Molecular Allergen-Specific IgE Recognition Profiles and Cumulative Specific IgE Levels Associated with Phenotypes of Cat Allergy
Abstract
:1. Introduction
2. Results
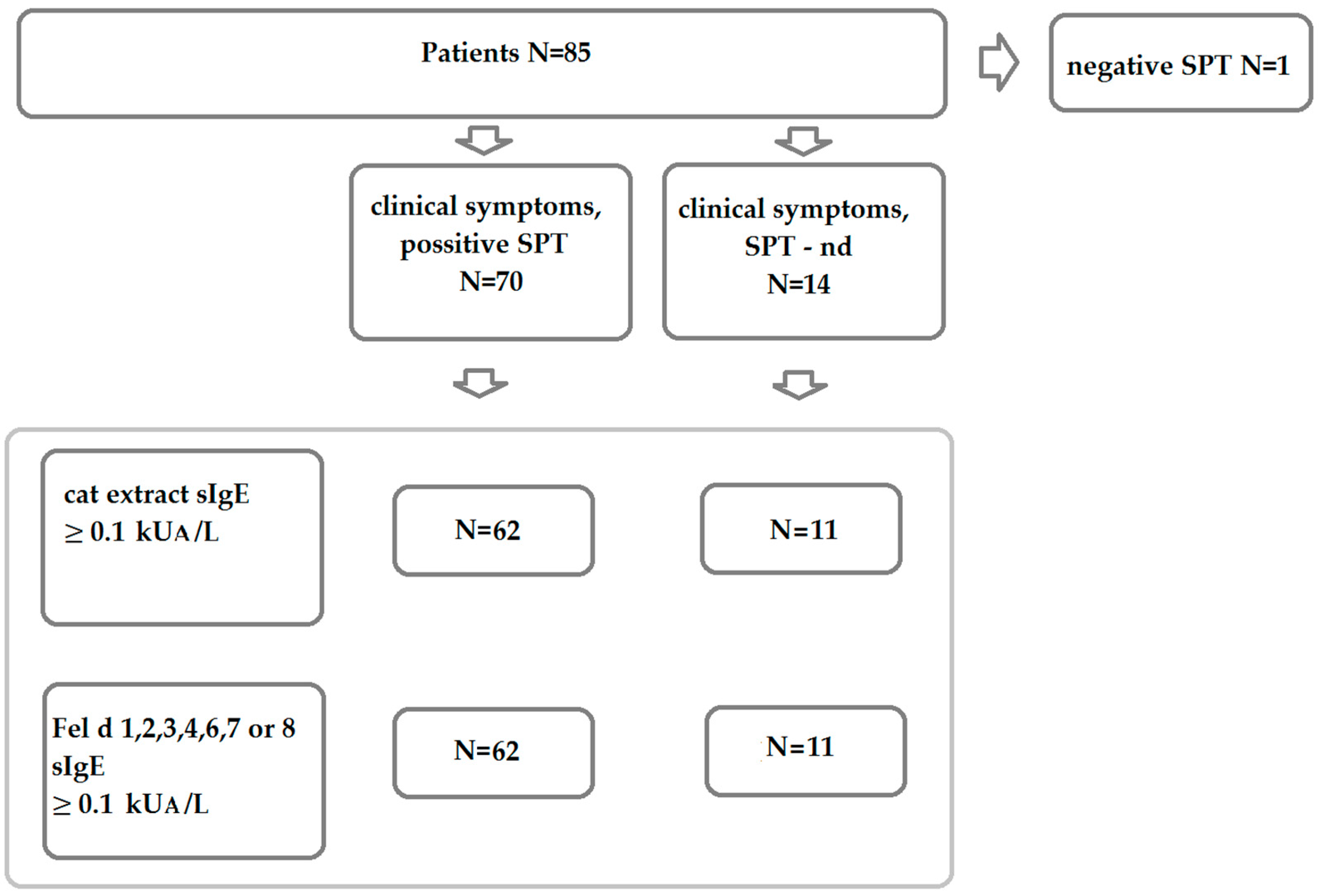
2.1. Characterization of Cat Allergic Patients
2.2. rFel d 1 Is the Major Cat Allergen but Cat Allergic Patients Are Also Sensitized to Several Other Cat Allergens
2.3. The Cumulative Sum of IgE Levels Specific to Allergen Molecules Is Correlated with Cat Allergen Extract-Specific IgE Levels
2.4. Association of Symptoms and Phenotypes of Cat Allergy with Molecular IgE Recognition Profiles and Cumulative Allergen-Specific IgE Levels
3. Discussion
4. Materials and Methods
4.1. Characterization of Cat-Allergic Patients
4.2. Expression and Purification of Allergens
4.3. Quantification of Allergen-Specific IgE Levels
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perzanowski, M.S.; Rönmark, E.; Platts-Mills, T.A.E.; Lundbäck, B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am. J. Respir. Crit. Care Med. 2002, 166, 696–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiou, A.; Holmdahl, I.; Asarnoj, A.; van Hage, M.; Ekenkrantz, T.; Rydell, N.; Sjölander, A.; Stenberg-Hammar, K.; Hedlin, G.; Konradsen, J.R.; et al. Development of sensitization to multiple allergen molecules from preschool to school age is related to asthma. Int. Arch. Allergy Immunol. 2022, 183, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Hoffmann, H.J.; Renz, H.; Demoly, P.; Werfel, T.; Matricardi, P.M.; Muraro, A.; Schmid-Grendelmeier, P.; Cardona, V.; Papadopoulos, N.G. Diagnostic test allergens used for in vivo diagnosis of allergic diseases are at risk: A European perspective. Allergy 2015, 70, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Valenta, R.; Puerta, L.; Pomés, A.; Zakzuk, J.; Fernandez-Caldas, E.; Acevedo, N.; Sanchez-Borges, M.; Ansotegui, I.; Zhang, L.; et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ. J. 2020, 13, 100118. [Google Scholar] [CrossRef]
