Silicon Controls Bacterial Wilt Disease in Tomato Plants and Inhibits the Virulence-Related Gene Expression of Ralstonia solanacearum
Abstract
1. Introduction
2. Results
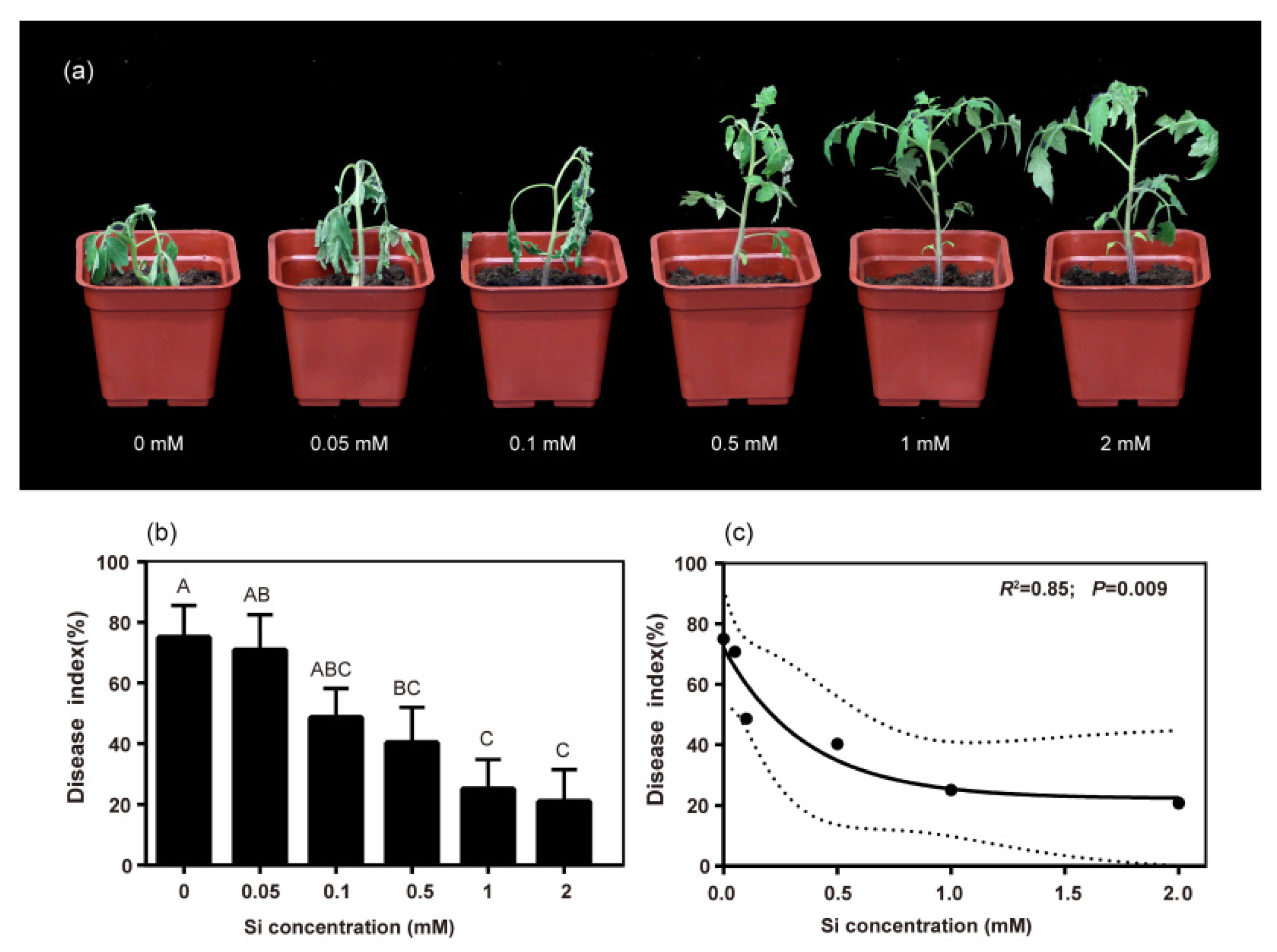
2.1. Silicon Application Reduced Disease Index of Bacterial Wilt in Tomato
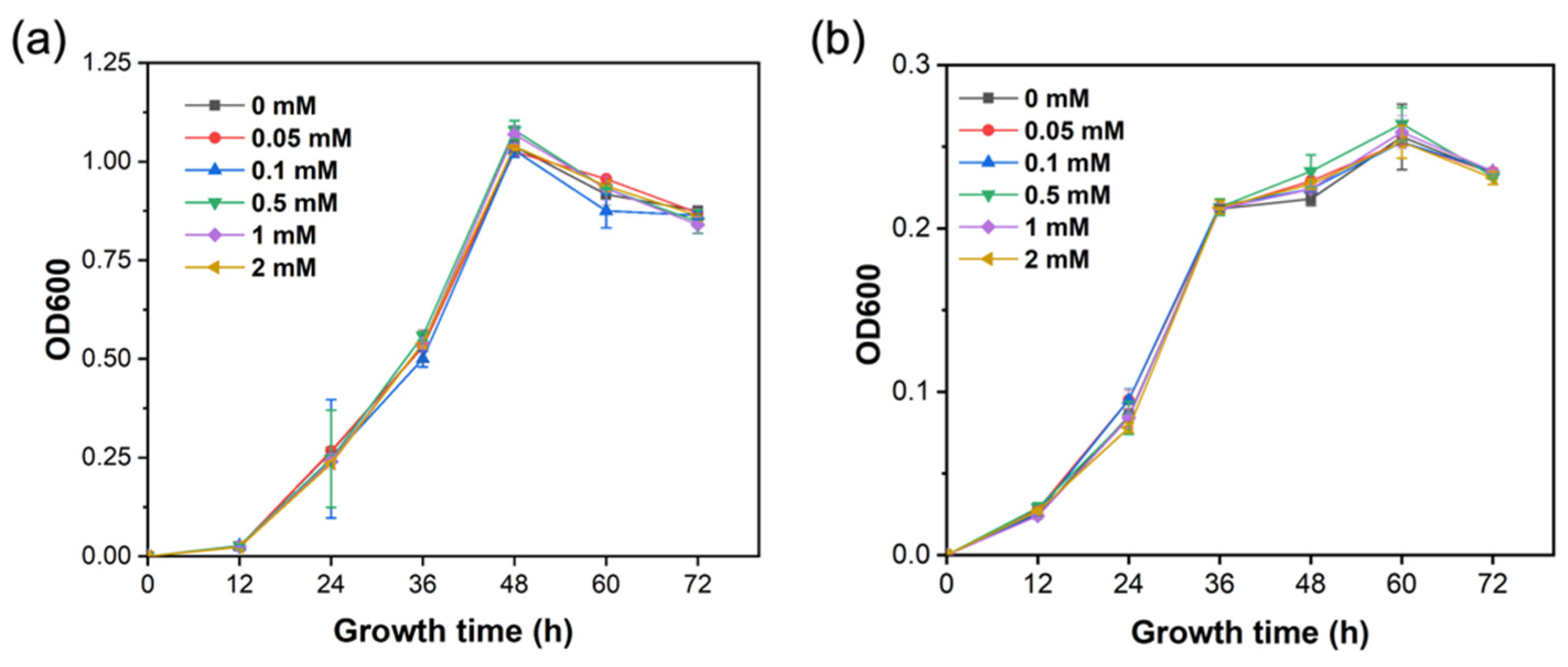
2.2. Silicon Did Not Affect the Growth of R. solanacearum
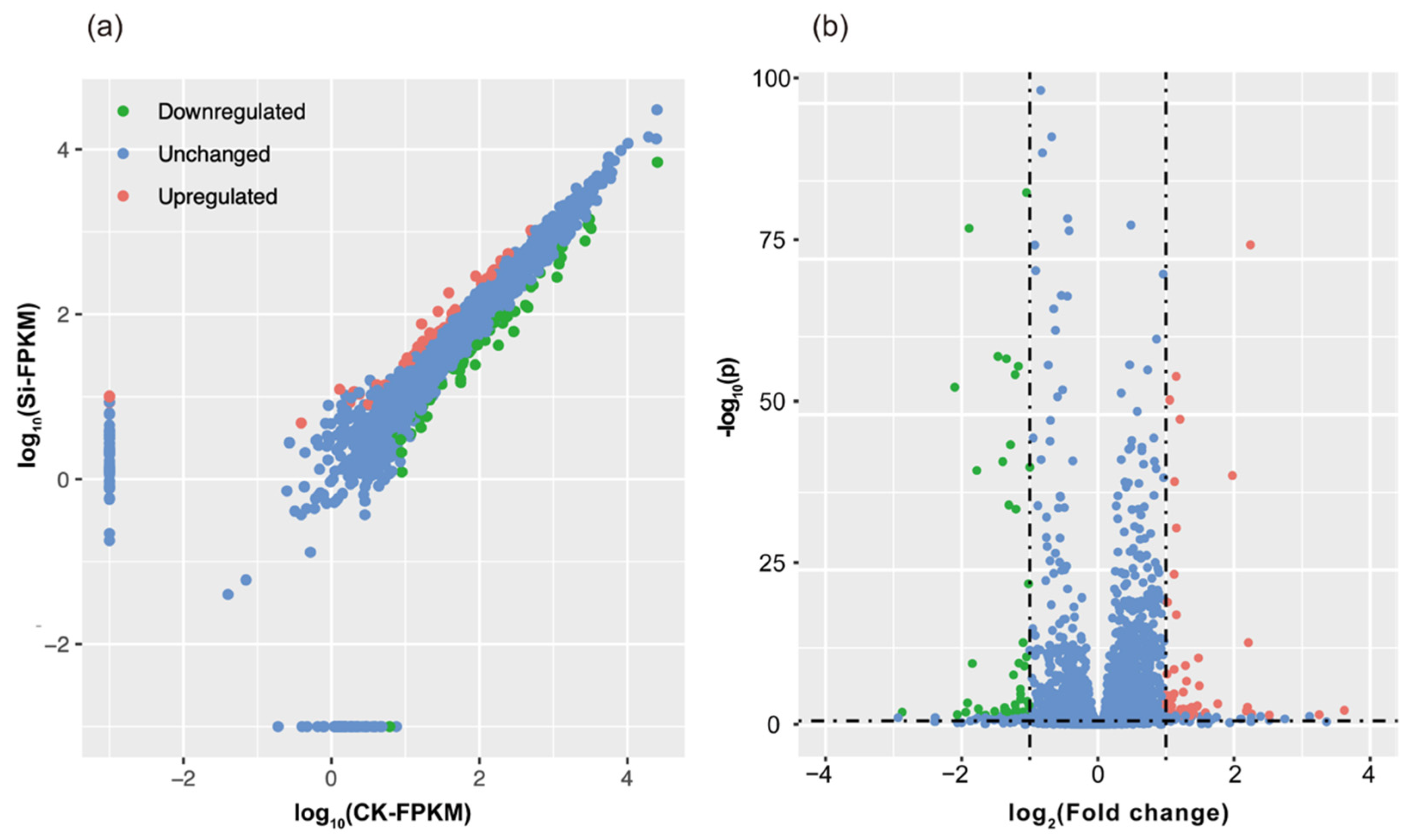
2.3. Silicon Regulated the Expression of 119 Genes of R. solanacearum In Vitro
2.4. Silicon Altered the Transcriptional Expression of xpsR, EPS, and T3SS in R. solanacearum
2.5. Silicon Disrupted EPS and Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Effects of Different Si Concentrations on Wilt Incidence
4.3. Effects of Different Silicon Concentrations on the Growth of R. solanacearum
4.4. Pathogen Symptom Evaluation
4.5. EPS Assay
4.6. Biofilm Assay
4.7. Transcriptome Analysis
4.8. Data Quality Check and Analysis
4.9. qRT-PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanto, T.; Maekawa, K.; Aino, M. Suppression of conidial germination and appressorial formation by silicate treatment in powdery mildew of strawberry. J. Gen. Plant Pathol. 2007, 73, 1–7. [Google Scholar] [CrossRef]
- Kvedaras, O.L.; Keeping, M.G. Silicon impedes stalk penetration by the borer Eldana saccharina in sugarcane. Entomol. Exp. Appl. 2007, 125, 103–110. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Wang, G.P.; Cai, K.Z. Silicon-mediated tomato resistance against Ralstonia solanacearum is associated with modification of soil microbial community structure and activity. Biol. Trace Elem. Res. 2013, 152, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Xue, G.; Cui, P.; Fan, F.; Liu, H.; Yin, C.; Sun, W.; Liang, Y. The role of silicon in enhancing resistance to bacterial blight of hydroponic-and soil-cultured rice. Sci. Rep. 2016, 6, 24640. [Google Scholar] [CrossRef]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and plant disease resistance against virulence fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Aconitate and methyl aconitate are modulated by silicon in powdery mildew-infected wheat plants. J. Plant Physiol. 2009, 166, 1413–1422. [Google Scholar] [CrossRef]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef]
