsenic-induced gut barrier dusfunction. Arch. Toxicol. 2022, 96, 987–1007. [Google Scholar] [CrossRef]
- Bereswill, S.; Munoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Loddenkemper, C.; Gobel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [Green Version]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of resveratrol in inflammatory arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef]
- Ma, Z.H.; Ma, Q.Y.; Wang, L.C.; Sha, H.C.; Wu, S.L.; Zhang, M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm. Res. 2005, 54, 522–527. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.H.; Cai, X.K.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.Y.; Li, Z.Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2020, 11, 1063–1073. [Google Scholar] [CrossRef]
- Jakus, P.B.; Kalman, N.; Antus, C.; Radnai, B.; Tucsek, Z.; Gallyas, F.; Sumegi, B.; Veres, B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by resveratrol. J. Nutr. Biochem. 2013, 24, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.Z.; Hwang, D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Fu, C.X.; Yan, M.L.; Xie, H.B.; Li, S.; Yu, Q.F.; He, S.P.; He, J.H. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.W.; Sun, S.S.; Luo, Z.; Shi, B.M.; Shan, A.S.; Cheng, B.J. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef]
- Samsami-kor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.; Jin, C.Y.; Lee, J.D.; Choi, Y.H.; Ahn, S.C.; Lee, C.M.; Jeong, S.C.; Park, Y.M.; Kim, G.Y. Curcumin decreases binding of shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-alpha and IL-1beta: Suppression of p38, JNK and NF-kappaB p65 as potential targets. Biol. Pharm. Bull. 2006, 29, 1470–1475. [Google Scholar] [CrossRef] [Green Version]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar]
- Lubbad, A.; Oriowo, M.A.; Khan, I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol. Cell. Biochem. 2009, 322, 127–135. [Google Scholar] [CrossRef]
- Wang, D.; Sun, M.H.; Zhang, Y.; Chen, Z.H.; Zang, S.Y.; Li, G.Y.; Li, G.; Clark, A.R.; Huang, J.G.; Si, L.Q. Enhanced therapeutic efficacy of a novel colon-specific nanosystem loading emodin on DSS-induced experimental colitis. Phytomedicine 2020, 78, 153293. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhong, J.; Zhou, Q.L.; Zhong, Y.; Liu, P.; Liu, Z.J. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int. Immunopharmacol. 2019, 71, 321–327. [Google Scholar] [CrossRef]
- Luo, S.; Deng, X.L.; Liu, Q.; Pan, Z.F.; Zhao, Z.X.; Zhou, L.; Luo, X. Emodin ameliorates ulcerative colitis by the flagellin-TLR5 dependent pathway in mice. Int. Immunopharmacol. 2018, 59, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Xu, Y.K.; Zhang, H.; Yin, J.T.; Fan, X.; Liu, D.D.; Fu, H.Y.; Wan, B. Emodin alleviates jejunum injury in rats with sepsis by inhibiting inflammation response. Biomed. Pharmacother. 2016, 84, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, X.T. Epigenetic regulation of the innate immune response to infection. Nat. Rev. Immunol. 2019, 19, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The anti-Inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Kazemi, S.; Shirafkan, F.; Hosseinzadeh, R.; Ebrahimpour, A.; Barary, M.; Sio, T.T.; Hosseini, S.M.; Moghadamnia, A.A. The protective effects of quercetin nano-emulsion on intestinal mucositis induced by 5-fluorouracil in mice. Biochem. Biophys. Res. Commun. 2021, 585, 75–81. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Rhodes, M.J.; Johnson, I.T. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic Biol Med 1998, 25, 19–25. [Google Scholar] [CrossRef]
- Kobayashi, S.; Konishi, Y. Transepithelial transport of flavanone in intestinal Caco-2 cell monolayers. Biochem. Bioph. Res. Co. 2008, 368, 23–29. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Gardana, C.; Guarnieri, S.; Riso, P.; Simonetti, P.; Porrini, M. Flavanone plasma pharmacokinetics from blood orange juice in human subjects. Brit. J. Nutr. 2007, 98, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Allijn, I.E.; Vaessen, S.F.C.; Quarles Van Ufford, L.C.; Beukelman, K.J.; de Winther, M.P.J.; Storm, G.; Schiffelers, R.M. Head-to-head comparison of anti-inflammatory performance of known natural products in vitro. PLoS ONE 2016, 11, e0155325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, L.W.; Stratton, M.S.; Ferguson, B.S. Dietary natural products as epigenetic modifiers in aging-associated inflammation and disease. Nat. Prod. Rep. 2020, 37, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Chang, J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.P.; Chu, P.M.; Tsai, S.Y.; Wu, M.H.; Hou, Y.C. Pharmacokinetics and tissue distribution of resveratrol, emodin and their metabolites after intake of Polygonum cuspidatum in rats. J. Ethnopharmacol 2012, 144, 671–676. [Google Scholar] [CrossRef] [PubMed]

 Inhibition;
Inhibition;  Promotion;
Promotion;  Promotion.
