Amitriptyline Accelerates SERT Binding Recovery in a Rat 3,4-Methylenedioxymethamphetamine (MDMA) Model: In Vivo 4-[18F]-ADAM PET Imaging
Abstract
:1. Introduction
2. Results
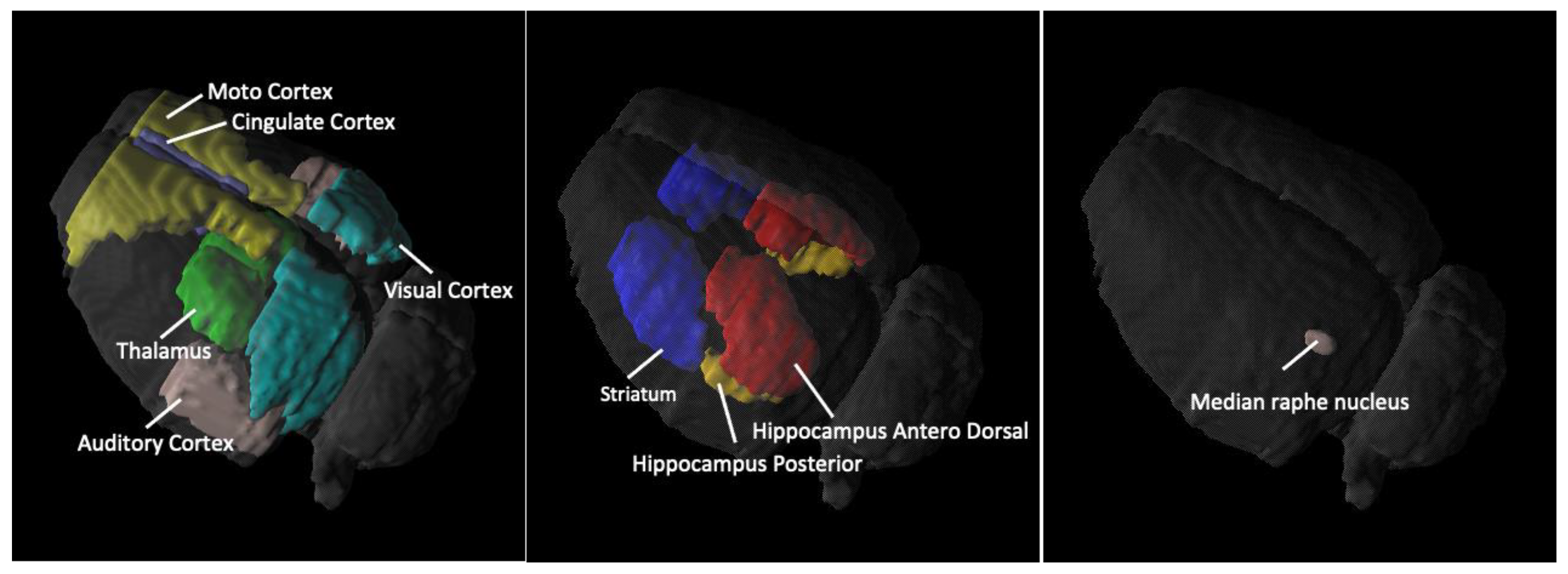
2.1. SERT Recovery Is Region-Specific and Time-Dependent
2.2. Amitriptyline Accelerates SERT Recovery after MDMA Induction
2.3. Amitriptyline Does Not Affect the Normal Brain
3. Discussion
Limitations
4. Materials and Methods
4.1. Animals
4.2. Drug Treatments and Study Design
4.3. Radiopharmaceutical
4.4. Image Data Acquisition and Analyses
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyles, J.; Cadet, J.L. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: Cellular and molecular mechanisms. Brain Res. Brain Res. Rev. 2003, 42, 155–168. [Google Scholar] [CrossRef]
- Aguirre, N.; Barrionuevo, M.; Lasheras, B.; Del Rio, J. The role of dopaminergic systems in the perinatal sensitivity to 3, 4-methylenedioxymethamphetamine-induced neurotoxicity in rats. J. Pharmacol. Exp. Ther. 1998, 286, 1159–1165. [Google Scholar] [PubMed]
- Ricaurte, G.A. Studies of MDMA-induced neurotoxicity in nonhuman primates: A basis for evaluating long-term effects in humans. NIDA Res. Monogr. 1989, 94, 306–322. [Google Scholar] [PubMed]
- Ma, K.H.; Liu, T.T.; Weng, S.J.; Chen, C.F.; Huang, Y.S.; Chueh, S.H.; Liao, M.H.; Chang, K.W.; Sung, C.C.; Hsu, T.H.; et al. Effects of dextromethorphan on MDMA-induced serotonergic aberration in the brains of non-human primates using [(123)I]-ADAM/SPECT. Sci. Rep. 2016, 6, 38695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, I.S.; Clemens, K.J.; Van der Plasse, G.; Li, K.M.; Hunt, G.E.; Chen, F.; Lawrence, A.J. Increased anxiety 3 months after brief exposure to MDMA (“Ecstasy”) in rats: Association with altered 5-HT transporter and receptor density. Neuropsychopharmacology 2003, 28, 1472–1484. [Google Scholar] [CrossRef] [Green Version]
- Vollenweider, F.X.; Gamma, A.; Liechti, M.; Huber, T. Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology 1998, 19, 241–251. [Google Scholar] [CrossRef]
- Kirilly, E. Long-term neuronal damage and recovery after a single dose of MDMA: Expression and distribution of serotonin transporter in the rat brain. Neuropsychopharmacol. Hung. 2010, 12, 413–423. [Google Scholar]
- Sanchez, V.; Camarero, J.; Esteban, B.; Peter, M.J.; Green, A.R.; Colado, M.I. The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA (‘ecstasy’)-induced degeneration of 5-HT nerve endings in rat brain. Br. J. Pharmacol. 2001, 134, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Renoir, T.; Paizanis, E.; El Yacoubi, M.; Saurini, F.; Hanoun, N.; Melfort, M.; Lesch, K.P.; Hamon, M.; Lanfumey, L. Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild-type mice. Int. J. Neuropsychopharmacol. 2008, 11, 1149–1162. [Google Scholar] [CrossRef] [Green Version]
- Li, I.H.; Huang, W.S.; Shiue, C.Y.; Huang, Y.Y.; Liu, R.S.; Chyueh, S.C.; Hu, S.H.; Liao, M.H.; Shen, L.H.; Liu, J.C.; et al. Study on the neuroprotective effect of fluoxetine against MDMA-induced neurotoxicity on the serotonin transporter in rat brain using micro-PET. Neuroimage 2010, 49, 1259–1270. [Google Scholar] [CrossRef]