- Ghareeb, H.; Bozsó, Z.; Ott, P.G.; Repenning, C.; Stahl, F.; Wydra, K. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011, 75, 83–89. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant-pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Maekawa, K.; Watanabe, K.; Kanto, T.; Aino, M.; Saigusa, M. Effect of soluble silicic acid on suppression of rice leaf blast. Int. J. Mol. Sci. 2003, 74, 293–299. [Google Scholar]
- Bekker, T.F.; Kaiser, C.; Merwe, R.; Labuschagne, N. In-vitro inhibition of mycelial growth of several phytovirulence fungi by soluble potassium silicate. S. Afr. J. Plant Soil. 2006, 23, 169–172. [Google Scholar] [CrossRef]
- Bekker, T.F.; Kaiser, C.; Labuschagne, N. The antifungal activity of potassium silicate and the role of pH against selected plant virulence fungi in vitro. S. Afr. J. Plant Soil. 2009, 26, 55–57. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Bi, Y.; Ge, Y.; Sun, X.J.; Wan, Y. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. J. Food Sci. 2009, 74, M213–M218. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.Z.; Tian, S.P. Enhancement of biocontrol activity of Cryptococcus laurentii by silicon and the possible mechanisms involved. Phytopathology 2005, 95, 69–75. [Google Scholar] [CrossRef]
- Denny, T.P. Ralstonia solanacearum—A plant pathogen in touch with its host. Trends Microbiol. 2000, 8, 486–489. [Google Scholar] [CrossRef]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef]
- Dannon, E.A.; Wydra, K. Interaction between silicon amendment, bacterial wilt development and phenotype of Ralstonia solanacearum in tomato genotypes. Physiol. Mol. Plant Pathol. 2004, 64, 233–243. [Google Scholar] [CrossRef]
- Diogo, R.V.C.; Wydra, K. Silicon-induced basal resistance in tomato against Ralstonia solanacearum is related to modification of pectic cell wall polysaccharide structure. Physiol. Mol. Plant Pathol. 2007, 70, 120–129. [Google Scholar] [CrossRef]