Promotion.
 Inhibition;
Inhibition;  Promotion;
Promotion;  Promotion.
Promotion.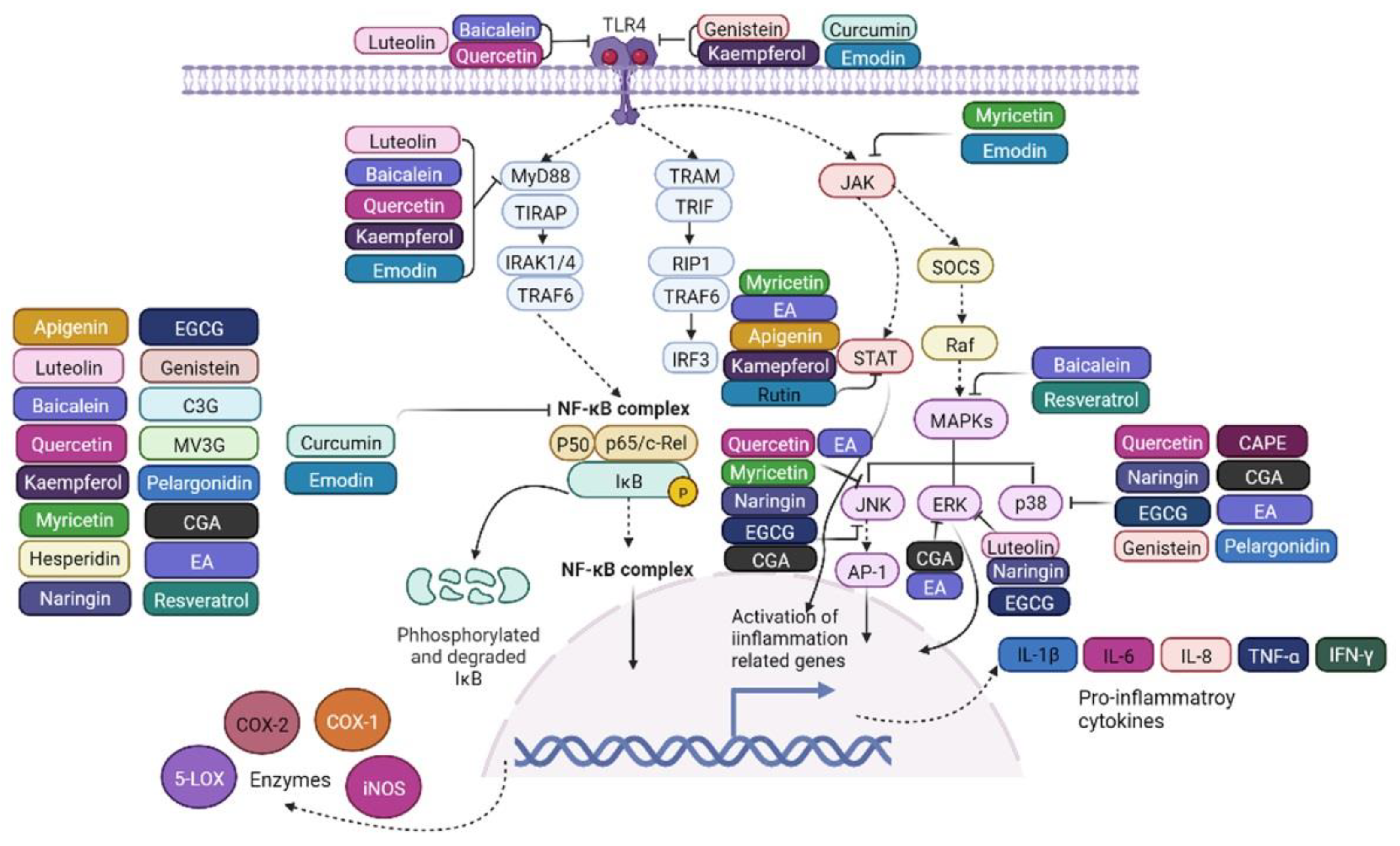
 Inhibition.
Inhibition.
 Inhibition.
