Friction in Myocardial Anoxia Leads to Negative Excess Entropy Production, Self-Organization, and Dissipative Structures
Abstract
:1. Introduction
2. Results
2.1. Mechanical Properties of Left Ventricular Papillary Muscles (LVPMs) and Molecular Myosin CB Characteristics
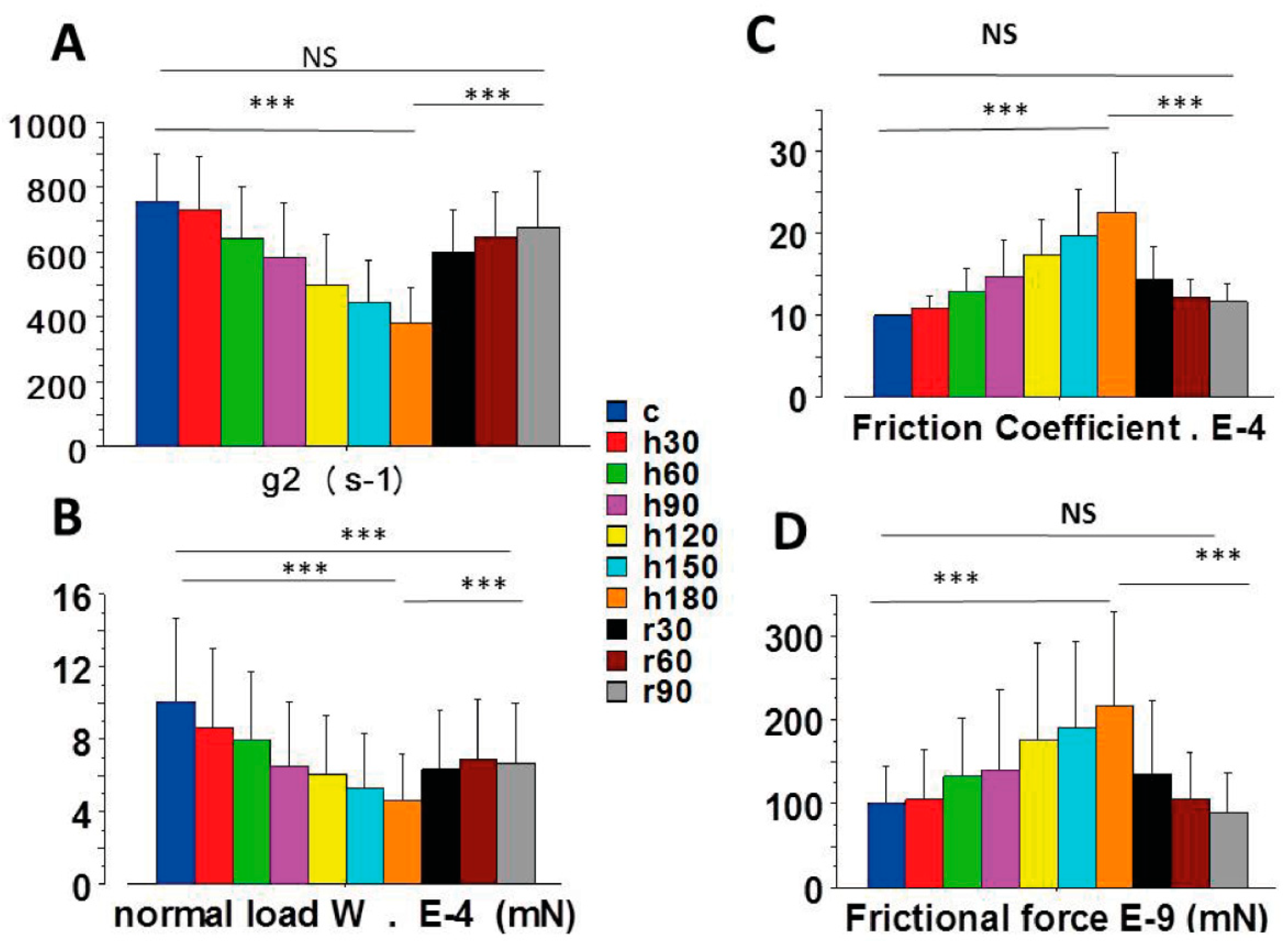
2.2. Coefficient of Friction, Frictional Force and Normal Load
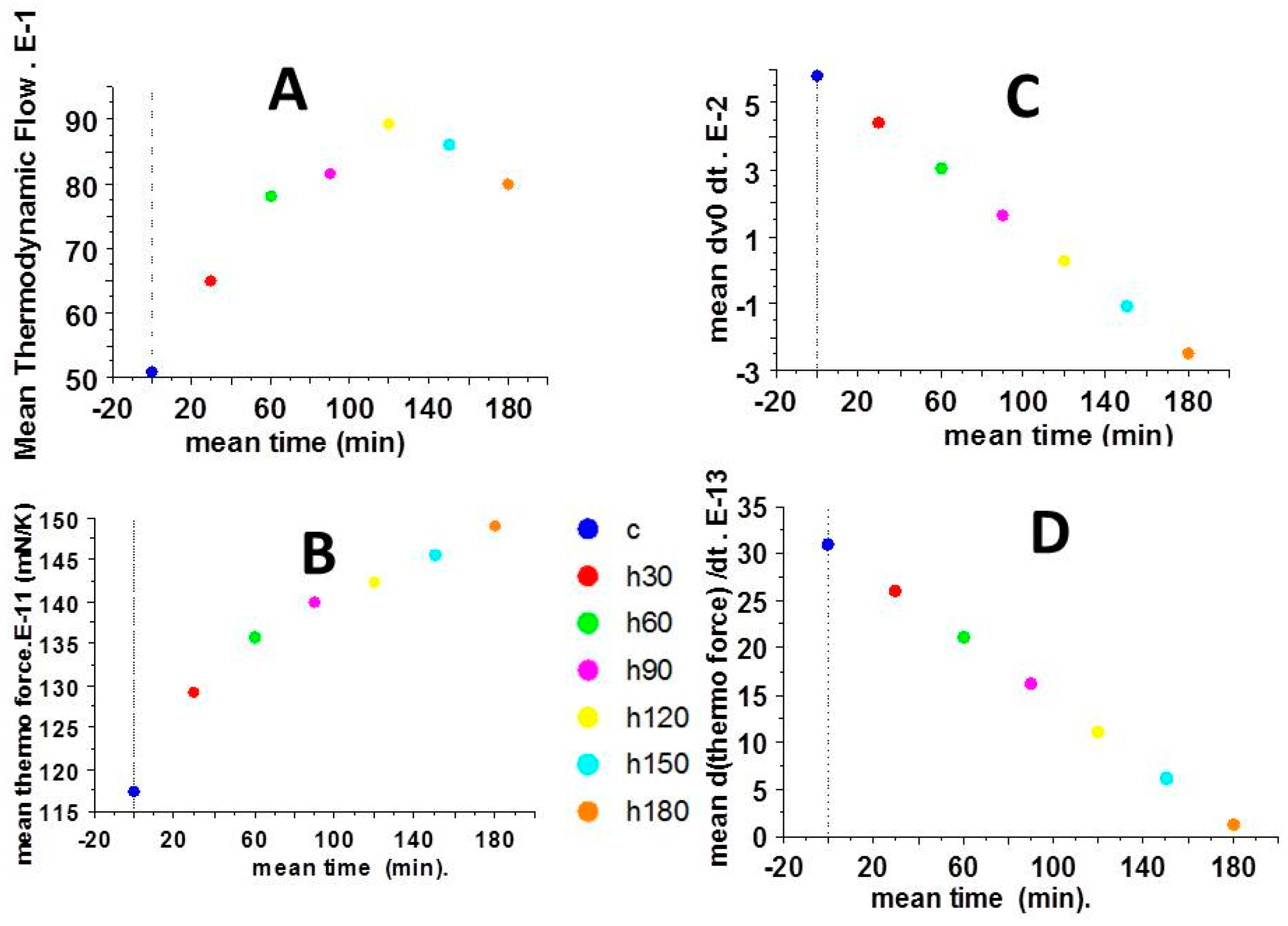
2.3. Thermodynamic Force and Thermodynamic Flow
2.4. Partial Time Derivatives of Thermodynamic Force and Thermodynamic Flow
2.5. Entropy Production Rate (EPR) and Excess Entropy Production (EEP)
2.6. Self-Organization and Dissipative Structures
2.7. Re-Oxygenation
3. Discussion
4. Methods
4.1. Ethical Statement
4.2. General Study Approach
4.3. Experimental Procedure
4.4. A. Huxley’s Formalism
4.5. Tribology and Heart Muscle
4.6. Stability Conditions
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huxley, A.F.; Niedergerke, R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 1954, 173, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.; Hanson, J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 1954, 173, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F. Muscle structure and theories of contraction. Prog. Biophys Biophys Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef]
- Nicolis, G.; Prigogine, I. Fluctuations in Nonequilibrium Systems. Proc. Natl. Acad. Sci. USA 1971, 68, 2102–2107. [Google Scholar] [CrossRef] [Green Version]
- Nicolis, G.; Auchmuty, J.F. Dissipative Structures, Catastrophes, and Pattern Formation: A Bifurcation Analysis. Proc. Natl. Acad. Sci. USA 1974, 71, 2748–2751. [Google Scholar] [CrossRef] [Green Version]
- Nicolis, G. Dissipative structures and biological order. Adv. Biol. Med. Phys. 1977, 16, 99–113. [Google Scholar]
- Nicolis, G.; Basios, V.; Nicolis, C. Pattern formation and fluctuation-induced transitions in protein crystallization. J. Chem. Phys. 2004, 120, 7708–7719. [Google Scholar] [CrossRef]
- Prigogine, I.; Nicolis, G.; Babloyantz, A. Nonequilibrium problems in biological phenomena. Ann. N. Y. Acad. Sci. 1974, 231, 99–105. [Google Scholar] [CrossRef]
- Prigogine, I.; Nicolis, G. Biological order, structure and instabilities. Q. Rev. Biophys 1971, 4, 107–148. [Google Scholar] [CrossRef] [Green Version]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics from Heat Engines to Dissipative Structures; Wiley & Sons: New York, NY, USA, 1999; pp. 1–486. [Google Scholar]
