Recombinant Cathepsin L of Tribolium castaneum and Its Potential in the Hydrolysis of Immunogenic Gliadin Peptides
Abstract
:1. Introduction
2. Results
2.1. Expression of rpTcCathL1
2.2. Autocatalytic Processing of rpTcCathL1
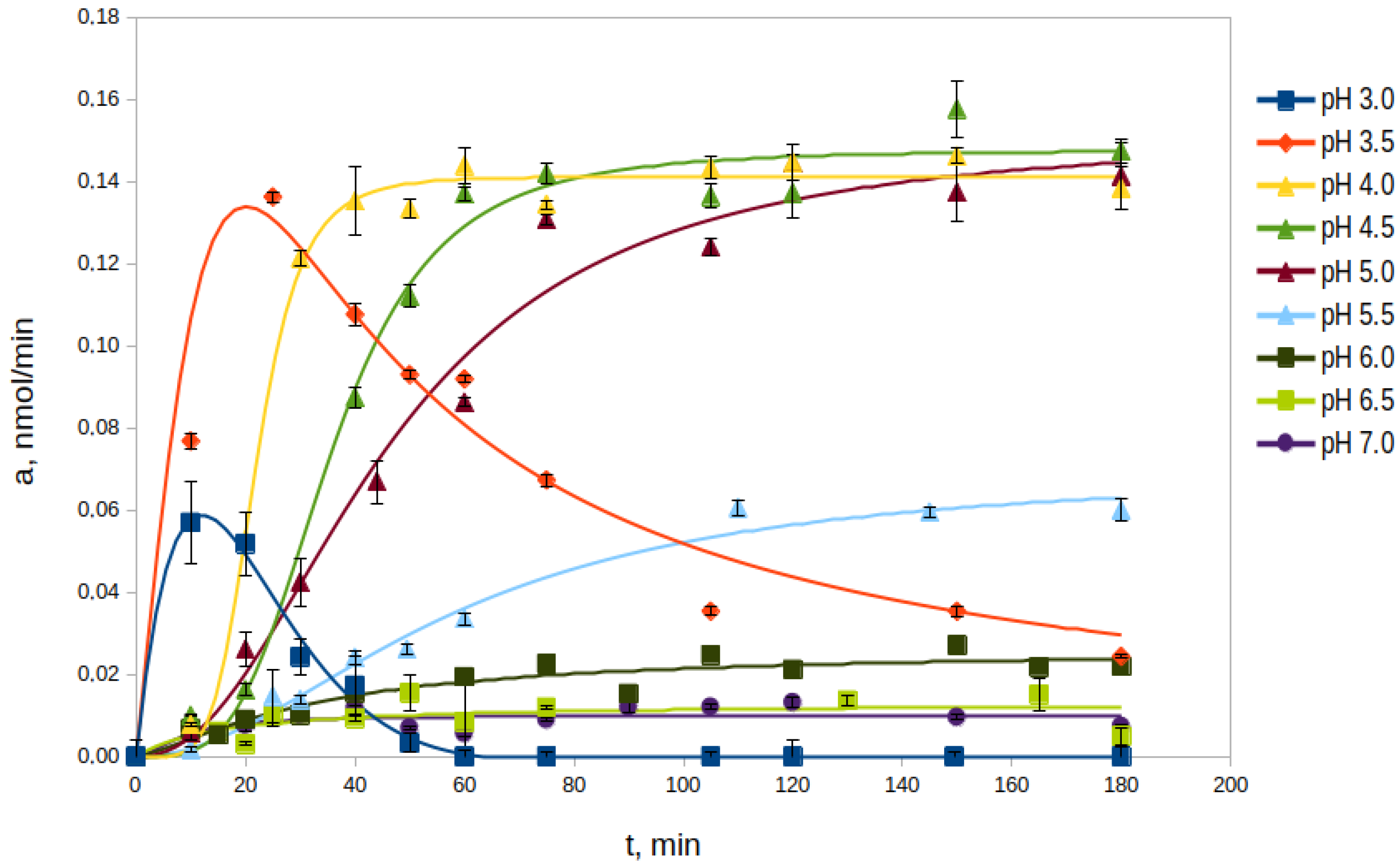
2.2.1. Effect of pH
2.2.2. Effect of Proenzyme Concentration on the Processing Rate
2.3. Electrophoretic Study of rpTcCathL1 Processing
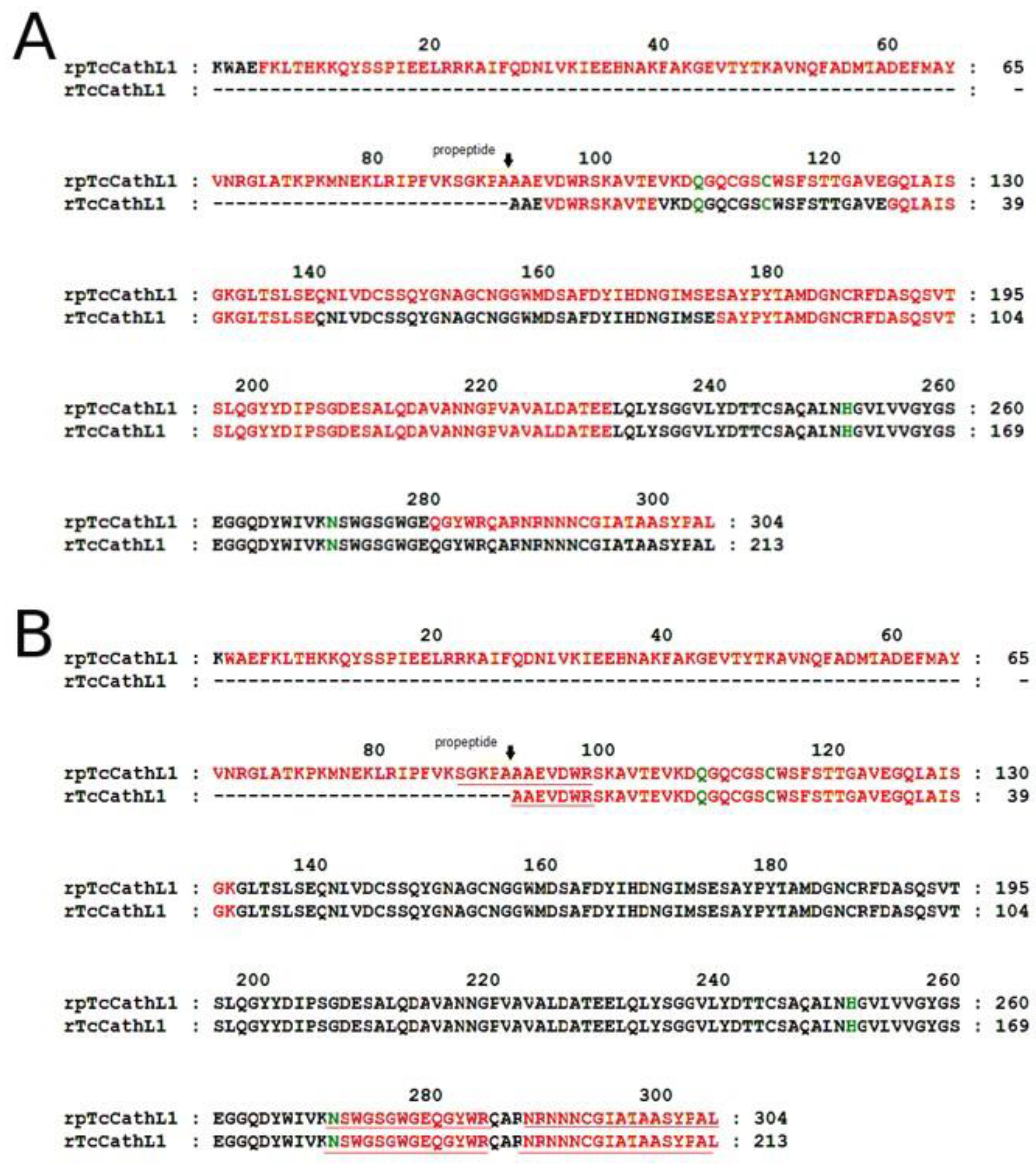
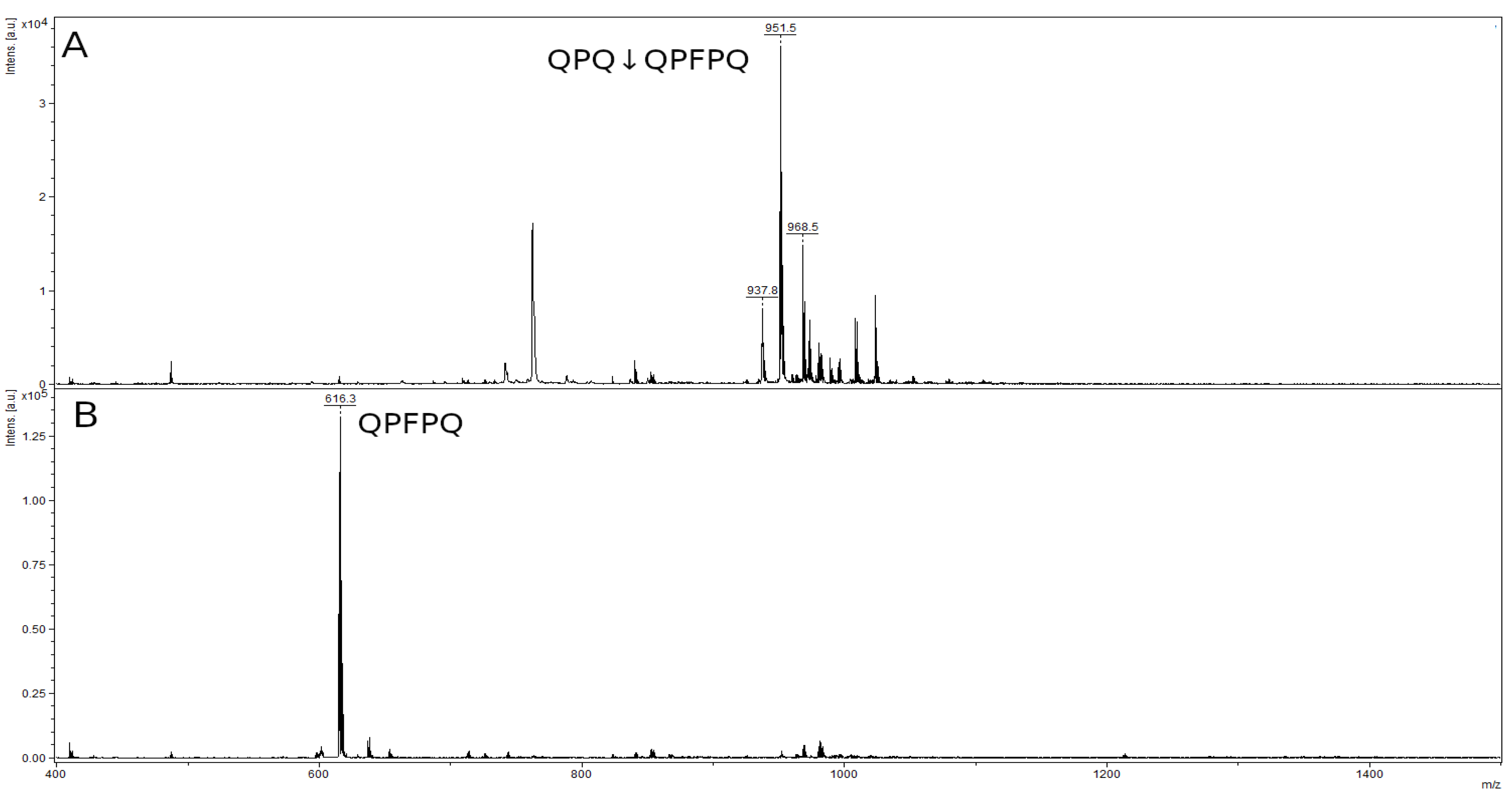
2.4. Mass-Spectrometry Analysis of rpTcCathL1 and rTcCathL1
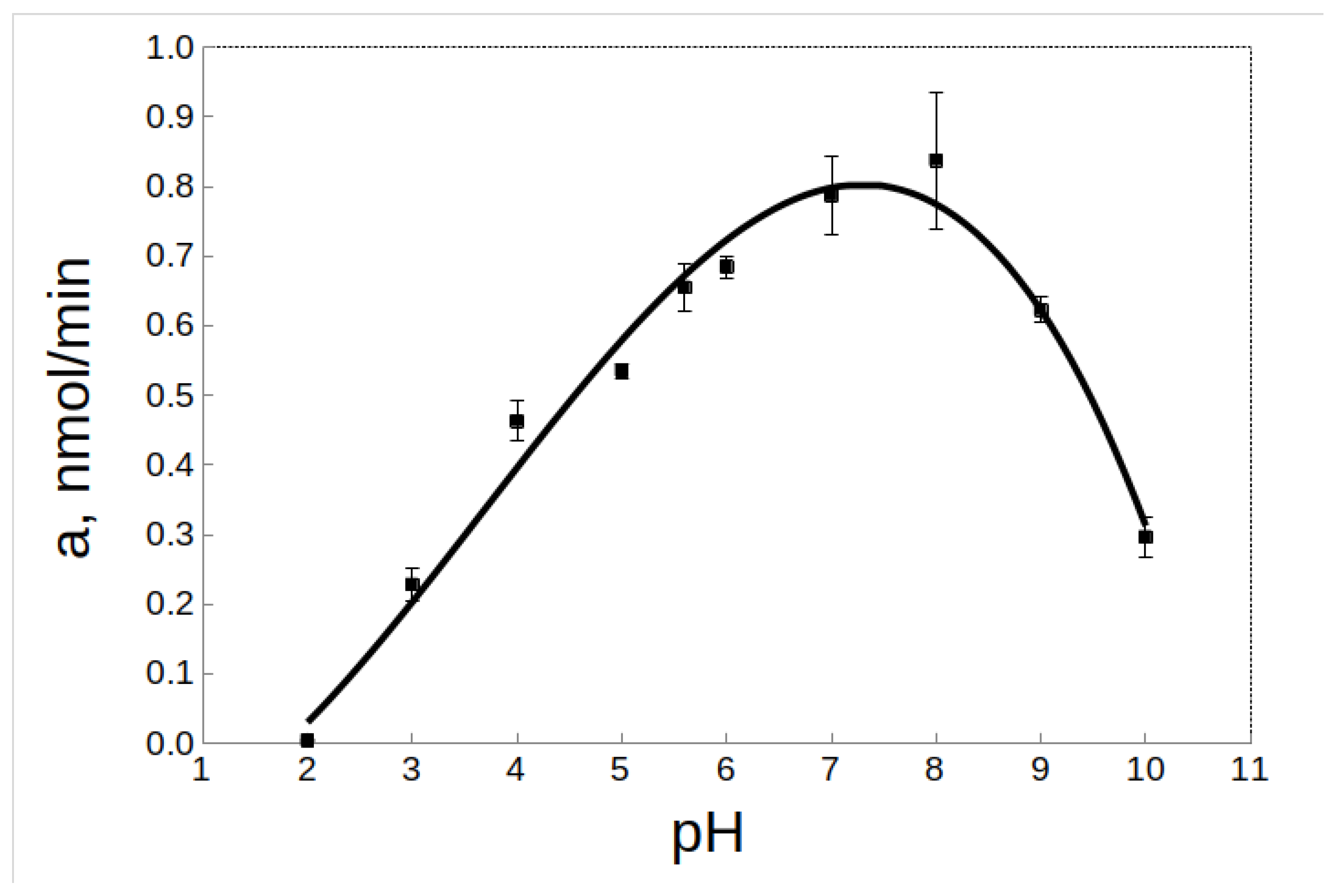
2.5. Effect of pH on the Activity and Stability of rTcCathL1
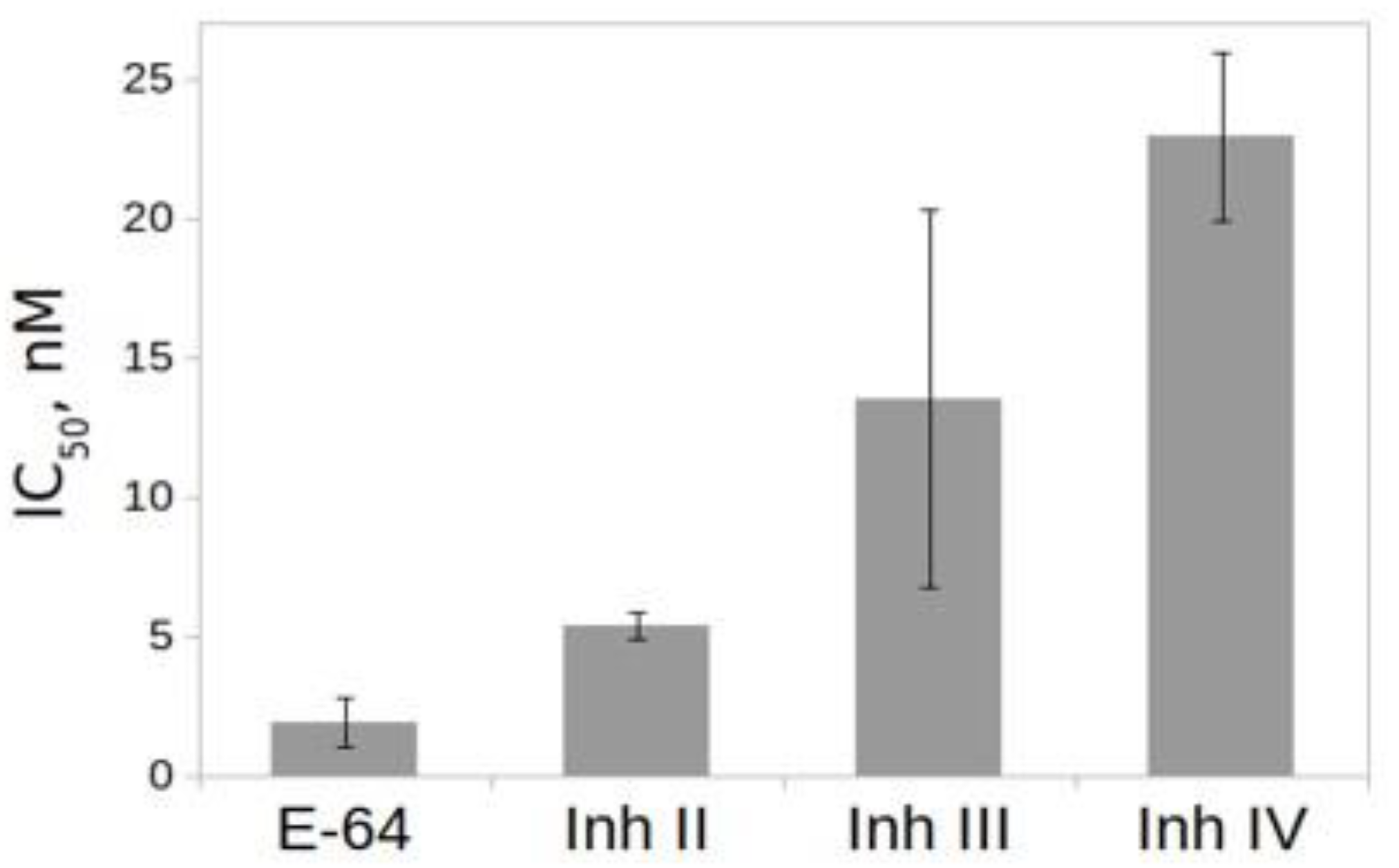
2.6. Effect of Inhibitors on the Activity of rTcCathL1
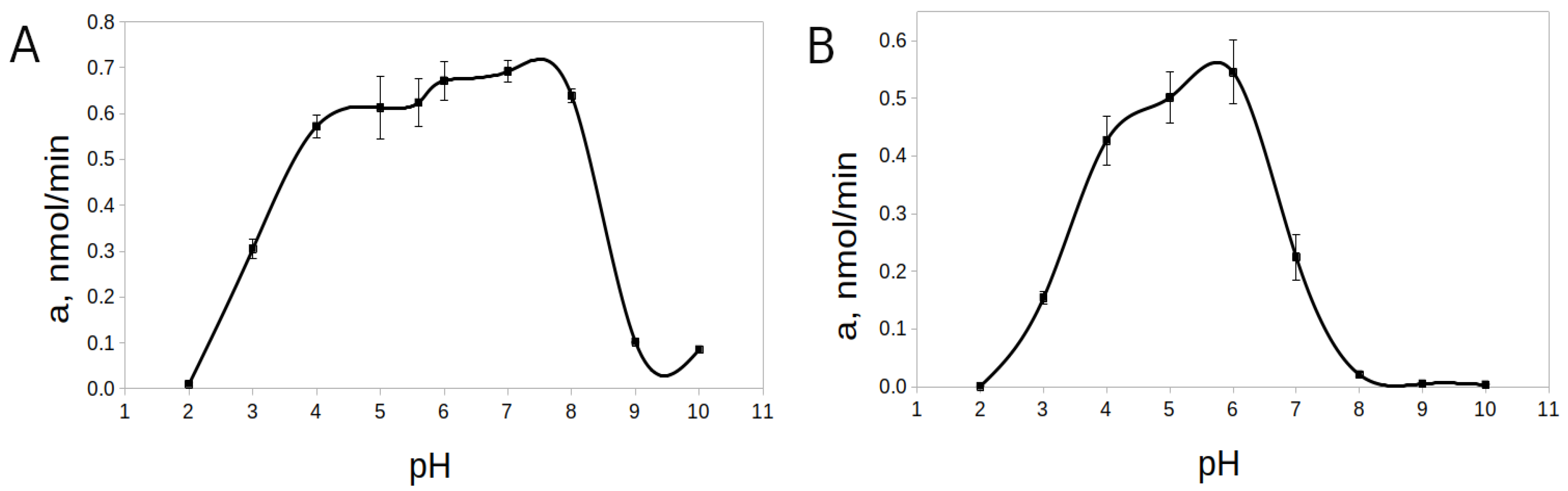
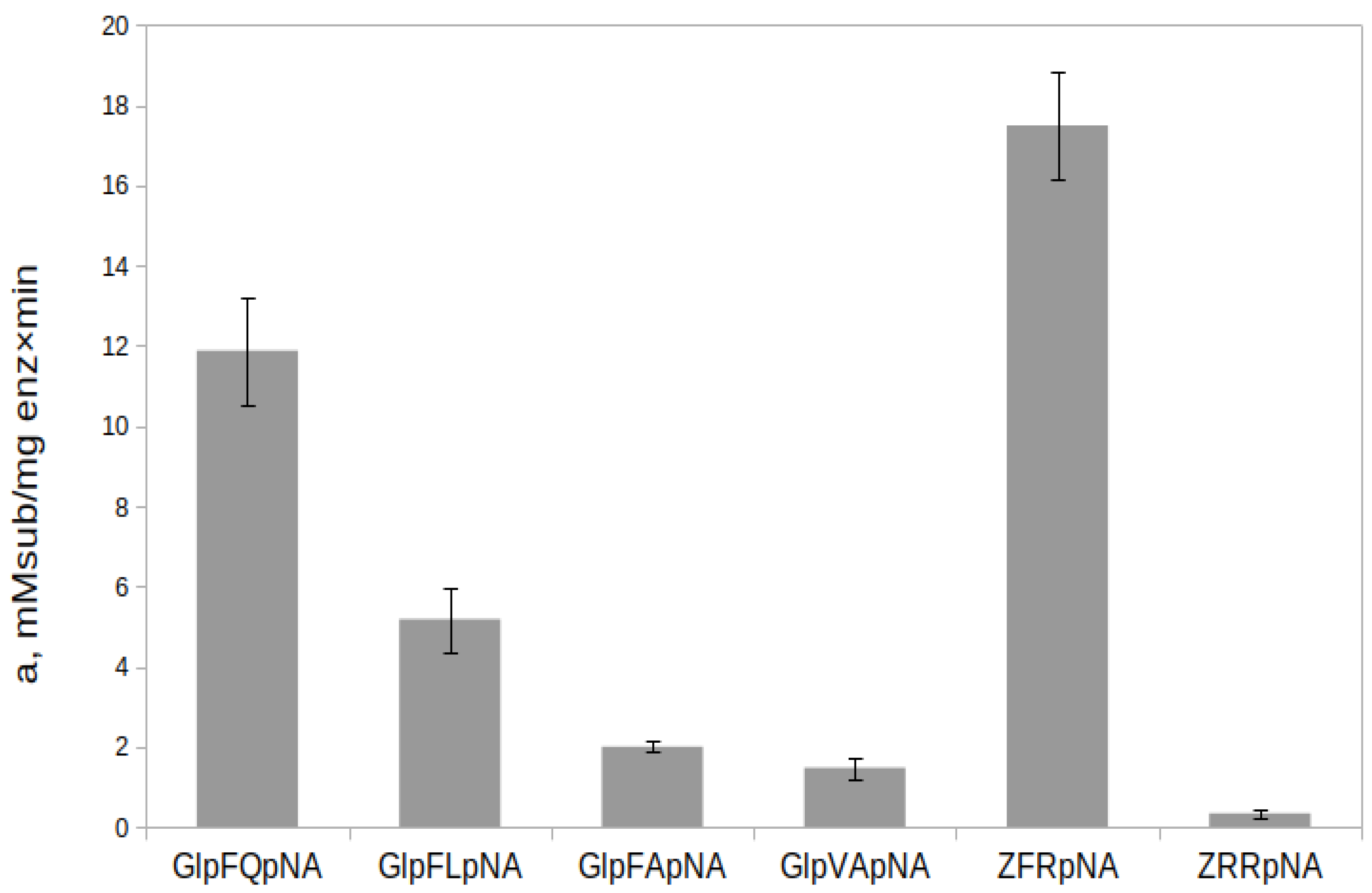
2.7. Hydrolysis of Chromogenic Substrates by rTcCathL1
2.8. Hydrolysis of Toxic Gliadin Peptides
3. Discussion
4. Materials and Methods
4.1. Cloning and Expression of rpTcCathL1
4.2. Isolation of rpTcCathL1
4.3. Protein Concentration Assay
4.4. Assay of Peptidase Activity with the Chromogenic Substrate Glp-Phe-Gln-pNA
4.5. Processing of rpTcCathL1
4.6. Effect of pH on the rpTcCathL1 Processing Rate
4.7. Effect of the Proenzyme Concentration on the rpTcCathL1 Processing Rate
4.8. Native PAGE and Post-Electrophoretic Detection of Proteolytic Activity
4.9. Mass Spectrometry Analysis of rpTcCathL1 and rTcCathL1
4.10. Effect of pH on rTcCathL1 Activity and Stability
4.11. Substrate Specificity of rTcCathL1
4.12. Effect of Inhibitors on Cysteine Cathepsin
4.13. Study of Immunogenic Peptides Hydrolysis
4.14. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | 4-amino-7-methylcoumaride |
| DMF | dimethylformamide |
| DMK | diazomethylketone |
| E-64 | N-(trans-Epoxysuccinyl)- L -leucine 4-guanidinobutylamide; |
| EDTA | ethylenediaminetetraacetic acid |
| Glp | pyroglutamyl |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| MALDI-TOF MS | matrix assisted laser desorption/ionization time of flight mass spectrometry |