- Konradsen, J.R.; Fujisawa, T.; Van Hage, M.; Hedlin, G.; Hilger, C.; Org Kleine-Tebbe, J.; Matsui, E.C.; Roberts, G.; Rönmark, E.; Platts-Mills, T.A.E. Allergy to furry animals: New insights, diagnostic approaches, and challenges. J. Allergy Clin. Immunol. 2015, 135, 616–625. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Suzuki, S.; Ekerljung, L.; Sjölander, S.; Mincheva, R.; Rönmark, E.P.; Rådinger, M.; Rönmark, E.; Borres, M.P.; Lundbäck, B.; et al. Furry animal allergen component sensitization and clinical outcomes in adult asthma and rhinitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 1230–1238.e4. [Google Scholar] [CrossRef]
- Suzuki, S.; Nwaru, B.I.; Ekerljung, L.; Sjölander, S.; Mincheva, R.; Rönmark, E.P.; Rönmark, E.; Lundbäck, B.; Borres, M.P.; Lötvall, J. Characterization of sensitization to furry animal allergen components in an adult population. Clin. Exp. Allergy 2019, 49, 495–505. [Google Scholar] [CrossRef]
- Siroux, V.; Lupinek, C.; Resch, Y.; Curin, M.; Just, J.; Keil, T.; Kiss, R.; Lødrup Carlsen, K.; Melén, E.; Nadif, R.; et al. Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: The EGEA study. J. Allergy Clin. Immunol. 2017, 139, 643–654.e6. [Google Scholar] [CrossRef] [Green Version]
- Siroux, V.; Boudier, A.; Nadif, R.; Lupinek, C.; Valenta, R.; Bousquet, J. Association between asthma, rhinitis, and conjunctivitis multimorbidities with molecular IgE sensitization in adults. Allergy 2019, 74, 824–827. [Google Scholar] [CrossRef]
- Grönlund, H.; Adédoyin, J.; Commins, S.P.; Platts-Mills, T.A.E.; van Hage, M. The carbohydrate galactose-α-1,3-galactose is a major IgE-binding epitope on cat IgA. J. Allergy Clin. Immunol. 2009, 123, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Arkestl, K.; Sibanda, E.; Thors, C.; Troye-Blomberg, M.; Mduluza, T.; Valenta, R.; Grönlund, H.; Van Hage, M. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-α 1,3-galactose. J. Allergy Clin. Immunol. 2011, 127, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Karsonova, A.V.; Riabova, K.A.; Khaitov, M.R.; Elisyutina, O.G.; Ilina, N.; Fedenko, E.S.; Fomina, D.S.; Beltyukov, E.; Bondarenko, N.L.; Evsegneeva, I.V.; et al. Milk-specific IgE reactivity without symptoms in albumin-sensitized cat allergic patients. Allergy Asthma Immunol. Res. 2021, 13, 668–670. [Google Scholar] [CrossRef] [PubMed]
- WHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 1 Allergen Details. 2003. Available online: http://allergen.org/viewallergen.php?aid=319 (accessed on 4 May 2022).
- WHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 2 Allergen Details. 2003. Available online: http://allergen.org/viewallergen.php?aid=320 (accessed on 4 May 2022).
- WHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 3 Allergen Details. Available online: http://allergen.org/viewallergen.php?aid=321 (accessed on 4 May 2022).
- WHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 4 Allergen Details. 2006. Available online: http://allergen.org/viewallergen.php?aid=322 (accessed on 4 May 2022).
- WWHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 5 Allergen Details. 2006. Available online: http://allergen.org/viewallergen.php?aid=323 (accessed on 4 May 2022).
- WHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 7 Allergen Details. Available online: http://www.allergen.org/viewallergen.php?aid=665 (accessed on 4 May 2022).
- WHO/IUIS Allergen Nomenclature Sub-Committee. Fel d 8 Allergen Details. 2010. Available online: http://www.allergen.org/viewallergen.php?aid=702 (accessed on 4 May 2022).
- Morgenstern, J.P.; Griffith, I.J.; Brauer, A.W.; Rogers, B.L.; Bond, J.F.; Chapman, M.D.; Kuo, M.C. Amino acid sequence of Fel dI, the major allergen of the domestic cat: Protein sequence analysis and cDNA cloning. Proc. Natl. Acad. Sci. USA 1991, 88, 9690–9694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reininger, R.; Swoboda, I.; Bohle, B.; Hauswirth, A.W.; Valent, P.; Rumpold, H.; Valenta, R.; Spitzauer, S. Characterization of recombinant cat albumin. Clin. Exp. Allergy 2003, 33, 1695–1702. [Google Scholar] [CrossRef]
- Ichikawa, K.; Vailes, L.D.; Pomés, A.; Chapman, M.D. Molecular cloning, expression and modelling of cat allergen, cystatin (Fel d 3), a cysteine protease inhibitor. Clin. Exp. Allergy 2001, 31, 1279–1286. [Google Scholar] [CrossRef]
- Smith, W.A.; Butler, A.J.L.; Hazell, L.A.; Chapman, M.D.; Pomés, A.; Nickels, D.G.; Thomas, W.R. Fel d 4, a cat lipocalin allergen. Clin. Exp. Allergy 2004, 34, 1732–1738. [Google Scholar] [CrossRef]
- Smith, W.; O’Neil, S.E.; Hales, B.J.; Chai, T.L.Y.; Hazell, L.A.; Tanyaratsrisakul, S.; Piboonpocanum, S.; Thomas, W.R. Two newly identified cat allergens: The von Ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int. Arch. Allergy Immunol. 2011, 156, 159–170. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Apostolovic, D.; Starkhammar, M.; Grundström, J.; Hamsten, C.; van Hage, M. Clinical and serological characterization of the α-gal syndrome—Importance of atopy for symptom severity in a european cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 2027–2034.e2. [Google Scholar] [CrossRef]
- Kaiser, L.; Grönlund, H.; Sandalova, T.; Ljunggren, H.G.; Van Hage-Hamsten, M.; Achour, A.; Schneider, G. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J. Biol. Chem. 2003, 278, 37730–37735. [Google Scholar] [CrossRef] [Green Version]
- Grönlund, H.; Adédoyin, J.; Reininger, R.; Varga, E.M.; Zach, M.; Fredriksson, M.; Kronqvist, M.; Szepfalusi, Z.; Spitzauer, S.; Grönneberg, R.; et al. Higher immunoglobulin E antibody levels to recombinant Fel d 1 in cat-allergic children with asthma compared with rhinoconjunctivitis. Clin. Exp. Allergy 2008, 38, 1275–1281. [Google Scholar] [CrossRef]
- Curin, M.; Khaitov, M.; Karaulov, A.; Namazova-Baranova, L.; Campana, R.; Garib, V.; Valenta, R. Next-generation of allergen-specific immunotherapies: Molecular approaches. Curr. Allergy Asthma Rep. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.S. Therapeutic potential of peptides in allergic disease. In Ann. Allergy; 1993; 71, pp. 330–333. Available online: https://pubmed.ncbi.nlm.nih.gov/8373007/ (accessed on 3 May 2022). [PubMed]
