Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response
Abstract
:1. Introduction
2. Disease Classification, Activity and Severity Assessment Tools
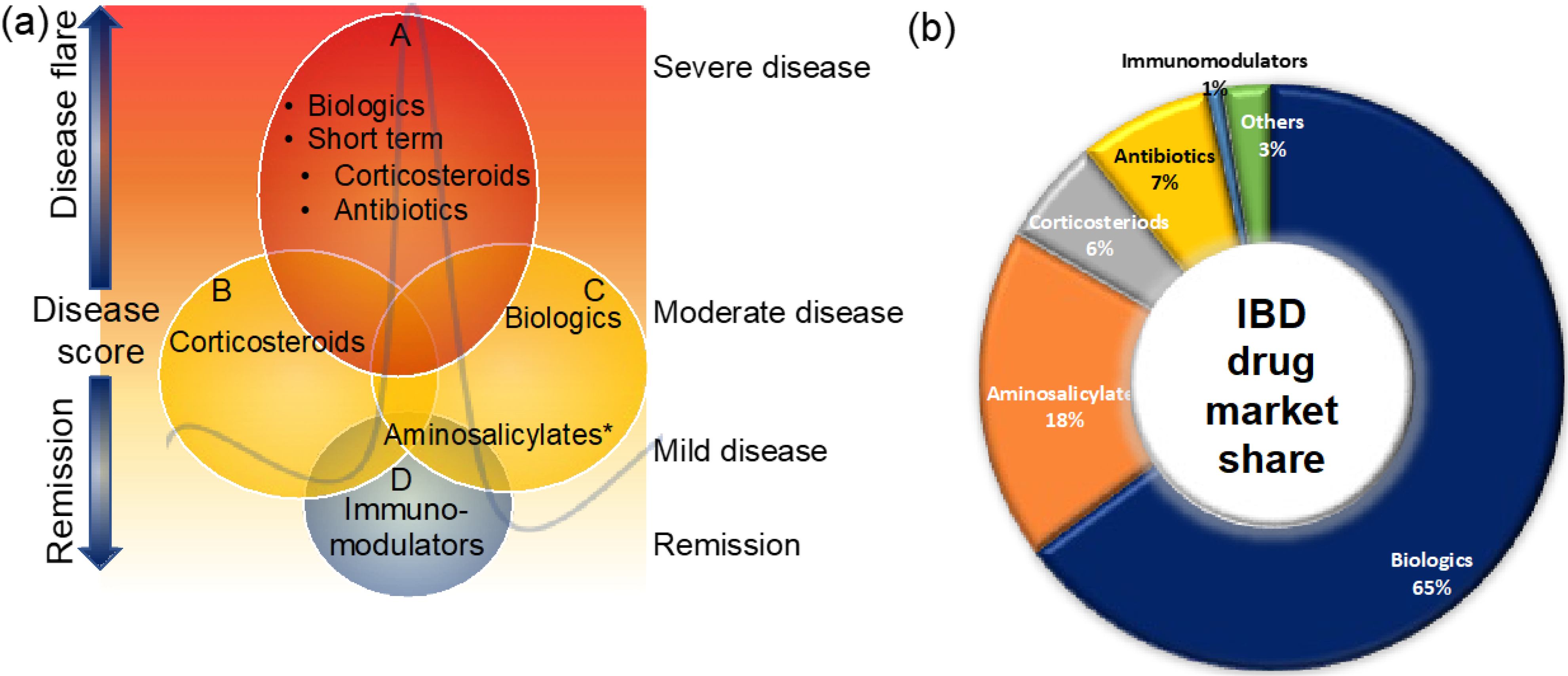
3. Treatment Options for CD and UC
3.1. Aminosalicylates
3.2. Corticosteroids
3.3. Immunomodulators
3.4. Antibiotics
3.5. Biologic Therapies
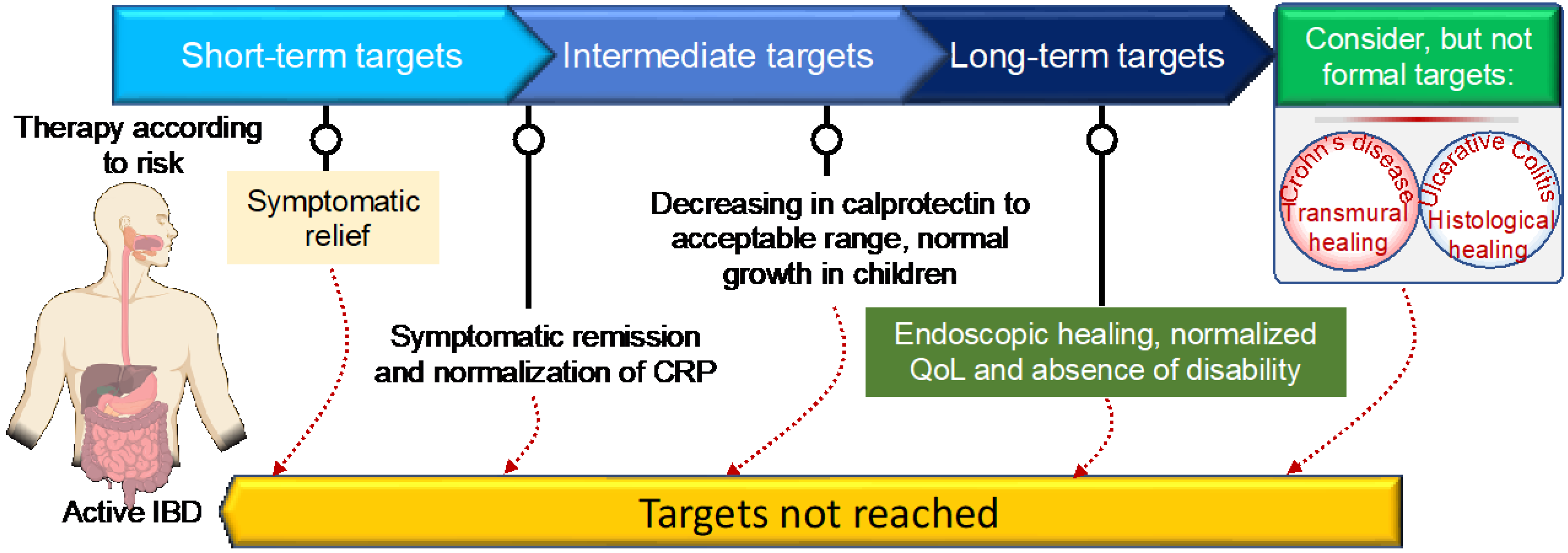
3.5.1. Specific Treatment Options for CD and UC: Treat-To-Target Approach
3.5.2. TNF-Inhibitors
3.5.3. CAM Inhibitors
3.5.4. Anti-Interleukin Inhibitors
3.6. JAK Inhibitors
3.7. Dietary Therapies
4. Emerging Therapies for CD and UC
4.1. Sphingosine-1-Phosphate Receptor
4.2. Stem-Cell Therapies
4.3. Antisense Nucleotide
4.4. Microbial-Based Therapeutics: To Decolonize the Bed Buds
4.5. Fecal Microbiota Transplantation
4.6. Bacterial Inhibitor
5. Predictor Biomarkers for Evaluating Therapeutic Response to Different IBD Treatments
5.1. Biomarkers for Response to Aminosalicylates
5.2. Biomarkers for Response to Corticosteroids
5.3. Biomarkers for Response to Biological Treatments
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase enzyme |
| 5-ASA | Aminosalicylates |
| BDP | Beclomethasone dipropionate |
| CS | Corticosteroids |
| IFX | Infliximab |
| VDZ | Vedolizumab |
| ADA | Adalimumab |
| USK | Ustekinumab |
| ETZ | Etrolizumab |
| FMT | Fecal microbiota transplantation |
| FC | Fecal calprotectin |
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Garand, M.; Al Khodor, S. Integrating omics for a better understanding of Inflammatory Bowel Disease: A step towards personalized medicine. J. Transl. Med. 2019, 17, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasant, D.H.; Ford, A.C. Functional gastrointestinal disorders in inflammatory bowel disease: Time for a paradigm shift? World J. Gastroenterol. 2020, 26, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [PubMed]
- Verstockt, B.; Bressler, B.; Martinez-Lozano, H.; McGovern, D.; Silverberg, M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology 2022, 162, 1370–1382. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Alsoud, D.; Verstockt, B.; Fiocchi, C.; Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 2021, 6, 589–595. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2018, 13, 144K–164K. [Google Scholar] [CrossRef] [Green Version]
- Plichta, D.R.; Graham, D.B.; Subramanian, S.; Xavier, R.J. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell 2019, 178, 1041–1056. [Google Scholar] [CrossRef]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef]
- Liverani, E.; Scaioli, E.; Digby, R.J.; Bellanova, M.; Belluzzi, A. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016, 22, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, L.; Tilley, M.; Afzelius, L.; Maciejewski, M.; Jelinsky, S.; Tian, C.; McIntyre, M.; Agee, M.; Auton, A.; et al. Distinct clinical phenotypes for Crohn’s disease derived from patient surveys. BMC Gastroenterol. 2021, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Williet, N.; Jardin, S.; Roblin, X. The Simplified Magnetic Resonance Index of Activity (MARIA) for Crohn’s Disease Is Strongly Correlated With the MARIA and Clermont Score: An External Validation. Gastroenterology 2020, 158, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Kambayashi, H.; Murasugi, S.; Ito, A.; Yonezawa, M.; Nakamura, S.; Tokushige, K. Comparison of Lewis Score and Capsule Endoscopy Crohn’s Disease Activity Index in Patients with Crohn’s Disease. Dig. Dis. Sci. 2020, 65, 1180–1188. [Google Scholar] [CrossRef]
- Bots, S.; Nylund, K.; Löwenberg, M.; Gecse, K.; D’Haens, G. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a novel UC-Ultrasound Index. J. Crohn’s Colitis 2021, 15, 1264–1271. [Google Scholar] [CrossRef]
- Buisson, A.; Pereira, B.; Goutte, M.; Reymond, M.; Allimant, C.; Obritin-Guilhen, H.; Bommelaer, G.; Hordonneau, C. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn’s disease. Dig. Liver Dis. 2017, 49, 1211–1217. [Google Scholar] [CrossRef]
- Gui, X.; Bazarova, A.; del Amor, R.; Vieth, M.; de Hertogh, G.; Villanacci, V.; Zardo, D.; Parigi, T.L.; Røyset, E.S.; Shivaji, U.N.; et al. PICaSSO Histologic Remission Index (PHRI) in ulcerative colitis: Development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut 2022, 71, 889–898. [Google Scholar] [CrossRef]
- D’Amico, F.; Chateau, T.; Laurent, V.; Danese, S.; Peyrin-Biroulet, L. Which MRI Score and Technique Should Be Used for Assessing Crohn’s Disease Activity? J. Clin. Med. 2020, 9, 1691. [Google Scholar] [CrossRef]
- Goodsall, T.M.; Nguyen, T.M.; Parker, C.E.; Ma, C.; Andrews, J.M.; Jairath, V.; Bryant, R.V. Systematic Review: Gastrointestinal Ultrasound Scoring Indices for Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 125–142. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; on behalf of theAGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Gotz, M.; Katsanos, K.H.; Kiesslich, R.; et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carman, N.; Tomalty, D.; Church, P.C.; Mack, D.R.; Benchimol, E.I.; Otley, A.R.; Jacobson, K.; Huynh, H.Q.; De Bruyn, J.C.; El-Matary, W.; et al. Clinical disease activity and endoscopic severity correlate poorly in children newly diagnosed with Crohn’s disease. Gastrointest. Endosc. 2019, 89, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Bruining, D.H.; Loftus, E.V., Jr.; Thia, K.T.; Schroeder, K.W.; Tremaine, W.J.; Faubion, W.A.; Kane, S.V.; Pardi, D.S.; de Groen, P.C.; et al. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin. Gastroenterol. Hepatol. 2013, 11, 49–54.e1. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Bozada-Gutierrez, K.E.; Sanchez-Rodriguez, A.; Bojalil-Romano, F.; Barreto-Zuniga, R.; Martinez-Benitez, B. Validation of a novel integral disease index for evaluating the grade of activity in Mexican patients with ulcerative colitis: A prospective cohort study. Rev. Gastroenterol. Mex. 2019, 84, 317–325. [Google Scholar] [CrossRef]