| pNA | p-nitroanilide |
| rpTcCathL1 | recombinant procathepsin L T. castaneum NP_001163996 (NP_001164001) |
| rTcCathL1 | recombinant cathepsin L T. castaneum NP_001163996 (NP_001164001) |
| TcCathL1 | cathepsin L T. castaneum NP_001163996 (NP_001164001) |
| TcCathL2 | cathepsin L T. castaneum NP_001164314 |
| UB | universal buffer |
| Z | benzyloxycarbonyl |
References
- Rawlings, N.D.; Barrett, A.J. Introduction: The Clans and Families of Cysteine Peptidases. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: London, UK, 2013; Volume 2, pp. 1743–1773. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine Cathepsins: From Structure, Function and Regulation to New Frontiers. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiljeva, O.; Reinheckel, T.; Peters, C.; Turk, D.; Turk, V.; Turk, B. Emerging Roles of Cysteine Cathepsins in Disease and Their Potential as Drug Targets. Curr. Pharm. Des. 2007, 13, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, K.S.; Elpidina, E.N.; Oppert, B.; Prabhakar, S.; Zhuzhikov, D.P.; Dunaevsky, Y.E.; Belozersky, M.A. Diversity of Digestive Proteinases in Tenebrio molitor (Coleoptera: Tenebrionidae) Larvae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 145, 126–137. [Google Scholar] [CrossRef]
- Vinokurov, K.S.; Elpidina, E.N.; Oppert, B.; Prabhakar, S.; Zhuzhikov, D.P.; Dunaevsky, Y.E.; Belozersky, M.A. Fractionation of Digestive Proteinases from Tenebrio molitor (Coleoptera: Tenebrionidae) Larvae and Role in Protein Digestion. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 145, 138–146. [Google Scholar] [CrossRef]
- Tribolium Genome Sequencing Consortium. The Genome of the Model Beetle and Pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinokurov, K.S.; Elpidina, E.N.; Zhuzhikov, D.P.; Oppert, B.; Kodrik, D.; Sehnal, F. Digestive Proteolysis Organization in Two Closely Related Tenebrionid Beetles: Red Flour Beetle (Tribolium castaneum) and Confused Flour Beetle (Tribolium confusum). Arch. Insect Biochem. Physiol. 2009, 70, 254–279. [Google Scholar] [CrossRef]