- Kiirika, L.M.; Stahl, F.; Wydra, K. Phenotypic and molecular characterization of resistance induction by single and combined application of chitosan and silicon in tomato against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 81, 1–12. [Google Scholar] [CrossRef]
- Coll, N.S.; Valls, M. Current knowledge on the Ralstonia solanacearum type III secretion system. Microb. Miotechnol. 2013, 6, 614–620. [Google Scholar]
- Yao, J.; Allen, C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 2007, 189, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A. Control of virulence and virulence genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 2000, 38, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, Y.; Yoshimochi, T.; Tsujimoto, S.; Shinohara, R.; Nakaho, K.; Kanda, A.; Kiba1, A.; Ohnishi, K. Global regulation of virulence mechanism of Ralstonia solanacearum. Curr. Top. Microbiol. Immunol. 2007, 24, 149–154. [Google Scholar]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef]
- Yoshihara, A.; Shimatani, M.; Sakata, M.; Takemura, C.; Kai, K. Quorum Sensing Inhibition Attenuates the Virulence of the Plant Pathogen Ralstonia solanacearum Species Complex. ACS Chem. Biol. 2020, 15, 3050–3059. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Wu, H.J.; Niu, Y.D.; Huo, R.; Gao, X.W. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef]
- Tans-Kersten, J.; Huang, H.; Allen, C. Ralstonia solanacearum needs motility for invasive virulence on tomato. J. Bacteriol. 2011, 183, 3597–3605. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, D.; Yu, X.; Wu, D.; Li, S.; Chen, J.; Bing, G. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules 2016, 21, 468. [Google Scholar]
- Wu, D.; Ding, W.; Zhang, Y.; Liu, X.; Liang, Y. Oleanolic acid induces the type III secretion system of Ralstonia solanacearum. Front. Microbiol. 2015, 6, 1466. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Qin, X.; Chen, J.; Li, B.; Yao, X.; Liang, P.; Zhang, Y.; Ding, W. Exposure to umbelliferone reduces Ralstonia solanacearum biofilm formation, transcription of type III secretion system regulators and effectors and virulence on tobacco. Front. Microbiol. 2017, 8, 1234. [Google Scholar] [CrossRef] [PubMed]