- Klamecky, B.E. A thermodynamic model of friction. Wear 1980, 63, 113–120. [Google Scholar] [CrossRef]
- Bershadsky, L. On self-organisation and concept of tribosystem self organizing. J. Fric. Wear 1992, 13, 101–114. [Google Scholar]
- Nosonovsky, M. Self-organization at the frictional interface for green tribology. Philos. Transactions. Ser. A Math. Phys. Eng. Sci. 2010, 368, 4755–4774. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Roy, P. Scaling in Colloidal and Biological Networks. Entropy 2020, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Bhushan, B. Green tribology: Principles, research areas and challenges. Philos. Transactions. Ser. A Math. Phys. Eng. Sci. 2010, 368, 4677–4694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosonovsky, M.; Bhushan, B. Thermodynamics of surface degradation, self-organization and self-healing for biomimetic surfaces. Philos. Transactions. Ser. A Math. Phys. Eng. Sci. 2009, 367, 1607–1627. [Google Scholar] [CrossRef]
- Naderi, M. On the evidence of thermodynamic self-organization during fatigue: A review. Entropy J. 2020, 22, 372. [Google Scholar] [CrossRef] [Green Version]
- Gershman, I.S.; Bushe, N.A. Elements of thermodynamics and self-organization during friction. In Self-Organization during Friction. Advanced Surface-Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G.E., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Fox-Rabinovich, G.; Gershman, I.S.; Yamamoto, K.; Biksa, A.; Veldhui, S.C.; Beak, B.D.; Kovalev, A.I. Self-Organization during Friction in Complex Surface Engineered Tribosystems. Entropy 2010, 12, 275–288. [Google Scholar] [CrossRef]
- Bakaeen, F.G.; Gaudino, M.; Whitman, G.; Doenst, T.; Ruel, M.; Taggart, D.P.; Stulak, J.M.; Benedetto, U.; Anyanwu, A.; Chikwe, J.; et al. 2021: The American Association for Thoracic Surgery Expert Consensus Document: Coronary artery bypass grafting in patients with ischemic cardiomyopathy and heart failure. J. Thorac. Cardiovasc. Surg. 2021, 162, 829–850.e1. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 197–215. [Google Scholar] [CrossRef]