- Beton, D.; Guzzo, C.R.; Ribeiro, A.F.; Farah, C.S.; Terra, W.R. The 3D Structure and Function of Digestive Cathepsin L-like Proteinases of Tenebrio molitor Larval Midgut. Insect Biochem. Mol. Biol. 2012, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.P.P.; Soares-Costa, A.; Ribeiro, A.F.; Rosa, J.C.; Terra, W.R.; Henrique-Silva, F. Recombinant Expression, Localization and in Vitro Inhibition of Midgut Cysteine Peptidase (Sl-CathL) from Sugarcane Weevil, Sphenophorus levis. Insect Biochem. Mol. Biol. 2012, 42, 58–69. [Google Scholar] [CrossRef]
- Schoville, S.D.; Chen, Y.H.; Andersson, M.N.; Benoit, J.B.; Bhandari, A.; Bowsher, J.H.; Brevik, K.; Cappelle, K.; Chen, M.J.M.; Childers, A.K.; et al. A Model Species for Agricultural Pest Genomics: The Genome of the Colorado Potato Beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Sci. Rep. 2018, 8, 1931. [Google Scholar] [CrossRef] [Green Version]
- Dvoryakova, E.A.; Vinokurov, K.S.; Tereshchenkova, V.F.; Dunaevsky, Y.E.; Belozersky, M.A.; Oppert, B.; Filippova, I.Y.; Elpidina, E.N. Primary Digestive Cathepsins L of Tribolium castaneum Larvae: Proteomic Identification, Properties, Comparison with Human Lysosomal Cathepsin L. Insect Biochem. Mol. Biol. 2022, 140, 103679. [Google Scholar] [CrossRef]
- Pimentel, A.C.; Dias, R.O.; Bifano, T.D.; Genta, F.A.; Ferreira, C.; Terra, W.R. Cathepsins L and B in Dysdercus peruvianus, Rhodnius prolixus, and Mahanarva fimbriolata. Looking for Enzyme Adaptations to Digestion. Insect Biochem. Mol. Biol. 2020, 127, 103488. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.E.; Bansal, R.; Benoit, J.B.; Blackburn, M.B.; Chao, H.; Chen, M.; Cheng, S.; Childers, C.; Dinh, H.; Doddapaneni, H.V.; et al. Brown Marmorated Stink Bug, Halyomorpha halys (Stål), Genome: Putative Underpinnings of Polyphagy, Insecticide Resistance Potential and Biology of a Top Worldwide Pest. BMC Genom. 