- Salanoubat, M.; Genin, S.; Artiguenave, F.; Gouzy, J.; Mangenot, S.; Arlat, M.; Billault, A.; Brottier, P.; Camus, J.C.; Cattolico, L. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 2002, 415, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Liu, M.; Wang, L.; Lin, W.P.; Fan, X.Y.; Cai, K.Z. Proteomic characterization of silicon-mediated resistance against R. solanacearum in tomato. Plant Soil 2015, 387, 425–440. [Google Scholar] [CrossRef]
- Jiang, N.H.; Fan, X.Y.; Lin, W.P.; Wang, G.P.; Cai, K.Z. Transcriptome analysis reveals new insights into the bacterial wilt resistance mechanism mediated by silicon in tomato. Int. J. Mol. Sci. 2019, 20, 761. [Google Scholar] [CrossRef]
- Sambanthamoorthy, K.; Gokhale, A.A.; Lao, W.W.; Parashar, V.; Neiditch, M.B.; Semmelhack, M.F. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. [Google Scholar] [CrossRef]
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.M.; Abderahmane, D.; Mokkapati, V.; Carina, S.; Katsuhiko, M.; Takashi, G.; Gao, X.; Fredrik, W. Low concentrations of vitamin C reduce the synthesis of extracellular polymers and destabilize bacterial biofilms. Front. Microbiol. 2017, 8, 2599. [Google Scholar] [CrossRef]
- Saile, E.; McGarvey, J.A.; Schell, M.A.; Denny, T.P. Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology 1997, 87, 1264–1271. [Google Scholar] [CrossRef]
- Bocsanczy, A.M.; Achenbach, U.C.M.; Mangravita-Novo, A.; Chow, M.; Norman, D.J. Proteomic comparison of Ralstonia solanacearum strains reveals temperature dependent virulence factors. BMC Genom. 2014, 15, 280. [Google Scholar] [CrossRef]
- Minic, Z.; Marie, C.; Delorme, C.; Faurie, J.M.; Mercier, G.; Ehrlich, D.; Renault, P. Control of epsE, the phosphoglycosyltransferase initiating exopolysaccharide synthesis in Streptococcus thermophilus, by EpsD tyrosine kinase. J. Bacteriol. 2007, 189, 1351–1357. [Google Scholar] [CrossRef]
- Denny, T.P.; Ganova-Raeva, L.M.; Huang, J.; Schell, M.A. Cloning and characterization of tek, the gene encoding the major extracellular protein of Pseudomonas solanacearum. Mol. Plant-Microbe Interact. 1996, 9, 272–281. [Google Scholar] [CrossRef]