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018, 1, Cd011450. [Google Scholar] [CrossRef]
- Chen, H.; Wu, L.; Wang, M.; Shao, B.; Ye, L.; Zhang, Y.; Cao, Q. Use of the ulcerative colitis endoscopic index of severity and Mayo endoscopic score for predicting the therapeutic effect of mesalazine in patients with ulcerative colitis. Laparosc. Endosc. Robot. Surg. 2021, 4, 33–39. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Balint, A.; Farkas, K.; Szepes, Z.; Nagy, F.; Szucs, M.; Tiszlavicz, L.; Bor, R.; Milassin, A.; Rutka, M.; Fabian, A.; et al. How disease extent can be included in the endoscopic activity index of ulcerative colitis: The panMayo score, a promising scoring system. BMC Gastroenterol. 2018, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Restellini, S.; Chao, C.Y.; Martel, M.; Barkun, A.; Kherad, O.; Seidman, E.; Wild, G.; Bitton, A.; Afif, W.; Bessissow, T.; et al. Clinical Parameters Correlate With Endoscopic Activity of Ulcerative Colitis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2019, 17, 1265–1275.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koliani-Pace, J.L.; Siegel, C.A. Beyond disease activity to overall disease severity in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2017, 2, 624–626. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, B.L.; Mazzaro, M.C.; Nagasako, C.K.; Ayrizono, M.d.L.S.; Fagundes, J.J.; Leal, R.F. Assessment of disease activity in inflammatory bowel diseases: Non-invasive biomarkers and endoscopic scores. World J. Gastrointest. Endosc. 2020, 12, 504–520. [Google Scholar] [CrossRef]
- Pabla, B.S.; Schwartz, D.A. Assessing Severity of Disease in Patients with Ulcerative Colitis. Gastroenterol. Clin. N. Am 2020, 49, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Jairath, V.; Ma, C.; Narula, N.; Vande Casteele, N.; Peyrin-Biroulet, L.; Vermeire, S.; D’Haens, G.; Feagan, B.G.; et al. Prevalence of endoscopic improvement and remission according to patient-reported outcomes in ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Kerur, B.; Litman, H.J.; Stern, J.B.; Weber, S.; Lightdale, J.R.; Rufo, P.A.; Bousvaros, A. Correlation of endoscopic disease severity with pediatric ulcerative colitis activity index score in children and young adults with ulcerative colitis. World J. Gastroenterol. 2017, 23, 3322–3329. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Nagahori, M.; Kochi, S.; Hanai, H.; Yamamoto, T.; Nakamura, S.; Omuro, S.; Watanabe, M.; Hibi, T.; Group, O.S. Real life results in using 5-ASA for maintaining mild to moderate UC patients in Japan, a multi-center study, OPTIMUM Study. BMC Gastroenterol. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Louis, E.; Paridaens, K.; Al Awadhi, S.; Begun, J.; Cheon, J.H.; Dignass, A.U.; Magro, F.; Márquez, J.R.; Moschen, A.R.; Narula, N.; et al. Modelling the benefits of an optimised treatment strategy for 5-ASA in mild-to-moderate ulcerative colitis. BMJ Open Gastroenterol. 2022, 9, e000853. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Maillard, M.H.; Schoepfer, A.M.; Seibold, F.; Van Assche, G.; Rivière, P.; Laharie, D.; Manz, M. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion 2020, 101 (Suppl. S1), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Folscn, U.; Schreiber, S. Immunopharmacology of 5-aminosalicylic acid and of glucocorticoids in the therapy of inflammatory bowel disease. Hepatogastroenterology 2000, 47, 71–82. [Google Scholar] [PubMed]
- Weber, C.K.; Liptay, S.; Wirth, T.; Adler, G.; Schmid, R.M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 2000, 119, 1209–1218. [Google Scholar] [CrossRef]
- Allgayer, H.; Kruis, W. Aminosalicylates: Potential antineoplastic actions in colon cancer prevention. Scand. J. Gastroenterol. 2002, 37, 125–131. [Google Scholar] [CrossRef]
- Greenfield, S.M.; Punchard, N.A.; Teare, J.P.; Thompson, R.P. Review article: The mode of action of the aminosalicylates in inflammatory bowel disease. Aliment. Pharmacol. Ther. 1993, 7, 369–383. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 2681. [Google Scholar] [CrossRef]
- Waljee, A.K.; Wiitala, W.L.; Govani, S.; Stidham, R.; Saini, S.; Hou, J.; Feagins, L.A.; Khan, N.; Good, C.B.; Vijan, S.; et al. Corticosteroid Use and Complications in a US Inflammatory Bowel Disease Cohort. PLoS ONE 2016, 11, e0158017. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids—All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef] [Green Version]
- Dorrington, A.M.; Selinger, C.P.; Parkes, G.C.; Smith, M.; Pollok, R.C.; Raine, T. The Historical Role and Contemporary Use of Corticosteroids in Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- An, Y.K. Common mistakes with steroids. J. Gastroenterol. Hepatol. 2021, 36 (Suppl. S1), 30–31. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489.e3. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.Y.; Spiegel, B.M.; Bressler, B.; Cheifetz, A.S.; Devlin, S.M.; Harrell, L.E.; Irving, P.M.; Jones, J.; Kaplan, G.G.; Kozuch, P.L.; et al. The Appropriateness of Concomitant Immunomodulators With Anti–Tumor Necrosis Factor Agents for Crohn’s Disease: One Size Does Not Fit All. Clin. Gastroenterol. Hepatol. 2010, 8, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Kennedy, N.A. Immunomodulator and Biologic Combination Therapy in IBD: The Debate That Just Won’t Go Away? J. Crohn’s Colitis 2020, 14, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Azimi, T.; Nasiri, M.J.; Chirani, A.S.; Pouriran, R.; Dabiri, H. The role of bacteria in the inflammatory bowel disease development: A narrative review. Apmis 2018, 126, 275–283. [Google Scholar] [CrossRef]