2020, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Martynov, A.G.; Elpidina, E.N.; Perkin, L.; Oppert, B. Functional Analysis of C1 Family Cysteine Peptidases in the Larval Gut of Tenebrio molitor and Tribolium castaneum. BMC Genom. 2015, 16, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkin, L.; Elpidina, E.N.; Oppert, B. Expression Patterns of Cysteine Peptidase Genes across the Tribolium Castaneum Life Cycle Provide Clues to Biological Function. PeerJ 2016, 4, e1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, K.; Lorenzen, M.D.; Hiromasa, Y.; Tomich, J.M.; Oppert, C.; Elpidina, E.N.; Vinokurov, K.; Jurat-Fuentes, J.L.; Fabrick, J.; Oppert, B. Tribolium Castaneum Larval Gut Transcriptome and Proteome: A Resource for the Study of the Coleopteran Gut. J. Proteome Res. 2009, 8, 3889–3898. [Google Scholar] [CrossRef]
- Goptar, I.A.; Semashko, T.A.; Danilenko, S.A.; Lysogorskaya, E.N.; Oksenoit, E.S.; Zhuzhikov, D.P.; Belozersky, M.A.; Dunaevsky, Y.E.; Oppert, B.; Filippova, I.Y.; et al. Cysteine Digestive Peptidases Function as Post-Glutamine Cleaving Enzymes in Tenebrionid Stored-Product Pests. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 148–154. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal Seed Storage Proteins: Structures, Properties and Role in Grain Utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Piper, J.L.; Gray, G.M.; Khosla, C. Effect of Prolyl Endopeptidase on Digestive-Resistant Gliadin Peptides In Vivo. J. Pharmacol. Exp. Ther. 2004, 311, 213–219. [Google Scholar] [CrossRef]
- Shan, L.; Marti, T.; Sollid, L.M.; Gray, G.M.; Khosla, C. Comparative Biochemical Analysis of Three Bacterial Prolyl Endopeptidases: Implications for Coeliac Sprue. Biochem. J. 2004, 383, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Kiyosaki, T.; Matsumoto, I.; Asakura, T.; Funaki, J.; Kuroda, M.; Misaka, T.; Arai, S.; Abe, K. Gliadain, a Gibberellin-Inducible Cysteine Proteinase Occurring in Germinating Seeds of Wheat, Triticum aestivum L., Specifically Digests Gliadin and Is Regulated by Intrinsic Cystatins. FEBS J. 2007, 274, 1908–1917. [Google Scholar] [CrossRef]