Inhibition.
| Polyphenol | Cell Type or Animal Model | Induction of Intestinal Inflammation | Anti-Inflammatory Mechanism | References |
|---|---|---|---|---|
| Apigenin | Swiss albino mice | Radiation-induced gastrointestinal damages | It inhibited NF-κB expression | Begum et al. [40] |
| HCT-116 human colonic epithelial cancer cells | 5 μg/mL LPS | It downregulated NF-κB and STAT3 expression, as well as IL-6 and IL-10 secretion in a dose dependent manner | Ai et al. [41] | |
| C57BL/6J mice | Oral administration of 1% DSS for 21 d | It reduced the severity of colitis by decreasing TNF-α, IL-1β, IL-6, and COX-2 levels | Ai et al. [41] | |
| Luteolin | Human Caco-2 cells | 5 μmol/L decabromodiphenyl ether (BDE-209) for 12 h | It inhibited ERK and NF-κB p50 expression and IκBα phosphorylation, as well as secretion of TNF-α, IL-6, IL-1β | Yuan et al. [42] |
| C57BL/6J mice | Drinking water containing 3.0% DSS | It decreased the levels of IL-6, IL-1β, and TNF-α in the serum and colon, and the protein levels of TLR4, MyD88, and NF-κB p65, and phosphorylation of NF-κB p65 | Zuo et al. [43] | |
| Caco-2/RAW264.7 co-culture model | LPS stimulation | It suppressed NF-κB nuclear translocation, and mRNA expression of IL-8 and TNF-α | Nishitani et al. [44] | |
| Baicalein | Female Balb/c mice | 2 mg of TNBS | It reduced TNF-α and IL-1β, and phosphorylation of NF-κB p65 and IκBα, and protein expression of TLR4 and MyD88 | Luo et al. [45] |
| Sprague-Dawley rats | Ulcerative colitis | It inhibited NF-κB and MAPK expression, as well as IL-1β, IL-6, and IL-17 | Liang et al. [46] | |
| Quercetin | IEC-6 cells | 300 μmol/L indomethacin for 24 h | It suppressed calcium-mediated JNK and Src activation | Fan et al. [47] |
| Human intestinal epithelial cell line Int407 | Vibrio cholerae | Pretreatment with it reduced the IL-8 secretion and NF-κB translocation into the nucleus | Das et al. [48] | |
| Male Sprague-Dawley rats | Acute necrotizing pancreatitis induced by 3.5% sodium taurocholate solution | It downregulated intestinal protein expression of TLR4 and MyD88, and phosphorylation of p38 MAPK | Zheng et al. [49] | |
| Sprague-Dawley rats | Indomethacin dissolved in 5% NaHCO3, at 40 mg/kg body weight | Its oxidation metabolite prevented NF-κB activation and IL-8 secretion | Fuentes et al. [50] | |
| Kaempferol | Rat intestinal microvascular endothelial cells | 10 µg/mL LPS for 12 h | It inhibited LPS-induced NF-κB, I-κB and STAT phosphorylation, decreased TLR4 overexpression, and LPS-induced IL-1β, IL-6 and TNF-α upregulation | Bian et al. [51] |
| C57BL/6J male mice | High fat diet | It reduced the protein expression of TLR4, MyD88 and NF-κB, and mRNA expression of TNF-α in the colon | Bian et al. [52] | |
| Rutin | Rag1 −/− mice | CD4+ CD62L+ T cells transfer model of colitis | It inhibited STAT4 and IκBαphosphorylation, as well as IL-1β and IFN-γ expression in CD4+ spleen cells of the mice | Mascaraque et al. [1] |
| Female Wistar rats | 10 mg of TNBS induced ileitis and colitis | Intragastric rutin resulted in reduced IL-1β and IL-17 mRNA expression in the treatment of ileitis rats, while just tended to decrease levels of IL-17 and IFN-γ in the colitis rats | Mascaraque et al. [53] | |
| Myricetin | IEC-6 cells | 300 μmol/L indomethacin for 24 h | It increased the expression of tight junction proteins, and reduced JNK/Src phosphorylation | Fan et al. [47] |
| Male Kunming mice | Oral administration of 3% DSS solution for 2 weeks | It suppressed TNF-α, NF-κB and COX-2 expression, and increased tight junction proteins expression | Li et al. [54] | |
| Myricetin-3-O-b-D-lactose sodium salt | Male C57BL/6 mice | Oral water containing 1.0% DSS | It reduced the protein expression of IL-6, and the phosphorylation of JAK2, STAT3 and NF-κB, as well as TNF-α pathway, increased IL-4 and IL-10 secretion | Zhou et al. [55] |
| Hesperidin | Wistar albino male rats | TNBS-induced colitis | It reduced the colonic levels of NF-κB, TNF-α and IL-6 | Polat et al. [56] |