- Stehlik, J.; Kobashigawa, J.; Hunt, S.A.; Reichenspurner, H.; Kirklin, J.K. Honoring 50 Years of Clinical Heart Transplantation in Circulation: In-Depth State-of-the-Art Review. Circulation 2018, 137, 71–87. [Google Scholar] [CrossRef]
- Kostetsky, B. Structural -energetic adaptation of materials at friction. J. Fric Wear 1985, 6, 201. [Google Scholar]
- Fox-Rabinovich, G.; Totten, G.E. Self-organization during friction: Advanced surface-engineered materials and systems design. In Endotext; CRC Press: BocaRaton, FL, USA, 2006. [Google Scholar]
- Mortazavi, V.; Nosonovsky, M. Friction-induced pattern formation and Turing systems. Langmuir ACS J. Surf. Colloids 2011, 27, 4772–4779. [Google Scholar] [CrossRef]
- Glansdorff, P.; Prigogine, I. Thermodynamics of Structure Stability and Fluctuations; Wiley: New York, NY, USA, 1971. [Google Scholar]
- Nicolis, G.; Prigogine, I. Self-Organization in Non-Equilibrium Systems: From Dissipative Structures to Order through Fluctuations; John & Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Palatnik, L.S. Pores in the Films; Energoizdat: Moscow, Russia, 1982. [Google Scholar]
- Davies, P.C.; Rieper, E.; Tuszynski, J.A. Self-organization and entropy reduction in a living cell. Bio. Syst. 2013, 111, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, N.S.; Leith, A.G. Self-sorting of anisotropic cells. J. Theor. Biol. 1970, 28, 469–482. [Google Scholar] [CrossRef]
- Gordon, R.; Goel, N.S.; Steinberg, M.S.; Wiseman, L.L. A rheological mechanism sufficient to explain the kinetics of cell sorting. J. Theor. Biol. 1972, 37, 43–73. [Google Scholar] [CrossRef]
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell. In StemBook; Cambridge University Press: Cambridge, UK, 1967. [Google Scholar]
- Lecarpentier, Y.; Chemla, D.; Blanc, F.X.; Pourny, J.C.; Joseph, T.; Riou, B.; Coirault, C. Mechanics, energetics, and crossbridge kinetics of rabbit diaphragm during congestive heart failure. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 981–989. [Google Scholar] [CrossRef]
- Brutsaert, D.L.; Sonnenblick, E.H. Force-velocity-length-time relations of the contractile elements in heart muscle of the cat. Circ. Res. 1969, 24, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Biol. Sci. 1938, 126, 136–195. [Google Scholar]
- Woledge, R.C.; Curtin, A.N.; Homsher, E. Energetic Aspects of Muscle Contraction; Academic Press: London, UK, 1985; Volume 41, pp. 1–357. [Google Scholar]