- Gerez, C.L.; Font de Valdez, G.; Rollán, G.C. Functionality of Lactic Acid Bacteria Peptidase Activities in the Hydrolysis of Gliadin-like Fragments. Lett. Appl. Microbiol. 2008, 47, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Savvateeva, L.V.; Gorokhovets, N.V.; Makarov, V.A.; Serebryakova, M.V.; Solovyev, A.G.; Morozov, S.Y.; Reddy, V.P.; Zernii, E.Y.; Zamyatnin, A.A.; Aliev, G. Glutenase and Collagenase Activities of Wheat Cysteine Protease Triticain-α: Feasibility for Enzymatic Therapy Assays. Int. J. Biochem. Cell Biol. 2015, 62, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Siegel, J.B.; Tinberg, C.; Camarca, A.; Gianfrani, C.; Paski, S.; Guan, R.; Montelione, G.; Baker, D.; Pultz, I.S. Engineering of Kuma030: A Gliadin Peptidase That Rapidly Degrades Immunogenic Gliadin Peptides in Gastric Conditions. J. Am. Chem. Soc. 2015, 137, 13106–13113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syage, J.A.; Murray, J.A.; Green, P.H.R.; Khosla, C. Latiglutenase Improves Symptoms in Seropositive Celiac Disease Patients While on a Gluten-Free Diet. Dig. Dis. Sci. 2017, 62, 2428–2432. [Google Scholar] [CrossRef]
- Martinez, M.; Gómez-Cabellos, S.; Giménez, M.J.; Barro, F.; Diaz, I.; Diaz-Mendoza, M. Plant Proteases: From Key Enzymes in Germination to Allies for Fighting Human Gluten-Related Disorders. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Helmerhorst, E.J.; Darwish, G.; Blumenkranz, G.; Schuppan, D. Gluten Degrading Enzymes for Treatment of Celiac Disease. Nutrients 2020, 12, 2095. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Belozersky, M.A.; Filippova, I.Y.; Oppert, B.; Elpidina, E.N. Effective Degradation of Gluten and Its Fragments by Gluten-Specific Peptidases: A Review on Application for the Treatment of Patients with Gluten Sensitivity. Pharmaceutics 2021, 13, 1603. [Google Scholar] [CrossRef]