- Worm, M.; Lee, H.H.; Kleine-Tebbe, J.; Hafner, R.P.; Laidler, P.; Healey, D.; Buhot, C.; Verhoef, A.; Maillre, B.; Kay, A.B.; et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J. Allergy Clin. Immunol. 2011, 127, 89–997. [Google Scholar] [CrossRef] [PubMed]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef]
- Shamji, M.H.; Singh, I.; Layhadi, J.A.; Ito, C.; Karamani, A.; Kouser, L.; Sharif, H.; Tang, J.; Handijiev, S.; Parkin, R.V.; et al. Passive prophylactic administration with a single dose of anti-Fel d 1 monoclonal antibodies REGN1908-1909 in cat allergen-induced allergic rhinitis: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2021, 204, 23–33. [Google Scholar] [CrossRef]
- Asarnoj, A.; Hamsten, C.; Wadén, K.; Lupinek, C.; Andersson, N.; Kull, I.; Curin, M.; Anto, J.; Bousquet, J.; Valenta, R.; et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: A BAMSE/MeDALL study. J. Allergy Clin. Immunol. 2016, 137, 813–821.e7. [Google Scholar] [CrossRef]
- Caraballo, L.; Valenta, R.; Acevedo, N.; Zakzuk, J. Are the terms major and minor allergens useful for precision allergology? Front. Immunol. 2021, 12, 651500. [Google Scholar] [CrossRef]
- van Hage, M.; Hamsten, C.; Valenta, R. ImmunoCAP assays: Pros and cons in allergology. J. Allergy Clin. Immunol. 2017, 140, 974–977. [Google Scholar] [CrossRef] [Green Version]
- Mizuma, H.; Tanaka, A.; Uchida, Y.; Fujiwara, A.; Manabe, R.; Furukawa, H.; Kuwahara, N.; Fukuda, Y.; Kimura, T.; Jinno, M.; et al. Influence of omalizumab on allergen-specific IgE in patients with adult asthma. Int. Arch. Allergy Immunol. 2015, 168, 165–172. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Bousquet, J.; Heinzerling, L.; Bachert, C.; Papadopoulos, N.G.; Bousquet, P.J.; Burney, P.G.; Canonica, G.W.; Carlsen, K.H.; Cox, L.; Haahtela, T.; et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 2012, 67, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI molecular allergology user’s guide. Pediatr. Allergy Immunol. 2016, 27, 1–250. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Initiative For Asthma. A GINA Pocket Guide for Health Professionals Difficult-to-Treat & Severe Asthma in Adolescent and Adult Patients Diagnosis and Management; Global Initiative for Asthma Bethesda: Fontana, WI, USA, 2018. [Google Scholar]
- Global Initiative For Asthma. A GINA Pocket Guide for Health Professionals Difficult-to-Treat & Severe Asthma in Adolescent and Adult Patients Diagnosis and Management; Global Initiative for Asthma Bethesda: Fontana, WI, USA, 2019. [Google Scholar]
- Roberts, G.; Xatzipsalti, M.; Borrego, L.M.; Custovic, A.; Halken, S.; Hellings, P.W.; Papadopoulos, N.G.; Rotiroti, G.; Scadding, G.; Timmermans, F.; et al. Paediatric rhinitis: Position paper of the European academy of allergy and clinical immunology. Allergy 2013, 68, 1102–1116. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment, development and evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef] [Green Version]
- Chan, V.F.; Yong, A.C.; Azuara-Blanco, A.; Gordon, I.; Safi, S.; Lingham, G.; Evans, J.; Keel, S. A systematic review of clinical practice guidelines for infectious and non-infectious conjunctivitis. Ophthalmic Epidemiol. 2021, 1–10. [Google Scholar] [CrossRef]
- Wollenberg, A.; Oranje, A.; Deleuran, M.; Simon, D.; Szalai, Z.; Kunz, B.; Svensson, A.; Barbarot, S.; Von Kobyletzki, L.; Taieb, A.; et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 729–747. [Google Scholar] [CrossRef] [Green Version]