- Satoh, K.; Okuyama, M.; Furuya, T.; Irie, Y.; Nakae, H. Severe Sepsis Caused by Bacteria That Entered via the Intestinal Tract: A Case of Crohn’s Disease in a Child. Cureus 2020, 12, e9822. [Google Scholar] [CrossRef]
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef] [Green Version]
- Dignass, A.; Lindsay, J.O.; Sturm, A.; Windsor, A.; Colombel, J.F.; Allez, M.; D’Haens, G.; D’Hoore, A.; Mantzaris, G.; Novacek, G.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. J. Crohns Colitis 2012, 6, 991–1030. [Google Scholar] [CrossRef] [Green Version]
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl. Pediatr. 2019, 8, 42–55. [Google Scholar] [CrossRef]
- Rabbenou, W.; Chang, S. Medical treatment of pouchitis: A guide for the clinician. Ther. Adv. Gastroenterol. 2021, 14, 17562848211023376. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Rosenstein, A.K.; Mehandru, S.; Colombel, J.F. The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal. Immunol. 2018, 11, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ali, R.A.R.; Wei, S.C.; Adsul, S. Biologics for the Management of Inflammatory Bowel Disease: A Review in Tuberculosis-Endemic Countries. Gut Liver 2020, 14, 685–698. [Google Scholar] [CrossRef]
- Siegel, C.A.; Yang, F.; Eslava, S.; Cai, Z. Treatment Pathways Leading to Biologic Therapies for Ulcerative Colitis and Crohn’s Disease in the United States. Clin. Transl. Gastroenterol. 2020, 11, e00128. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Gecse, K.; Halfvarson, J.; Irving, P.M.; Jahnsen, J.; Peyrin-Biroulet, L.; Rogler, G.; Schreiber, S.; Danese, S. Clinical Practice of Adalimumab and Infliximab Biosimilar Treatment in Adult Patients With Crohn’s Disease. Inflamm. Bowel Dis. 2021, 27, 106–122. [Google Scholar] [CrossRef]
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic Dis. 2019, 10, 2040622319838443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Rutella, S.; Fiorino, G.; Vetrano, S.; Correale, C.; Spinelli, A.; Pagano, N.; Arena, V.; Maggiano, N.; Repici, A.; Malesci, A.; et al. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn’s disease. Am. J. Gastroenterol. 2011, 106, 762–770. [Google Scholar] [CrossRef]
- Gunther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Van den Brande, J.M.; Koehler, T.C.; Zelinkova, Z.; Bennink, R.J.; te Velde, A.A.; ten Cate, F.J.; van Deventer, S.J.; Peppelenbosch, M.P.; Hommes, D.W. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut 2007, 56, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Rudrapatna, V.A.; Velayos, F. Biosimilars for the Treatment of Inflammatory Bowel Disease. Pract. Gastroenterol. 2019, 43, 84–91. [Google Scholar] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picarella, D.; Hurlbut, P.; Rottman, J.; Shi, X.; Butcher, E.; Ringler, D.J. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J. Immunol. 1997, 158, 2099–2106. [Google Scholar]
- Kurmaeva, E.; Lord, J.D.; Zhang, S.; Bao, J.R.; Kevil, C.G.; Grisham, M.B.; Ostanin, D.V. T cell-associated α4β7 but not α4β1 integrin is required for the induction and perpetuation of chronic colitis. Mucosal. Immunol. 2014, 7, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Colombel, J.F.; Enns, R.; Feagan, B.G.; Hanauer, S.B.; Lawrance, I.C.; Panaccione, R.; Sanders, M.; Schreiber, S.; Targan, S.; et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2005, 353, 1912–1925. [Google Scholar] [CrossRef]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Targan, S.R.; Feagan, B.G.; Fedorak, R.N.; Lashner, B.A.; Panaccione, R.; Present, D.H.; Spehlmann, M.E.; Rutgeerts, P.J.; Tulassay, Z.; Volfova, M.; et al. Natalizumab for the treatment of active Crohn’s disease: Results of the ENCORE Trial. Gastroenterology 2007, 132, 1672–1683. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Vedolizumab and etrolizumab for ulcerative colitis: Twins or simple cousins? Expert Opin. Biol. Ther. 2020, 20, 353–361. [Google Scholar] [CrossRef]
- Rutgeerts, P.J.; Fedorak, R.N.; Hommes, D.W.; Sturm, A.; Baumgart, D.C.; Bressler, B.; Schreiber, S.; Mansfield, J.C.; Williams, M.; Tang, M.; et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013, 62, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhu, J.; Mi, L.Z.; Walz, T.; Sun, H.; Chen, J.; Springer, T.A. Structural specializations of alpha(4)beta(7), an integrin that mediates rolling adhesion. J. Cell Biol. 2012, 196, 131–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyant, T.; Yang, L.; Fedyk, E. In vitro assessment of the effects of vedolizumab binding on peripheral blood lymphocytes. MAbs 2013, 5, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, N.; Watanabe, M.; Motoya, S.; Tominaga, K.; Matsuoka, K.; Iwakiri, R.; Watanabe, K.; Hibi, T.; Group, A.J.M.S. Safety and Efficacy of AJM300, an Oral Antagonist of alpha4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology 2015, 149, 1775–1783.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggeletopoulou, I.; Assimakopoulos, S.F.; Konstantakis, C.; Triantos, C. Interleukin 12/interleukin 23 pathway: Biological basis and therapeutic effect in patients with Crohn’s disease. World J. Gastroenterol. 2018, 24, 4093–4103. [Google Scholar] [CrossRef]
- Harris, K.A.; Horst, S.; Gadani, A.; Nohl, A.; Annis, K.; Duley, C.; Beaulieu, D.; Ghazi, L.; Schwartz, D.A. Patients with Refractory Crohn’s Disease Successfully Treated with Ustekinumab. Inflamm. Bowel Dis. 2016, 22, 397–401. [Google Scholar] [CrossRef]
- Khorrami, S.; Ginard, D.; Marin-Jimenez, I.; Chaparro, M.; Sierra, M.; Aguas, M.; Sicilia, B.; Garcia-Sanchez, V.; Suarez, C.; Villoria, A.; et al. Ustekinumab for the Treatment of Refractory Crohn’s Disease: The Spanish Experience in a Large Multicentre Open-label Cohort. Inflamm. Bowel Dis. 2016, 22, 1662–1669. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Carcamo, C.V.; Fleming, M.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Feagan, B.G.; Panes, J.; Ferrante, M.; Kaser, A.; D’Haens, G.R.; Sandborn, W.J.; Louis, E.; Neurath, M.F.; Franchimont, D.; Dewit, O.; et al. Risankizumab in patients with moderate to severe Crohn’s disease: An open-label extension study. Lancet Gastroenterol. Hepatol. 2018, 3, 671–680. [Google Scholar] [CrossRef]