- Schmidt, C.J. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1987, 240, 1–7. [Google Scholar] [PubMed]
- Berger, U.V.; Gu, X.F.; Azmitia, E.C. The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur. J. Pharmacol. 1992, 215, 153–160. [Google Scholar] [CrossRef]
- Shankaran, M.; Gudelsky, G.A. A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology 1999, 147, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Yeh, S.Y.; De Souza, E.B. MDMA-induced neurotoxicity: Parameters of degeneration and recovery of brain serotonin neurons. Pharmacol. Biochem. Behav. 1988, 29, 269–274. [Google Scholar] [CrossRef]
- Gorska, A.M.; Kaminska, K.; Wawrzczak-Bargiela, A.; Costa, G.; Morelli, M.; Przewlocki, R.; Kreiner, G.; Golembiowska, K. Neurochemical and Neurotoxic Effects of MDMA (Ecstasy) and Caffeine After Chronic Combined Administration in Mice. Neurotox. Res. 2018, 33, 532–548. [Google Scholar] [CrossRef] [Green Version]
- Budzynska, B.; Wnorowski, A.; Kaszubska, K.; Biala, G.; Kruk-Slomka, M.; Kurzepa, J.; Boguszewska-Czubara, A. Acute MDMA and Nicotine Co-administration: Behavioral Effects and Oxidative Stress Processes in Mice. Front. Behav. NeuroSci. 2018, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, M.; Groshan, K.; Blakely, R.D.; Richelson, E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997, 340, 249–258. [Google Scholar] [CrossRef]
- Sharma, H.; Santra, S.; Dutta, A. Triple reuptake inhibitors as potential next-generation antidepressants: A new hope? Future Med. Chem. 2015, 7, 2385–2406. [Google Scholar] [CrossRef] [Green Version]
- Leucht, C.; Huhn, M.; Leucht, S. Amitriptyline versus placebo for major depressive disorder. Cochrane Database Syst. Rev. 2012, 12, CD009138. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, 2015, CD008242. [Google Scholar] [CrossRef]
- Anderson, I.M. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: A meta-analysis of efficacy and tolerability. J. Affect. Disord. 2000, 58, 19–36. [Google Scholar] [CrossRef]
- Barbui, C.; Hotopf, M. Amitriptyline v. the rest: Still the leading antidepressant after 40 years of randomised controlled trials. Br. J. Psychiatry 2001, 178, 129–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadwick, W.; Mitchell, N.; Caroll, J.; Zhou, Y.; Park, S.S.; Wang, L.; Becker, K.G.; Zhang, Y.; Lehrmann, E.; Wood, W.H., 3rd; et al. Amitriptyline-mediated cognitive enhancement in aged 3xTg Alzheimer’s disease mice is associated with neurogenesis and neurotrophic activity. PLoS ONE 2011, 6, e21660. [Google Scholar] [CrossRef] [PubMed]
- Parrott, A.C. Human psychobiology of MDMA or ‘Ecstasy’: An overview of 25 years of empirical research. Hum. Psychopharmacol. 2013, 28, 289–307. [Google Scholar] [CrossRef]
- Xu, H.; Steven Richardson, J.; Li, X.M. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology 2003, 28, 53–62. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Chan, C.B.; Weinshenker, D.; Hall, R.A.; Xiao, G.; Ye, K. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem. Biol. 2009, 16, 644–656. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, K.; Lenda, T.; Konieczny, J.; Wardas, J.; Lorenc-Koci, E. Interactions of the tricyclic antidepressant drug amitriptyline with L-DOPA in the striatum and substantia nigra of unilaterally 6-OHDA-lesioned rats. Relevance to motor dysfunction in Parkinson’s disease. Neurochem. Int. 2018, 121, 125–139. [Google Scholar] [CrossRef]