| Hesperidin methyl chalcone | Male Swiss mice | Acetic acid-induced colitis | It reduced acetic acid-induced TNF-α, IL-6, IL-1β, and IL-33 production and inhibited NF-κB activation by blocking Ser276 | Guazelli et al. [57] |
| Naringin | Mice | Cecal ligation and puncture-induced intestinal sepsis | It inhibited the release of TNF-α and IL-6, increased IL-10, inhibited NF-κB expression | Li et al. [58] |
| RAW 264.7 macrophages | LPS (1 μg/mL /mL) stimulation for 24 h | It reduced NF-κB translocation and phosphorylation of p38, ERK, and JNK, as well as the expressions of COX-2, IL-1β and TNF-α | Ha et al. [59] | |
| EGCG | Male C57BL/6J mice | High fat diet | It protected against gut barrier dysfunction, and decreased ileal and colonic mRNA expression of TNF-α | Dey et al. [60] |
| Rat intestinal epithelial cells | LPS (1 μg/mL) stimulation for 24 h | It blocked NF-κB signaling via degradation of IκBα and inhibition of NF-κB nuclear translocation, thereby suppressed the expression of adhesion molecules ICAM-1 and VCAM-1 | Myung et al. [61] | |
| Bone marrow-derived macrophages | LPS (1 μg/mL) incubation for 0–1 h | It prevented LPS-induced inflammation through inhibiting IκBα phosphorylation/degradation, NF-κB RelA nuclear translocation, and phosphorylation of ERK1/2, JNK and p38 expression | Joo et al. [62] | |
| Genistein | Male Arbor Acre broilers | Escherichia coli O78 | It improves intestinal mucosa barrier function by modulating apoptosis and secretion of TNF-α and IL-6 | Zhang et al. [63] |
| Caco-2 cells | 3% DSS for 7 d | It reduced nuclear NF-κB p65 and upstream TLR4 expression | Zhang et al. [64] | |
| RAW 264.7 macrophage cells | LPS stimulation | It down-regulated TLR4 and NF-κB expression, IκBα degradation and phosphorylation of ERK1/2 and p38, as well as COX-2, TNF-α, IL-6 and IL-1β expression | Byun et al. [65] | |
| Cyanidin-3-glucoside | Caco-2 cells | Exposed for 3 h to 50 ng/mL TNF-α | It inhibited NF-κB translocation into the nucleus, and IκBα degradation, as well as IL-6 and COX-2 expression | Ferrari et al. [66] |
| Caco-2-HUVECs coculture model | Exposed for 1 h to 50 ng/mL TNF-α | It prevented translocation of NF-κB into the nucleus and inhibited leukocyte adhesion in a dose dependent manner | Ferrari et al. [67] | |
| Balbc mice | Drinking water containing 2.5% DSS | It suppressed NF-κB phosphorylation, thereby inhibited IL-1β, IL-6, IL-8, COX-2 and TNF-α mRNA expression | Tan et al. [68] | |
| Malvidin 3-glucoside | HUVECs | TNF-α (10 μg/L) stimulation for 6 h | It suppressed IκBα degradation and blocked the nuclear translocation of NF-κB p65 | Huang et al. [69] |
| Male Wistar rats | TNBS-induced colitis | It reduced leukocyte infiltration, downregulated iNOS and COX-2 expression | Pereira et al. [70] | |
| Caco-2-HUVECs coculture model | TNF-α (1 ng/mL) stimulation for 3h | It reduced NF-κB mRNA expression, and IL-8 and IL-6 secretion | Kuntz et al. [71] | |
| Pelargonidin | Balb/c mice | TNBS-induced colitis | It decreased the colonic expression of IL-6, TNF-α, IL-1β, and IFN-γ, and increased IL-10 expression | Biagioli et al. [72] |
| Female C57BL/6 mice | Drinking water containing 2.5% DSS for 8 d | It inhibited the activation of NF-κB p65 and IκBα degradation, as well as reduced the serum level of IL-6, IFN-γ and TNF-α | Zhang et al. [73] | |
| Myofibroblasts-like cell line | 1 ng/mL IL-1β stimulation for 24 h | It reduced the IL-8 and COX-2 expression | Zielińska et al. [74] | |
| Pelargonidin-3-O-glucoside | RAW 264.7 Macrophages | 1 μg/mL LPS stimulation for 24 h | It inhibited nuclear translocation of NF-κB p65, phosphorylation and degradation of IκBα, as well as phosphorylation of JNK, thereby reduced the expression of pro-inflammatory cytokines, including IL-1α, TNF-α, IL-27, and IL-6, and enzymes related to inflammation, such as COX-2 and iNOS | Zhang et al. [75] |