- Weisshof, R.; Golan, M.A.; Yvellez, O.V.; Rubin, D.T. The use of tofacitinib in the treatment of inflammatory bowel disease. Immunotherapy 2018, 10, 837–849. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W.; Study, A.I. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.N.; Ananthakrishnan, A.N.; Raman, M. Diet in Treatment of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 425–435.e3. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef] [Green Version]
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Ther. Adv. Gastroenterol. 2019, 12, 1756284819890534. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [Green Version]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef] [Green Version]
- Suskind, D.L.; Lee, D.; Kim, Y.M.; Wahbeh, G.; Singh, N.; Braly, K.; Nuding, M.; Nicora, C.D.; Purvine, S.O.; Lipton, M.S.; et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients 2020, 12, 3749. [Google Scholar] [CrossRef]
- Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients 2020, 12, 3793. [Google Scholar] [CrossRef]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults with Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kuhbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 550–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagan, B.G.; Sandborn, W.J.; Danese, S.; Wolf, D.C.; Liu, W.J.; Hua, S.Y.; Minton, N.; Olson, A.; D’Haens, G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020, 5, 819–828. [Google Scholar] [CrossRef]
- Argollo, M.; Furfaro, F.; Gilardi, D.; Roda, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Modulation of sphingosine-1-phosphate in ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 413–420. [Google Scholar] [CrossRef]
- Okamoto, R.; Watanabe, M. Investigating cell therapy for inflammatory bowel disease. Expert Opin. Biol. Ther. 2016, 16, 1015–1023. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119 Pt 11, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, D.P.; Kalia, N. Hematopoietic stem cell homing to injured tissues. Stem Cell Rev. Rep. 2011, 7, 672–682. [Google Scholar] [CrossRef]
- Lopez-Garcia, A.; Rovira, M.; Jauregui-Amezaga, A.; Marin, P.; Barastegui, R.; Salas, A.; Ribas, V.; Feu, F.; Elizalde, J.I.; Fernandez-Aviles, F.; et al. Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn’s Disease: Efficacy in a Single-Centre Cohort. J. Crohns Colitis 2017, 11, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2018, 154, 1334–1342.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, J.O.; Allez, M.; Clark, M.; Labopin, M.; Ricart, E.; Rogler, G.; Rovira, M.; Satsangi, J.; Farge, D.; Hawkey, C.J.; et al. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: An analysis of pooled data from the ASTIC trial. Lancet Gastroenterol. Hepatol. 2017, 2, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Dalal, J.; Gandy, K.; Domen, J. Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatr. Res. 2012, 71, 445–451. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bevivino, G.; Monteleone, G. Mongersen, an oral Smad7 antisense oligonucleotide, in patients with active Crohn’s disease. Ther. Adv. Gastroenterol. 2016, 9, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marafini, I.; Stolfi, C.; Troncone, E.; Lolli, E.; Onali, S.; Paoluzi, O.A.; Fantini, M.C.; Biancone, L.; Calabrese, E.; Di Grazia, A.; et al. A Pharmacological Batch of Mongersen that Downregulates Smad7 is Effective as Induction Therapy in Active Crohn’s Disease: A Phase II, Open-Label Study. BioDrugs 2021, 35, 325–336. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, P.; Murugesan, S.; Vetizou, M.; McCulloch, J.; Badger, J.H.; Trinchieri, G.; Al Khodor, S. Microbiome as an Immunological Modifier. Methods Mol. Biol. 2020, 2055, 595–638. [Google Scholar] [CrossRef]
- Elhag, D.A.; Kumar, M.; Al Khodor, S. Exploring the Triple Interaction between the Host Genome, the Epigenome, and the Gut Microbiome in Type 1 Diabetes. Int. J. Mol. Sci. 2020, 22, 125. [Google Scholar] [CrossRef]
- Kumar, M.; Murugesan, S.; Singh, P.; Saadaoui, M.; Elhag, D.A.; Terranegra, A.; Kabeer, B.S.A.; Marr, A.K.; Kino, T.; Brummaier, T.; et al. Vaginal Microbiota and Cytokine Levels Predict Preterm Delivery in Asian Women. Front. Cell. Infect. Microbiol. 2021, 11, 639665. [Google Scholar] [CrossRef]
- Augustine, T.; Kumar, M.; Al Khodor, S.; van Panhuys, N. Microbial Dysbiosis Tunes the Immune Response Towards Allergic Disease Outcomes. Clin. Rev. Allergy Immunol. 2022. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Borody, T.J.; Paramsothy, S.; Agrawal, G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr. Gastroenterol. Rep. 2013, 15, 337. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Sipe, B.; Cheng, Y.W.; Phelps, E.; Rogers, N.; Sagi, S.; Bohm, M.; Xu, H.; Kassam, Z. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: A promising treatment approach. Gut Microbes 2017, 8, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Mathur, T.; Joshi, V.; Upadhyay, D.J.; Inoue, S.I.; Masuda, N. Effect of DS-2969b, a novel GyrB inhibitor, on rat and monkey intestinal microbiota. Anaerobe 2018, 51, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.; Eysenbach, L.M.; El-Nachef, N.; Fischer, M.; Kelly, C.; Kassam, Z. The Current Landscape and Lessons from Fecal Microbiota Transplantation for Inflammatory Bowel Disease: Past, Present, and Future. Inflamm. Bowel Dis. 2017, 23, 1710–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: Systematic review and meta-analysis. Gut Microbes 2017, 8, 574–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, C.L.; Reid-Yu, S.A.; McPhee, J.B.; Coombes, B.K. Persistent infection with Crohn’s disease-associated adherent-invasive Escherichia coli leads to chronic inflammation and intestinal fibrosis. Nat. Commun. 2013, 4, 1957. [Google Scholar] [CrossRef]