- Kutyshenko, V.P.; Mikoulinskaia, G.V.; Chernyshov, S.V.; Yegorov, A.Y.; Prokhorov, D.A.; Uversky, V.N. Effect of C-Terminal His-Tag and Purification Routine on the Activity and Structure of the Metalloenzyme, l-Alanyl-d-Glutamate Peptidase of the Bacteriophage T5. Int. J. Biol. Macromol. 2019, 124, 810–818. [Google Scholar] [CrossRef]
- Shan, L.; Qiao, S.W.; Arentz-Hansen, H.; Molberg, Ø.; Gray, G.M.; Sollid, L.M.; Khosla, C. Identification and Analysis of Multivalent Proteolytically Resistant Peptides from Gluten: Implications for Celiac Sprue. J. Proteome Res. 2005, 4, 1732–1741. [Google Scholar] [CrossRef] [Green Version]
- Gujral, N. Celiac Disease: Prevalence, Diagnosis, Pathogenesis and Treatment. World J. Gastroenterol. 2012, 18, 6036. [Google Scholar] [CrossRef]
- Walter, T.; Wieser, H.; Koehler, P. Production of Gluten-Free Wheat Starch by Peptidase Treatment. J. Cereal Sci. 2014, 60, 202–209. [Google Scholar] [CrossRef]
- Brömme, D.; Nallaseth, F.S.; Turk, B. Production and Activation of Recombinant Papain-like Cysteine Proteases. Methods 2004, 32, 199–206. [Google Scholar] [CrossRef]
- Turk, B.; Turk, D.; Turk, V. Lysosomal Cysteine Proteases: More than Scavengers. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 98–111. [Google Scholar] [CrossRef]
- Ménard, R.; Carmona, E.; Takebe, S.; Dufour, É.; Plouffe, C.; Mason, P.; Mort, J.S. Autocatalytic Processing of Recombinant Human Procathepsin L. J. Biol. Chem. 1998, 273, 4478–4484. [Google Scholar] [CrossRef] [Green Version]
- Ishidoh, K.; Kominami, E. Multi-Step Processing of Procathepsin L in Vitro. FEBS Lett. 1994, 352, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.R.; Stack, C.M.; O’Neill, S.M.; Doyle, S.; Ryan, T.; Brennan, G.P.; Mousley, A.; Stewart, M.; Maule, A.G.; Dalton, J.P.; et al. Cathepsin L1, the Major Protease Involved in Liver Fluke (Fasciola hepatica) Virulence. J. Biol. Chem. 2004, 279, 17038–17046. [Google Scholar] [CrossRef] [Green Version]
- Turk, B.; Dolenc, I.; Turk, V.; Bieth, J.G. Kinetics of the PH-Induced Inactivation of Human Cathepsin L. Biochemistry 1993, 32, 375–380. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Frugoni, J.A.C. Tampone Universale Di Britton e Robinson a Forza Ionica Constante. Gazz. Chim. Ital. 1957, 87, 403–407. [Google Scholar]