- Curin, M.; Huang, H.J.; Garmatiuk, T.; Gutfreund, S.; Resch-Marat, Y.; Chen, K.W.; Fauland, K.; Keller, W.; Zieglmayer, P.; Zieglmayer, R.; et al. IgE epitopes of the house dust mite allergen der p 7 are mainly discontinuous and conformational. Front. Immunol. 2021, 12, 1820. [Google Scholar] [CrossRef]
- Erwin, E.A.; Custis, N.J.; Satinover, S.M.; Perzanowski, M.S.; Woodfolk, J.A.; Crane, J.; Wickens, K.; Platts-Mills, T.A.E. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J. Allergy Clin. Immunol. 2005, 115, 1029–1035. [Google Scholar] [CrossRef]
- Huang, H.J.; Resch-Marat, Y.; Rodriguez-Dominguez, A.; Chen, K.W.; Kiss, R.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P.; Horak, F.; Valenta, R.; et al. Underestimation of house dust mite-specific IgE with extract-based ImmunoCAPs compared with molecular ImmunoCAPs. J. Allergy Clin. Immunol. 2018, 142, 1656–1659.e9. [Google Scholar] [CrossRef] [Green Version]
- Karsonova, A.; Riabova, K.; Villazala-Merino, S.; Campana, R.; Niederberger, V.; Eckl-Dorna, J.; Fröschl, R.; Perkmann, T.; Zhernov, Y.V.; Elisyutina, O.G.; et al. Highly sensitive ELISA-based assay for quantification of allergen-specific IgE antibody levels. Allergy 2020, 75, 2668. [Google Scholar] [CrossRef]
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119. [Google Scholar] [CrossRef] [Green Version]




| Sex | Age Mean (Min–Max) | Symptoms Upon Cat Exposure | ||||
|---|---|---|---|---|---|---|
| m | f | Asthma | Rhinitis | Conjunctivitis | Dermatitis | |
| 57 | 27 | 27.19 (18–53) | 67 | 81 | 70 | 20 |
| Patients with specific IgE antibodies > 0.1 kUA/L (N = 73) | ||||||
| 50 | 23 | 26.71 (18–52) | 59 | 70 | 61 | 19 |
| A | |||||||
|---|---|---|---|---|---|---|---|
| Cat extract kUA/L mean | rFel d 1 kUA/L mean | nFel d 2 kUA/L mean | rFel d 3 kUA/L mean | rFel d 4 kUA/L mean | nFel d 6 kUA/L mean | rFel d 7 kUA/L mean | rFel d 8 kUA/L mean |
| 27.06 (0.12–100) | 16.94 (0–100) | 2.7 (0–100) | 2.18 (0–73.8) | 5.69 (0–100) | 0.82 (0–41.3) | 4.53 (0–100) | 0.37 (0–8.14) |
| B | |||||||
| Cat extract N % | rFel d 1 N % | nFel d 2 N % | rFel d 3 N % | rFel d 4 N % | nFel d 6 N % | rFel d 7 N % | rFel d 8 N % |
| N = 73 100% | N = 71 97.2% | N = 22 30.1% | N = 36 50.6% | N = 38 52% | N = 24 32.8% | N = 40 54.7% | N = 32 42.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riabova, K.; Karsonova, A.V.; van Hage, M.; Käck, U.; Konradsen, J.R.; Grönlund, H.; Fomina, D.; Beltyukov, E.; Glazkova, P.A.; Semenov, D.Y.; et al. Molecular Allergen-Specific IgE Recognition Profiles and Cumulative Specific IgE Levels Associated with Phenotypes of Cat Allergy. Int. J. Mol. Sci. 2022, 23, 6984. https://doi.org/10.3390/ijms23136984
Riabova K, Karsonova AV, van Hage M, Käck U, Konradsen JR, Grönlund H, Fomina D, Beltyukov E, Glazkova PA, Semenov DY, et al. Molecular Allergen-Specific IgE Recognition Profiles and Cumulative Specific IgE Levels Associated with Phenotypes of Cat Allergy. International Journal of Molecular Sciences. 2022; 23(13):6984. https://doi.org/10.3390/ijms23136984
Chicago/Turabian StyleRiabova, Ksenja, Antonina V. Karsonova, Marianne van Hage, Ulrika Käck, Jon R. Konradsen, Hans Grönlund, Daria Fomina, Evgeny Beltyukov, Polina A. Glazkova, Dmitry Yu. Semenov, and et al. 2022. "Molecular Allergen-Specific IgE Recognition Profiles and Cumulative Specific IgE Levels Associated with Phenotypes of Cat Allergy" International Journal of Molecular Sciences 23, no. 13: 6984. https://doi.org/10.3390/ijms23136984