- Kumar, J.S.; Umesha, S.; Prasad, K.S.; Niranjana, P. Detection of quorum sensing molecules and biofilm formation in Ralstonia solanacearum. Curr. Microbiol. 2016, 72, 297–305. [Google Scholar] [CrossRef]
- Kong, C.; Chee, C.F.; Richter, K.; Thomas, N.; Rahman, N.A.; Nathan, S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, J.A.; Thomas, W.J.; Jiang, Y.; Creason, A.L.; Chang, J.H. Mutualistic co-evolution of type III effector genes in Sinorhizobium fredii and Bradyrhizobium japonicum. PLoS Pathog. 2013, 9, e1003204. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, F.; Schulz, S.; Hausner, J.; Marillonnet, S.; Büttner, D. Type III-dependent translocation of HrpB2 by a nonvirulence hpaABC mutant of the plant-virulence bacterium Xanthomonas campestris pv. vesicatoria. Appl. Environ. Microbiol. 2016, 82, 3331–3347. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Lee, K.E.; Schell, M.A.; Denny, T.P. A two-component system in Ralstonia solanacearum modulates production of PhcA-regulated virulence factors in response to 3-hydroxypalmitic acid methyl ester. J. Bacteriol. 1997, 179, 3639–3648. [Google Scholar] [CrossRef]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef]
- Chen, D.; Li, C.; Wu, K.; Xun, G.H.; Yuan, S.F.; Shen, Q.R.; Shen, B. A PhcA-marker-free mutant of Ralstonia solanacearum as potential biocontrol agent of tomato bacterial wilt. Biol. Control. 2015, 80, 96–102. [Google Scholar] [CrossRef]
- Yoshimochi, T.; Hikichi, Y.; Kiba, A.; Ohnishi, K. The global virulence regulator PhcA negatively controls the Ralstonia solanacearum hrp regulatory cascade by repressing expression of the PrhIR signaling proteins. J. Bacteriol. 2009, 191, 3424–3428. [Google Scholar] [CrossRef]
- Corral, J.; Sebastià, P.; Coll, N.S.; Barbé, J.; Valls, M. Twitching and swimming motility play a role in Ralstonia solanacearum virulence. mSphere 2020, 5, e00740-19. [Google Scholar] [CrossRef]
- Bremer, H.; Dennis, P.P. Modulation of chemical composition and other parameters of the cell by growth rate. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology; Neidhardt, F.C., Ed.; American Society for Microbiology: Washington, DC, USA, 1996; pp. 1553–1569. [Google Scholar]