- Filippova, I.Y.; Dvoryakova, E.A.; Sokolenko, N.I.; Simonyan, T.R.; Tereshchenkova, V.F.; Zhiganov, N.I.; Dunaevsky, Y.E.; Belozersky, M.A.; Oppert, B.; Elpidina, E.N. New Glutamine-Containing Substrates for the Assay of Cysteine Peptidases From the C1 Papain Family. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- McLellan, T. Electrophoresis Buffers for Polyacrylamide Gels at Various PH. Anal. Biochem. 1982, 126, 94–99. [Google Scholar] [CrossRef]
- Semashko, T.A.; Vorotnikova, E.A.; Sharikova, V.F.; Vinokurov, K.S.; Smirnova, Y.A.; Dunaevsky, Y.E.; Belozersky, M.A.; Oppert, B.; Elpidina, E.N.; Filippova, I.Y. Selective Chromogenic and Fluorogenic Peptide Substrates for the Assay of Cysteine Peptidases in Complex Mixtures. Anal. Biochem. 2014, 449, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Elpidina, E.N.; Semashko, T.A.; Smirnova, Y.A.; Dvoryakova, E.A.; Dunaevsky, Y.E.; Belozersky, M.A.; Serebryakova, M.V.; Klyachko, E.V.; Abd El-latif, A.O.; Oppert, B.; et al. Direct Detection of Cysteine Peptidases for MALDI-TOF MS Analysis Using Fluorogenic Substrates. Anal. Biochem. 2019, 567, 45–50. [Google Scholar] [CrossRef]
- Prabhakar, S.; Chen, M.-S.; Elpidina, E.N.; Vinokurov, K.S.; Smith, C.M.; Marshall, J.; Oppert, B. Sequence Analysis and Molecular Characterization of Larval Midgut CDNA Transcripts Encoding Peptidases from the Yellow Mealworm, Tenebrio molitor L. Insect Mol. Biol. 2007, 16, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.N.; Pappin, D.J.C.; Creasy, D.M.; Cottrell, J.S. Probability-Based Protein Identification by Searching Sequence Databases Using Mass Spectrometry Data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Stepanov, V.M.; Lysogorskaya, E.N.; Filippova, I.Y.; Oksenoit, E.S.; Lyublinskaya, L.A. L-Pyroglutamyl-L-Phenylalanyl-L-Alanyne P-Nitroanilide—A Chromogenic Substrate of Thiol Proteinases. Russian Inventor’s Certificate No. 1198082, 15 December 1985. (In Russian). [Google Scholar]