- Kumar, M.; Saadaoui, M.; Al Khodor, D. Infections and Pregnancy: Effects on Maternal and Child Health. Front. Cell. Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef]
- Poole, N.M.; Green, S.I.; Rajan, A.; Vela, L.E.; Zeng, X.L.; Estes, M.K.; Maresso, A.W. Role for FimH in Extraintestinal Pathogenic Escherichia coli Invasion and Translocation through the Intestinal Epithelium. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [Green Version]
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational design strategies for FimH antagonists: New drugs on the horizon for urinary tract infection and Crohn’s disease. Expert Opin. Drug. Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef]
- Chervy, M.; Barnich, N.; Denizot, J. Adherent-Invasive E. coli: Update on the Lifestyle of a Troublemaker in Crohn’s Disease. Int. J. Mol. Sci. 2020, 21, 3734. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Short, K.; Catto-Smith, A.G.; Cameron, D.J.; Bishop, R.F.; Kirkwood, C.D. Identification and characterisation of Pseudomonas 16S ribosomal DNA from ileal biopsies of children with Crohn’s disease. PLoS ONE 2008, 3, e3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Huang, T.; Dalwadi, H.; Sutton, C.L.; Bruckner, D.; Braun, J. Pseudomonas fluorescens encodes the Crohn’s disease-associated I2 sequence and T-cell superantigen. Infect. Immun. 2002, 70, 6567–6575. [Google Scholar] [CrossRef] [Green Version]
- Mottawea, W.; Chiang, C.K.; Muhlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef]
- Qasem, A.; Safavikhasraghi, M.; Naser, S.A. A single capsule formulation of RHB-104 demonstrates higher anti-microbial growth potency for effective treatment of Crohn’s disease associated with Mycobacterium avium subspecies paratuberculosis. Gut Pathog. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerich, M.E.; McGovern, D.P. Towards personalized care in IBD. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 287–299. [Google Scholar] [CrossRef]
- Bitton, A.; Peppercorn, M.A.; Antonioli, D.A.; Niles, J.L.; Shah, S.; Bousvaros, A.; Ransil, B.; Wild, G.; Cohen, A.; Edwardes, M.D.; et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology 2001, 120, 13–20. [Google Scholar] [CrossRef]
- Hoie, O.; Wolters, F.; Riis, L.; Aamodt, G.; Solberg, C.; Bernklev, T.; Odes, S.; Mouzas, I.A.; Beltrami, M.; Langholz, E.; et al. Ulcerative colitis: Patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am. J. Gastroenterol. 2007, 102, 1692–1701. [Google Scholar] [CrossRef]
- Bello, C.; Belaiche, J.; Louis, E.; Reenaers, C. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. J. Crohns Colitis 2011, 5, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Jung, E.S.; Lee, J.H.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with mild-to-moderate ulcerative colitis. Hepatogastroenterology 2012, 59, 1415–1420. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shimoyama, T.; Matsumoto, K. Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis. Aliment. Pharmacol. Ther. 2015, 42, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Planella, E.; Manosa, M.; Chaparro, M.; Beltran, B.; Barreiro-de-Acosta, M.; Gordillo, J.; Ricart, E.; Bermejo, F.; Garcia-Sanchez, V.; Piqueras, M.; et al. Serial semi-quantitative measurement of fecal calprotectin in patients with ulcerative colitis in remission. Scand. J. Gastroenterol. 2018, 53, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Marti-Aguado, D.; Ballester, M.P.; Minguez, M. Risk factors and management strategies associated with non-response to aminosalicylates for maintenance treatment in ulcerative colitis. Rev. Esp. Enferm. Dig. 2021, 113, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Davis Thomas, S.; Gotman, N.; Haberman, Y.; Karns, R.; Schirmer, M.; Mo, A.; Mack, D.R.; Boyle, B.; Griffiths, A.M.; et al. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): A multicentre inception cohort study. Lancet 2019, 393, 1708–1720. [Google Scholar] [CrossRef]
- Cravo, M.L.; Ferreira, P.A.; Sousa, P.; Moura-Santos, P.; Velho, S.; Tavares, L.; de Deus, J.R.; Ministro, P.; Peixe, P.; Correia, L.A.; et al. IL23R polymorphisms influence phenotype and response to therapy in patients with ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 26–32. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Renbaum, P.; Beeri, R.; Ledder, O.; Mevorach, R.; Karban, A.; Koifman, E.; Efrati, E.; Muise, A.M.; Chowers, Y.; et al. Rac1 Polymorphisms and Thiopurine Efficacy in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 404–407. [Google Scholar] [CrossRef]
- Al-Judaibi, B.; Schwarz, U.I.; Huda, N.; Dresser, G.K.; Gregor, J.C.; Ponich, T.; Chande, N.; Mosli, M.; Kim, R.B. Genetic Predictors of Azathioprine Toxicity and Clinical Response in Patients with Inflammatory Bowel Disease. J. Popul. Ther. Clin. Pharmacol. 2016, 23, e26–e36. [Google Scholar]
- Li, J.; Wang, F.; Zhang, H.J.; Sheng, J.Q.; Yan, W.F.; Ma, M.X.; Fan, R.Y.; Gu, F.; Li, C.F.; Chen, D.F.; et al. Corticosteroid therapy in ulcerative colitis: Clinical response and predictors. World J. Gastroenterol. 2015, 21, 3005–3015. [Google Scholar] [CrossRef]
- Xie, T.; Zhao, C.; Ding, C.; Zhang, T.; Dai, X.; Lv, T.; Li, Y.; Guo, Z.; Gong, J.; Zhu, W. Fecal calprotectin as an alternative to ulcerative colitis endoscopic index of severity to predict the response to corticosteroids of acute severe ulcerative colitis: A prospective observational study. Dig. Liver Dis. 2017, 49, 984–990. [Google Scholar] [CrossRef]