- Boucher, C.A.; Barberis, P.A.; Trigalet, A.P.H.; Demery, D.A. Transposon mutagenesis of Pseudomonas solanacearum: Isolation of Tn5-induced avirulent mutants. Microbiology 1985, 131, 2449–2457. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Hendrich, C.G.; von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R.; Dalsing, B.L.; Patrizia Ricca, P.; Naidoo, J.; David Cook, D.; et al. Metabolomics of tomato xylem sap during bacterial wilt reveals Ralstonia solanacearum produces abundant putrescine, a metabolite that accelerates wilt disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef] [PubMed]
- Milling, A.; Babujee, L.; Allen, C. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS ONE 2011, 6, e15853. [Google Scholar] [CrossRef] [PubMed]
- Elson, L.A.; Morgan, W.T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem. J. 1933, 27, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2012, 25, 402–408. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Gao, Y.; Jiang, N.; Yan, J.; Lin, W.; Cai, K. Silicon Controls Bacterial Wilt Disease in Tomato Plants and Inhibits the Virulence-Related Gene Expression of Ralstonia solanacearum. Int. J. Mol. Sci. 2022, 23, 6965. https://doi.org/10.3390/ijms23136965
Wang L, Gao Y, Jiang N, Yan J, Lin W, Cai K. Silicon Controls Bacterial Wilt Disease in Tomato Plants and Inhibits the Virulence-Related Gene Expression of Ralstonia solanacearum. International Journal of Molecular Sciences. 2022; 23(13):6965. https://doi.org/10.3390/ijms23136965
Chicago/Turabian StyleWang, Lei, Yang Gao, Nihao Jiang, Jian Yan, Weipeng Lin, and Kunzheng Cai. 2022. "Silicon Controls Bacterial Wilt Disease in Tomato Plants and Inhibits the Virulence-Related Gene Expression of Ralstonia solanacearum" International Journal of Molecular Sciences 23, no. 13: 6965. https://doi.org/10.3390/ijms23136965
APA StyleWang, L., Gao, Y., Jiang, N., Yan, J., Lin, W., & Cai, K. (2022). Silicon Controls Bacterial Wilt Disease in Tomato Plants and Inhibits the Virulence-Related Gene Expression of Ralstonia solanacearum. International Journal of Molecular Sciences, 23(13), 6965. https://doi.org/10.3390/ijms23136965