- Veech, R.L.; Lawson, J.W.; Cornell, N.W.; Krebs, H.A. Cytosolic phosphorylation potential. J. Biol. Chem. 1979, 254, 6538–6547. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Claes, V.; Krokidis, X.; Vallée, A.A. Comparative Statistical Mechanics of Muscle and Non-Muscle Contractile Systems: Stationary States of Near-Equilibrium Systems in A Linear Regime. Entropy J. 2017, 19, 558. [Google Scholar] [CrossRef] [Green Version]
- Dowson, D. Bio-tribology. Faraday Discuss. 2012, 156, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wei, L.; Cai, W.; Lin, Z.; Wu, Z.; Peng, Y.; Kohmoto, T.; Moss, R.L.; Ge, Y. Complete Characterization of Cardiac Myosin Heavy Chain (223 kDa) Enabled by Size-Exclusion Chromatography and Middle-Down Mass Spectrometry. Anal. Chem. 2017, 89, 4922–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Dowson, D. Bio-friction. Friction 2013, 1, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Suda, H. Molecular friction in an actomyosin molecular machine. J. Theor. Biol. 1990, 146, 341–346. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecarpentier, Y.; Claes, V.; Hébert, J.-L.; Krokidis, X.; Schussler, O.; Vallée, A. Friction in Myocardial Anoxia Leads to Negative Excess Entropy Production, Self-Organization, and Dissipative Structures. Int. J. Mol. Sci. 2022, 23, 6967. https://doi.org/10.3390/ijms23136967
Lecarpentier Y, Claes V, Hébert J-L, Krokidis X, Schussler O, Vallée A. Friction in Myocardial Anoxia Leads to Negative Excess Entropy Production, Self-Organization, and Dissipative Structures. International Journal of Molecular Sciences. 2022; 23(13):6967. https://doi.org/10.3390/ijms23136967
Chicago/Turabian StyleLecarpentier, Yves, Victor Claes, Jean-Louis Hébert, Xénophon Krokidis, Olivier Schussler, and Alexandre Vallée. 2022. "Friction in Myocardial Anoxia Leads to Negative Excess Entropy Production, Self-Organization, and Dissipative Structures" International Journal of Molecular Sciences 23, no. 13: 6967. https://doi.org/10.3390/ijms23136967
APA StyleLecarpentier, Y., Claes, V., Hébert, J.-L., Krokidis, X., Schussler, O., & Vallée, A. (2022). Friction in Myocardial Anoxia Leads to Negative Excess Entropy Production, Self-Organization, and Dissipative Structures. International Journal of Molecular Sciences, 23(13), 6967. https://doi.org/10.3390/ijms23136967