- Rai, T.; Choudhury, B.N.; Kedia, S.; Bopanna, S.; Venigalla, P.M.; Garg, S.K.; Singla, V.; Makharia, G.; Ahuja, V. Short-Term Clinical Response to Corticosteroids Can Predict Long-Term Natural History of Ulcerative Colitis: Prospective Study Experience. Dig. Dis. Sci. 2017, 62, 1025–1034. [Google Scholar] [CrossRef]
- Barnes, A.; Spizzo, P.; Mountifield, R. Corticosteroid exposure prior to admission and predicting need for rescue therapy in acute severe ulcerative colitis. Intern. Med. J. 2020, 52, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Seidman, E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2016, 9, 513–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FGC, E.P.; Rosa, R.M.; da Cunha, P.F.S.; de Souza, S.C.S.; de Abreu Ferrari, M.L. Faecal calprotectin is the biomarker that best distinguishes remission from different degrees of endoscopic activity in Crohn’s disease. BMC Gastroenterol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819; quiz 820. [Google Scholar] [CrossRef]
- Frin, A.C.; Filippi, J.; Boschetti, G.; Flourie, B.; Drai, J.; Ferrari, P.; Hebuterne, X.; Nancey, S. Accuracies of fecal calprotectin, lactoferrin, M2-pyruvate kinase, neopterin and zonulin to predict the response to infliximab in ulcerative colitis. Dig. Liver Dis. 2017, 49, 11–16. [Google Scholar] [CrossRef]
- Ho, G.T.; Lee, H.M.; Brydon, G.; Ting, T.; Hare, N.; Drummond, H.; Shand, A.G.; Bartolo, D.C.; Wilson, R.G.; Dunlop, M.G.; et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 673–678. [Google Scholar] [CrossRef]
- Pauwels, R.W.M.; van der Woude, C.J.; Erler, N.S.; de Vries, A.C. Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2020, 13, 1756284820979765. [Google Scholar] [CrossRef]
- Billiet, T.; Cleynen, I.; Ballet, V.; Claes, K.; Princen, F.; Singh, S.; Ferrante, M.; Van Assche, G.; Gils, A.; Vermeire, S. Evolution of cytokines and inflammatory biomarkers during infliximab induction therapy and the impact of inflammatory burden on primary response in patients with Crohn’s disease. Scand. J. Gastroenterol. 2017, 52, 1086–1092. [Google Scholar] [CrossRef]
- Baird, A.C.; Mallon, D.; Radford-Smith, G.; Boyer, J.; Piche, T.; Prescott, S.L.; Lawrance, I.C.; Tulic, M.K. Dysregulation of innate immunity in ulcerative colitis patients who fail anti-tumor necrosis factor therapy. World J. Gastroenterol. 2016, 22, 9104–9116. [Google Scholar] [CrossRef]
- Bertani, L.; Caviglia, G.P.; Antonioli, L.; Pellicano, R.; Fagoonee, S.; Astegiano, M.; Saracco, G.M.; Bugianesi, E.; Blandizzi, C.; Costa, F.; et al. Serum Interleukin-6 and -8 as Predictors of Response to Vedolizumab in Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 1323. [Google Scholar] [CrossRef]
- Bertani, L.; Baglietto, L.; Antonioli, L.; Fornai, M.; Tapete, G.; Albano, E.; Ceccarelli, L.; Mumolo, M.G.; Pellegrini, C.; Lucenteforte, E.; et al. Assessment of serum cytokines predicts clinical and endoscopic outcomes to vedolizumab in ulcerative colitis patients. Br. J. Clin. Pharmacol. 2020, 86, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rabizadeh, S.; Jossen, J.; Pittman, N.; Check, M.; Hashemi, G.; Phan, B.L.; Hyams, J.S.; Dubinsky, M.C. Multi-Center Experience of Vedolizumab Effectiveness in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hall, Y.L.; Nakatsu, C.H. The Intersection of TNF, IBD and the Microbiome. Gut Microbes 2016, 7, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Colombel, J.F.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: The CHARM trial. Gastroenterology 2007, 132, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyams, J.; Crandall, W.; Kugathasan, S.; Griffiths, A.; Olson, A.; Johanns, J.; Liu, G.; Travers, S.; Heuschkel, R.; Markowitz, J.; et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007, 132, 863–873. [Google Scholar] [CrossRef]
- Moran, G.W.; Dubeau, M.F.; Kaplan, G.G.; Yang, H.; Seow, C.H.; Fedorak, R.N.; Dieleman, L.A.; Barkema, H.W.; Ghosh, S.; Panaccione, R.; et al. Phenotypic features of Crohn’s disease associated with failure of medical treatment. Clin. Gastroenterol. Hepatol. 2014, 12, 434–442.e1. [Google Scholar] [CrossRef]
- Peters, C.P.; Eshuis, E.J.; Toxopeus, F.M.; Hellemons, M.E.; Jansen, J.M.; D’Haens, G.R.; Fockens, P.; Stokkers, P.C.; Tuynman, H.A.; van Bodegraven, A.A.; et al. Adalimumab for Crohn’s disease: Long-term sustained benefit in a population-based cohort of 438 patients. J. Crohns Colitis 2014, 8, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Khalili, H.; Garber, J.J.; Stevens, B.W.; Cleland, T.; Xavier, R.J. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe 2017, 21, 603–610.e3. [Google Scholar] [CrossRef] [Green Version]
- Wolbink, G.J.; Aarden, L.A.; Dijkmans, B.A. Dealing with immunogenicity of biologicals: Assessment and clinical relevance. Curr. Opin. Rheumatol. 2009, 21, 211–215. [Google Scholar] [CrossRef] [Green Version]
- West, R.L.; Zelinkova, Z.; Wolbink, G.J.; Kuipers, E.J.; Stokkers, P.C.; van der Woude, C.J. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 28, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Bortlik, M.; Duricova, D.; Malickova, K.; Machkova, N.; Bouzkova, E.; Hrdlicka, L.; Komarek, A.; Lukas, M. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J. Crohns Colitis 2013, 7, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubinsky, M.C.; Mei, L.; Friedman, M.; Dhere, T.; Haritunians, T.; Hakonarson, H.; Kim, C.; Glessner, J.; Targan, S.R.; McGovern, D.P.; et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Kevans, D.; Waterman, M.; Milgrom, R.; Xu, W.; Van Assche, G.; Silverberg, M. Serological markers associated with disease behavior and response to anti-tumor necrosis factor therapy in ulcerative colitis. J. Gastroenterol. Hepatol. 2015, 30, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Vermeire, S.; Katsanos, K.H.; Noman, M.; Van Assche, G.; Schnitzler, F.; Arijs, I.; De Hertogh, G.; Hoffman, I.; Geboes, J.K.; et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm. Bowel Dis. 2007, 13, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Koder, S.; Repnik, K.; Ferkolj, I.; Pernat, C.; Skok, P.; Weersma, R.K.; Potocnik, U. Genetic polymorphism in ATG16L1 gene influences the response to adalimumab in Crohn’s disease patients. Pharmacogenomics 2015, 16, 191–204. [Google Scholar] [CrossRef]