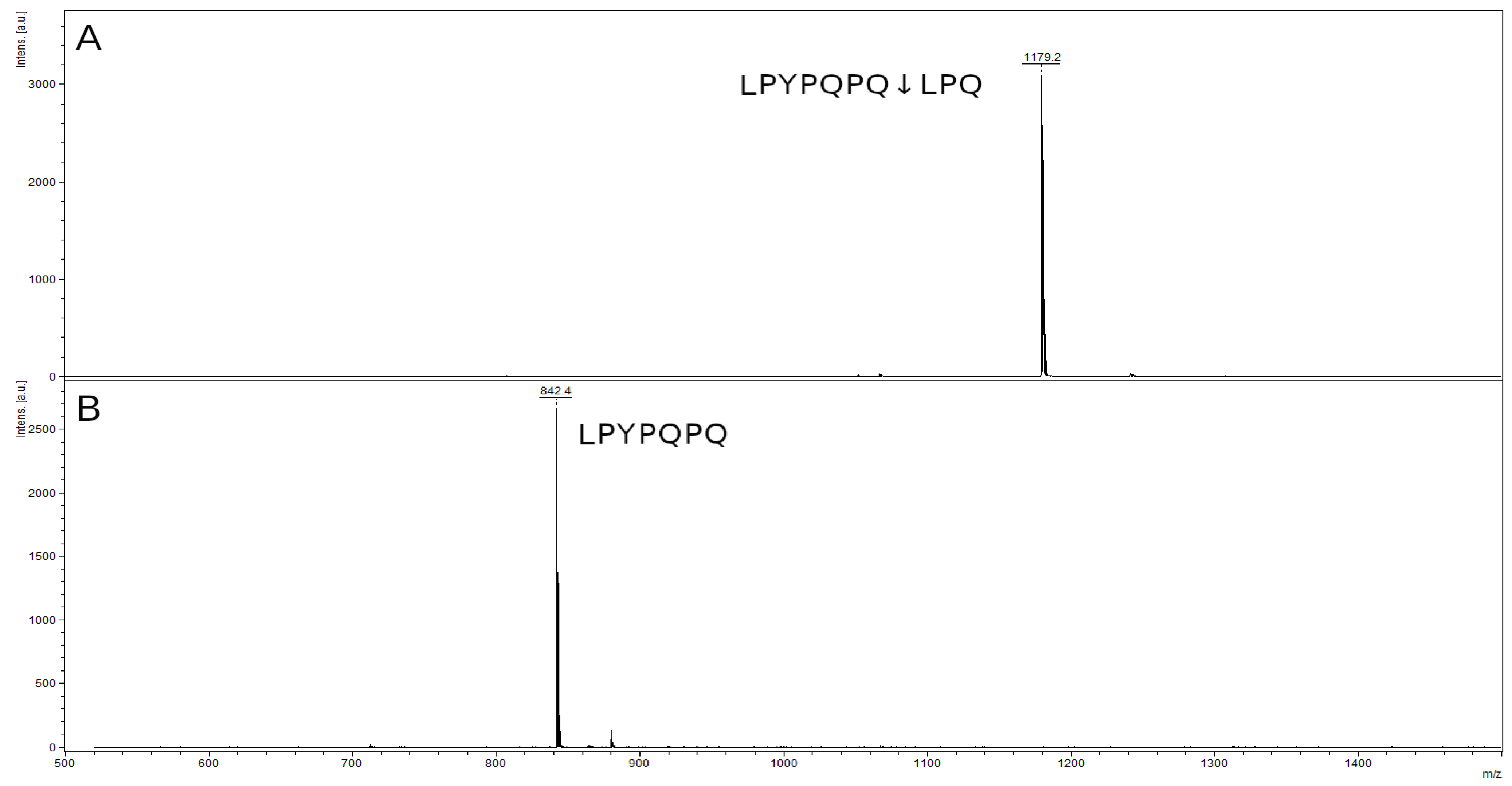

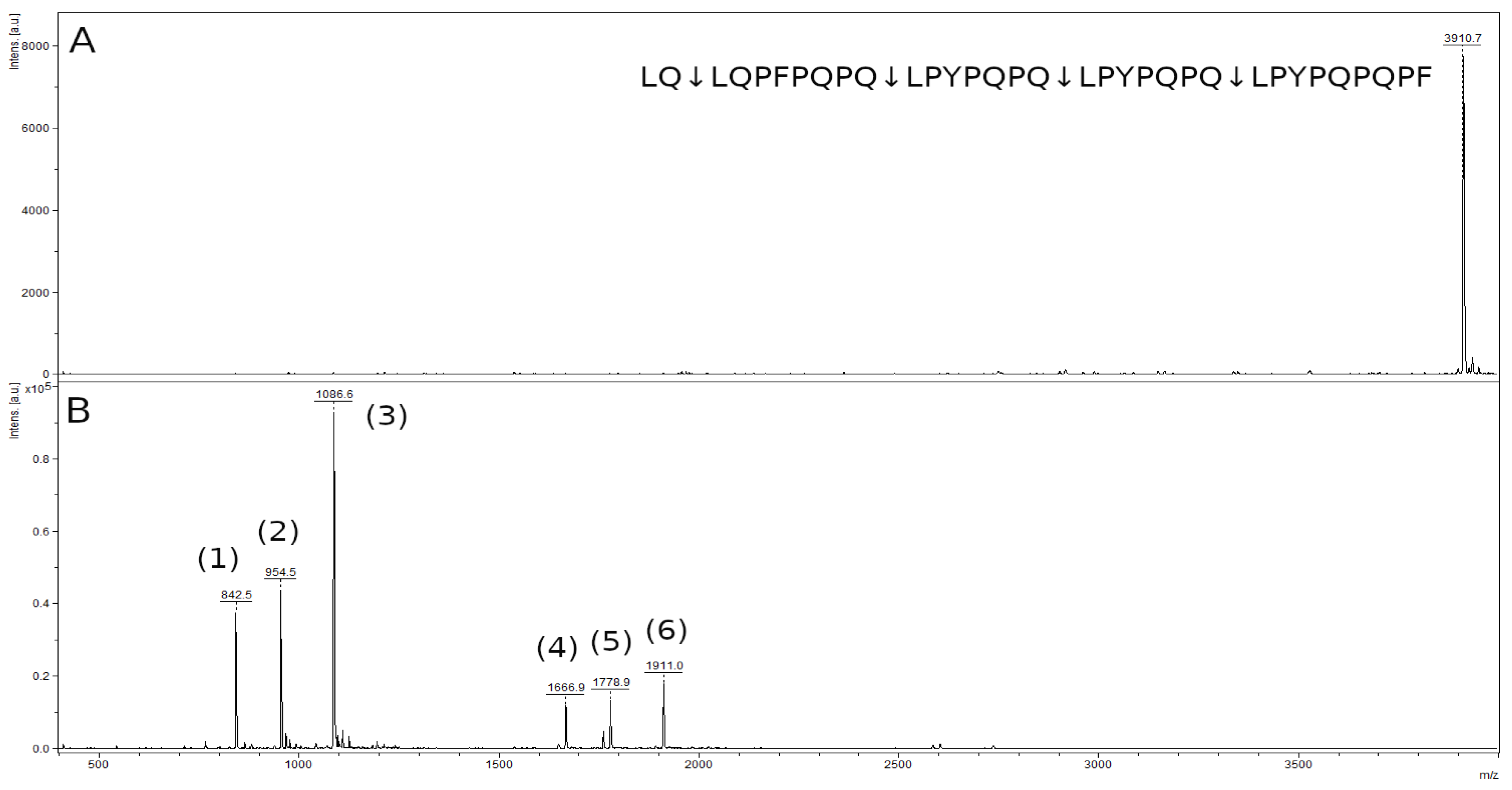
| Peptide | m/z Value |
|---|---|
| LPYPQPQ | 842.5 |
| LQPFPQPQ | 954.5 |
| LPYPQPQPF | 1086.6 |
| LPYPQPQLPYPQPQ | 1666.9 |
| LQPFPQPQLPYPQPQ | 1778.9 |
| LPYPQPQLPYPQPQPF | 1911.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoryakova, E.A.; Klimova, M.A.; Simonyan, T.R.; Dombrovsky, I.A.; Serebryakova, M.V.; Tereshchenkova, V.F.; Dunaevsky, Y.E.; Belozersky, M.A.; Filippova, I.Y.; Elpidina, E.N. Recombinant Cathepsin L of Tribolium castaneum and Its Potential in the Hydrolysis of Immunogenic Gliadin Peptides. Int. J. Mol. Sci. 2022, 23, 7001. https://doi.org/10.3390/ijms23137001
Dvoryakova EA, Klimova MA, Simonyan TR, Dombrovsky IA, Serebryakova MV, Tereshchenkova VF, Dunaevsky YE, Belozersky MA, Filippova IY, Elpidina EN. Recombinant Cathepsin L of Tribolium castaneum and Its Potential in the Hydrolysis of Immunogenic Gliadin Peptides. International Journal of Molecular Sciences. 2022; 23(13):7001. https://doi.org/10.3390/ijms23137001
Chicago/Turabian StyleDvoryakova, Elena A., Maria A. Klimova, Tatiana R. Simonyan, Ivan A. Dombrovsky, Marina V. Serebryakova, Valeriia F. Tereshchenkova, Yakov E. Dunaevsky, Mikhail A. Belozersky, Irina Y. Filippova, and Elena N. Elpidina. 2022. "Recombinant Cathepsin L of Tribolium castaneum and Its Potential in the Hydrolysis of Immunogenic Gliadin Peptides" International Journal of Molecular Sciences 23, no. 13: 7001. https://doi.org/10.3390/ijms23137001
APA StyleDvoryakova, E. A., Klimova, M. A., Simonyan, T. R., Dombrovsky, I. A., Serebryakova, M. V., Tereshchenkova, V. F., Dunaevsky, Y. E., Belozersky, M. A., Filippova, I. Y., & Elpidina, E. N. (2022). Recombinant Cathepsin L of Tribolium castaneum and Its Potential in the Hydrolysis of Immunogenic Gliadin Peptides. International Journal of Molecular Sciences, 23(13), 7001. https://doi.org/10.3390/ijms23137001