| RAW 264.7 Macrophages | 1 μg/mL LPS stimulation for 24 h | It suppressed phosphorylation of JNK, p38 MAPK, IκBα and NF-κB p65, and reduced TNF-α and IL-6 production | Duarte et al. [76] | |
| Caffeic acid phenethyl ester | Male Sprague-Dawley rats | X-ray irradiation (9 Gy) | It reduced the plasma level of TNF-α, and phosphorylation of p38MAPK | Jin et al. [77] |
| Male Balb/c mice | Drinking water containing 3.5% DSS for 7 d | It reduced the production of key cytokines and expression of NF-κB p65 | Pandurangan et al. [78] | |
| Chlorogenic acid | IPEC-J2 cells | 50 ng/mL TNF- α for 3 h | It inhibited the phosphorylation of NF-κB p65 and IκBα | Chen et al. [79] |
| Caco-2 cells | LPS (0.1 mg/mL) stimulation for 24 h | It blocked nuclear translocation of NF-κB p65, and suppressed TNF-α, IL-1β and IL-6 production | Yu et al. [80] | |
| Ellagic acid | C57BL/6 mice | Drinking water containing 5% DSS for 7 d | It reduced the protein expression and phosphorylation of ERK1/2, p38, and JNK | Gao et al. [81] |
| Wistar Albino rats | 3% acetic acid (2 mLintrarectal) induced colitis | It decreased the protein levels of TNF-α, COX-2, and NF-κB | Yipel et al. [82] | |
| Female Balb/C mice | Drinking water containing 5% DSS for 7 d | It reduced the production of IL-6, TNF-α, and IFN-γ | Marín et al. [83] | |
| Female C57BL/6 mice | Four week-long cycles of DSS (1% and 2%) | It inhibited p38 MAPK and STAT3 phosphorylation, IκBα degradation, NF-κB p65 activation, as well as IL-6, COX-2 and iNOS expression | Marín et al. [83] | |
| Four-week-old male Wistar rats | TNBS-induced colitis | It decreased the expression of TNF-α, COX-2, and iNOS, and p38 MAPK, p-JNK and p-ERK1/2, as well as the nuclear translocation of NF-κB p65 | Rosillo et al. [84] | |
| Resveratrol | Black-boned chickens | Circular heat stress | It reduced the jejunal protein expression of NF-κB | Liu et al. [85] |
| Weaned piglets | Weaning stress | It downregulated MAPK pathway and reduced the levels of intestinal pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α | Meng et al. [86] | |
| 50 eligible patients | Ulcerative colitis | It reduced plasma levels of TNF-α and activity of NF-κB in peripheral blood mononuclear cells (PBMC) | Samsami-kor et al. [87] | |
| Curcumin | Male Sprague-Dawley rats | Diarrhea and constipation induced by intracolonic acetic acid instillation or cold water gavage | It inhibited IκBα degradation and NF-κB phosphorylation, as well as IL-1β and TNF-α | Yao et al. [88] |
| Male Sprague-Dawley rats | Experimental colitis induced by intra-rectal administration of TNBS | It Inhibited TLR4, MyD88 and NF-κB protein expression | Lubbad et al. [89] | |
| Emodin | IEC-6 cells | TNF-α (50 ng/mL) stimulation | It inhibited the expression of TLR4, NF-κB and NLRP3, also the production of IL-1β and IL-6 | Zhuang et al. [90] |
| HT-29 cells | Flagellin (500 mg/L) stimulation for 24 h | It increased the expression of IκB, but inhibited the expression of TLR5 and MyD88, nuclear translocation of NF-κB p65, as well as the IL-8 production in flagellin-stimulated HT-29 cells | Luo et al. [91] | |
| Male Wistar rats | Cecal ligation and puncture induced jejunal sepsis | It decreased the levels of IL-6 and TNF-α, and increased the phosphorylated levels of JAK1 and STAT3 | Chen et al. [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. https://doi.org/10.3390/ijms23136939
Yu C, Wang D, Yang Z, Wang T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(13):6939. https://doi.org/10.3390/ijms23136939
Chicago/Turabian StyleYu, Caiyun, Dong Wang, Zaibin Yang, and Tian Wang. 2022. "Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway" International Journal of Molecular Sciences 23, no. 13: 6939. https://doi.org/10.3390/ijms23136939
APA StyleYu, C., Wang, D., Yang, Z., & Wang, T. (2022). Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. International Journal of Molecular Sciences, 23(13), 6939. https://doi.org/10.3390/ijms23136939