- Moroi, R.; Endo, K.; Kinouchi, Y.; Shiga, H.; Kakuta, Y.; Kuroha, M.; Kanazawa, Y.; Shimodaira, Y.; Horiuchi, T.; Takahashi, S.; et al. FCGR3A-158 polymorphism influences the biological response to infliximab in Crohn’s disease through affecting the ADCC activity. Immunogenetics 2013, 65, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; El Ghoul, Z.; Vermeire, S.; Dall’Ozzo, S.; Rutgeerts, P.; Paintaud, G.; Belaiche, J.; De Vos, M.; Van Gossum, A.; Colombel, J.F.; et al. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment. Pharmacol. Ther. 2004, 19, 511–519. [Google Scholar] [CrossRef]
- Garand, M.; Kumar, M.; Huang, S.S.Y.; Al Khodor, S. A literature-based approach for curating gene signatures in multifaceted diseases. J. Transl. Med. 2020, 18, 279. [Google Scholar] [CrossRef]
- Netz, U.; Carter, J.V.; Eichenberger, M.R.; Dryden, G.W.; Pan, J.; Rai, S.N.; Galandiuk, S. Genetic polymorphisms predict response to anti-tumor necrosis factor treatment in Crohn’s disease. World J. Gastroenterol. 2017, 23, 4958–4967. [Google Scholar] [CrossRef]
- Taylor, K.D.; Plevy, S.E.; Yang, H.; Landers, C.J.; Barry, M.J.; Rotter, J.I.; Targan, S.R. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn’s disease. Gastroenterology 2001, 120, 1347–1355. [Google Scholar] [CrossRef]
- Medrano, L.M.; Taxonera, C.; Marquez, A.; Barreiro-de Acosta, M.; Gomez-Garcia, M.; Gonzalez-Artacho, C.; Perez-Calle, J.L.; Bermejo, F.; Lopez-Sanroman, A.; Martin Arranz, M.D.; et al. Role of TNFRSF1B polymorphisms in the response of Crohn’s disease patients to infliximab. Hum. Immunol. 2014, 75, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Genetically determined high activity of IL-12 and IL-18 in ulcerative colitis and TLR5 in Crohns disease were associated with non-response to anti-TNF therapy. Pharm. J. 2018, 18, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.J.; Watier, H.E.; Schreiber, S.; Hampe, J.; Taillard, F.; Olson, A.; Thorne, N.; Zhang, H.; Colombel, J.F. Polymorphism in IgG Fc receptor gene FCGR3A and response to infliximab in Crohn’s disease: A subanalysis of the ACCENT I study. Pharm. Genom. 2006, 16, 911–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, G.E.; Yajnik, V.; Khalili, H.; Giallourakis, C.; Garber, J.; Xavier, R.; Ananthakrishnan, A.N. Genetic Markers Predict Primary Non-Response and Durable Response To Anti-TNF Biologic Therapies in Crohn’s Disease. Am. J. Gastroenterol. 2016, 111, 1816–1822. [Google Scholar] [CrossRef] [Green Version]
- Florholmen, J.R.; Johnsen, K.-M.; Meyer, R.; Olsen, T.; Moe, Ø.K.; Tandberg, P.; Gundersen, M.D.; Kvamme, J.-M.; Johnsen, K.; Løitegård, T.; et al. Discovery and validation of mucosal TNF expression combined with histological score—A biomarker for personalized treatment in ulcerative colitis. BMC Gastroenterol. 2020, 20, 321. [Google Scholar] [CrossRef]
- Cui, G.; Florholmen, J.; Goll, R. Could Mucosal TNF Transcript as a Biomarker Candidate Help Optimize Anti-TNF Biological Therapy in Patients With Ulcerative Colitis? Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Cui, G.; Fan, Q.; Li, Z.; Goll, R.; Florholmen, J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine 2021, 66, 103329. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Barbera Betancourt, A.; Lyons, P.A.; Zhang, Z.; Suo, C.; Lee, J.C.; McKinney, E.F.; Modis, L.K.; Ellson, C.; Smith, K.G.C. miR-374a-5p regulates inflammatory genes and monocyte function in patients with inflammatory bowel disease. J. Exp. Med. 2022, 219. [Google Scholar] [CrossRef]
- He, C.; Shi, Y.; Wu, R.; Sun, M.; Fang, L.; Wu, W.; Liu, C.; Tang, M.; Li, Z.; Wang, P.; et al. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut 2016, 65, 1938–1950. [Google Scholar] [CrossRef]
- Batra, S.K.; Heier, C.R.; Diaz-Calderon, L.; Tully, C.B.; Fiorillo, A.A.; van den Anker, J.; Conklin, L.S. Serum miRNAs Are Pharmacodynamic Biomarkers Associated With Therapeutic Response in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1597–1606. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Bergemalm, D.; Vatn, S.; Kennedy, N.A.; Ricanek, P.; Lindstrom, J.; Ocklind, A.; Hjelm, F.; Ventham, N.T.; et al. Serum proteomic profiling at diagnosis predicts clinical course, and need for intensification of treatment in inflammatory bowel disease. J. Crohn’s Colitis 2020, 15, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Salomon, B.; Verstockt, B.; Ungaro, R.; Aden, K.; D’Haens, G.; Komori, K.; Guay, H.; Silverberg, M.; Vermeire, S.; et al. DOP79 Biomarkers for IBD using OLINK Proteomics inflammation panel: Preliminary results from the COLLIBRI consortium. J. Crohn’s Colitis 2022, 16 (Suppl. S1), i123–i124. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Clinical Usefulness of Proteomics in Inflammatory Bowel Disease: A Comprehensive Review. J. Crohns Colitis 2019, 13, 374–384. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Kelly, O.; Battat, R.; Silverberg, M.S.; Laharie, D.; Louis, E.; Savarino, E.; Bodini, G.; Yarur, A.; Boland, B.S.; et al. Development and Validation of a Test to Monitor Endoscopic Activity in Patients With Crohn’s Disease Based on Serum Levels of Proteins. Gastroenterology 2020, 158, 515–526.e10. [Google Scholar] [CrossRef] [Green Version]

| CD and IBD-U Activity Indexes | UC Activity Indexes |
|---|---|
Crohn’s Disease Activity index (CDAI)
|
Ulcerative colitis disease activity index (UCDAI)
|
Pediatric Crohn’s Disease Activity index (PCDAI):
|
Pediatric Ulcerative Colitis Activity Index (PUCAI)
|
Weighted Pediatric Crohn’s Disease Activity index (wPCDAI)
|
Ulcerative Colitis Endoscopic Index of Severity (UCEIS)
|
Harvey-Bradshaw index (HBI) or simple endoscopic score
|
Mayo clinic score
|
Mucosal Inflammation Non-invasive index (MINI):
| Simple Clinical Colitis Activity Index (SCCAI)
|
The simple endoscopic score for CD (SES-CD)
|
The Modified Baron Score
|
The magnetic resonance index of activity (MARIA) and the Clermont score
|
Novel integral disease index of UC activity (NIDI) or Yamamoto-Furusho Index
|
The Lewis score (LS) and Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI)
|
UC Colonoscopic Index of Severity (UCC)
The Walmsley index
|
| Drug Name | Mechanism of Action | Route | Indications | Development Status |
|---|---|---|---|---|
Aminosalicylates
| * Anti-inflammatory CXY and LXY inhibitor * Anti-inflammatory Prostaglandins inhibitor | PO PO, rectal PO PO | Mild-to-mod UC Mild-to-mod UC UC UC | Approved Approved Approved Approved |
Corticosteroides
| GRs inhibitor Anti-inflammatory Anti-inflammatory Anti-inflammatory | PO PO, IV PO PO | Mild-to-mod CD, UC Mod-to-severe CD, UC Mod-to-severe CD, UC Mod-to-severe CD, UC | Approved Approved Approved Approved |
Immunomodulators
| Purine synthesis inhibitor T-cells inhibitor (IL-2) Purine synthesis inhibitor DHFR inhibitor Inhibits IL-2 transcription | PO PO, IV PO PO, SC PO, IV | CD, UC UC CD, UC Active CD Mod-to-severe CD, UC | Approved Approved Approved Approved Approved |
Antibiotics
| Topo and gyr inhibitor Bacterial DNA synthesis Cell wall synthesis inhibitor Protein synthesis inhibitor Cell wall synthesis inhibitor Bacterial DNA synthesis Protein synthesis inhibitor Cell wall synthesis inhibitor Bacterial DNA synthesis | PO, IV PO PO PO PO PO | Active CD and pouchitis Active CD and pouchitis Active CD Active CD Acute severe or chronic UC Acute severe UC | Approved Approved Approved Approved Approved Approved |
TNF-α inhibitors
| Anti-TNF-α ab (IgG1) Anti-TNF-α ab Anti-TNF-α ab Anti-TNF-α ab | SC SC, IV SC SC | CD, UC Mod-to-severe CD, UC Mod-to-severe CD Mod-to-severe UC (adult) | Approved Approved Approved Approved |
CAM inhibitors
| Anti-α4β1-integrin Anti-α4β7-integrin | IV SC, IV | Mod-to-severe CD CD, UC | Approved Approved |
IL-12/-23 inhibitors
| Anti-IL-12/IL-23 (p40) ab | IV | CD | Approved |
JAK inhibitors
| Janus Kinase | PO | UC | Approved |
| Drug Name | Mechanism of Action | Route | Indication | Development Status |
|---|---|---|---|---|
Immunomodulators
| Activate T-cells Inhibits signalling pathways | IV PO | Mod-to-severe UC Active UC | Ph-II Ph-II |
Antibiotics
| FimH inhibitor Anti-E. coli bacteriophage Antibiotics Antibiotics Antibiotics Antibiotics Antibiotics | PO PO PO PO PO PO PO | Active CD Inactive CD UC CD CD CD UC | Ph-II Ph-II Ph-II Ph-II Ph-II Ph-III Ph-II |
TNF-α inhibitors
| Anti-TNF-α ab Anti-TNF-α ab CD40 antagonist | SC SC, IV SC, IV | Ped UC Mod-to-severe UC Mod-to-severe UC | Ph-III Ph-IIA Ph-II |
CAM inhibitors
| α4ß7 and αEß7 α4 integrin receptor Anti-MADCAM1 ab | SC PO SC | CD/UC Active UC Mod-to-severe UC, CD | Ph-I/II Ph-III Ph-Ib |
IL-12/IL-23 inhibitors
| IL-23 antagonist Anti-IL-23 (p19) ab Anti-IL-23 (p19) ab Anti-IL-23 (p19) ab Anti-IL-23p (p19) ab | PO SC SC IV, SC SC | Mod-to-severe UC Mod-to-severe UC, CD Mod-to-severe UC, CD Mod-to-severe CD CD | Ph-II Ph-III Ph-II Ph-II Ph-II |
IL-22 inhibitors
| IL-22 inhibitor | IV | CD/UC | Ph-II |
IL-36 inhibitors
| Anti-IL-36R ab | IV | Mod-to-severe UC, CD | Ph-II/III |
IL-6 inhibitors
| Anti-IL-6 ab | SC | Mod-to-severe CD | Ph-II |
JAK/TYK inhibitors
| JAK-3 inhibitor JAK-1/TYK2 inhibitor JAK-1 inhibitor TYK-2 JAK-1 inhibitor JAK-1 inhibitor JAK-1 inhibitor JAK inhibitor | PO PO PO PO PO PO PO PO | Mod-to-severe UC, CD Mod-to-severe UC, CD Mod-to-severe UC, CD Mod-to-severe UC, CD Mod-to-severe CD Mod-to-severe UC Mod-to-severe UC, CD Mod-to-severe UC, CD | Ph-II Ph-II Ph-II/III Ph-II Ph-II Ph-II Ph-II Ph-II/III |
Stem-cell therapies
| Immune modulation | * IV | CD | Ph-III |
S1P inhibitors
| S1P receptor modulator S1P-1/5 receptor modulator | PO PO | Mod-to-severe CD, UC Mod-to-severe CD, UC | Ph-III Ph-III |
Antisense nucleotides
| Immune modulation | PO | CD | Ph-III |
| IMU-838 | Inhibit DHODH | PO | Mod-to-severe UC | Ph-II |
NKG
| Anti-NKG2D antibody | SC | Mod-to-severe CD | Ph-II |
FMT
| Probiotics (microbiome) | PO | Mild-to-moderate UC | Ph-1b |
| Biomarker | Anti-TNF Therapy: CD Patients | Anti-TNF Therapy: UC Patients | ||
|---|---|---|---|---|
| Expression in Responder | Expression in Mucosal Healing | Expression in Responder | Expression in Mucosal Healing | |
Mucosal transcripts
| ↓ | ↓ | ↓ | ↓ |
| ↓ | ↓ | ↓ | ↓ |
| - | - | ↓ | ↓ |
| ↓ | - | ↓ | - |
| ↓ | - | ↓ | - |
| ↓ | - | ↓ | - |
| Proteomics | ↓ | - | - | - |
| Genomic | ↓ | - | ↓ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int. J. Mol. Sci. 2022, 23, 6966. https://doi.org/10.3390/ijms23136966
Elhag DA, Kumar M, Saadaoui M, Akobeng AK, Al-Mudahka F, Elawad M, Al Khodor S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. International Journal of Molecular Sciences. 2022; 23(13):6966. https://doi.org/10.3390/ijms23136966
Chicago/Turabian StyleElhag, Duaa Ahmed, Manoj Kumar, Marwa Saadaoui, Anthony K. Akobeng, Fatma Al-Mudahka, Mamoun Elawad, and Souhaila Al Khodor. 2022. "Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response" International Journal of Molecular Sciences 23, no. 13: 6966. https://doi.org/10.3390/ijms23136966
APA StyleElhag, D. A., Kumar, M., Saadaoui, M., Akobeng, A. K., Al-Mudahka, F., Elawad, M., & Al Khodor, S. (2022). Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. International Journal of Molecular Sciences, 23(13), 6966. https://doi.org/10.3390/ijms23136966