- Buck, A.; Gucker, P.M.; Schonbachler, R.D.; Arigoni, M.; Kneifel, S.; Vollenweider, F.X.; Ametamey, S.M.; Burger, C. Evaluation of serotonergic transporters using PET and [11C](+)McN-5652: Assessment of methods. J. Cereb. Blood Flow Metab. 2000, 20, 253–262. [Google Scholar] [CrossRef] [Green Version]
- McCann, U.D.; Szabo, Z.; Seckin, E.; Rosenblatt, P.; Mathews, W.B.; Ravert, H.T.; Dannals, R.F.; Ricaurte, G.A. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology 2005, 30, 1741–1750. [Google Scholar] [CrossRef] [Green Version]
- Ginovart, N.; Wilson, A.A.; Meyer, J.H.; Hussey, D.; Houle, S. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: Modeling strategies. J. Cereb. Blood Flow Metab. 2001, 21, 1342–1353. [Google Scholar] [CrossRef] [Green Version]
- Urban, N.B.; Girgis, R.R.; Talbot, P.S.; Kegeles, L.S.; Xu, X.; Frankle, W.G.; Hart, C.L.; Slifstein, M.; Abi-Dargham, A.; Laruelle, M. Sustained recreational use of ecstasy is associated with altered pre and postsynaptic markers of serotonin transmission in neocortical areas: A PET study with [11C]DASB and [11C]MDL 100907. Neuropsychopharmacology 2012, 37, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Gobert, M.; Epinat, J.; Metereau, E.; Duperrier, S.; Neumane, S.; Ballanger, B.; Lavenne, F.; Liger, F.; Tourvielle, C.; Bonnefoi, F.; et al. Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain 2015, 138, 2632–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naganawa, M.; Nabulsi, N.; Planeta, B.; Gallezot, J.D.; Lin, S.F.; Najafzadeh, S.; Williams, W.; Ropchan, J.; Labaree, D.; Neumeister, A.; et al. Tracer kinetic modeling of [11C]AFM, a new PET imaging agent for the serotonin transporter. J. Cereb. Blood Flow Metab. 2013, 33, 1886–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suehiro, M.; Greenberg, J.H.; Shiue, C.Y.; Gonzalez, C.; Dembowski, B.; Reivich, M. Radiosynthesis and biodistribution of the S-[18F]fluoroethyl analog of McN5652. Nucl. Med. Biol. 1996, 23, 407–412. [Google Scholar] [CrossRef]
- Brust, P.; Hinz, R.; Kuwabara, H.; Hesse, S.; Zessin, J.; Pawelke, B.; Stephan, H.; Bergmann, R.; Steinbach, J.; Sabri, O. In vivo measurement of the serotonin transporter with (S)-([18F]fluoromethyl)-(+)-McN5652. Neuropsychopharmacology 2003, 28, 2010–2019. [Google Scholar] [CrossRef]
- Oya, S.; Choi, S.R.; Coenen, H.; Kung, H.F. New PET imaging agent for the serotonin transporter: [(18)F]ACF (2-[(2-amino-4-chloro-5-fluorophenyl)thio]-N,N-dimethyl-benzenmethanamine). J. Med. Chem. 2002, 45, 4716–4723. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.H.; Huang, W.S.; Kuo, Y.Y.; Peng, C.J.; Liou, N.H.; Liu, R.S.; Hwang, J.J.; Liu, J.C.; Chen, H.J.; Shiue, C.Y. Validation of 4-[18F]-ADAM as a SERT imaging agent using micro-PET and autoradiography. Neuroimage 2009, 45, 687–693. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Huang, W.S.; Chu, T.C.; Shiue, C.Y. An improved synthesis of 4-[18F]-ADAM, a potent serotonin transporter imaging agent. Appl. Radiat. Isot. 2009, 67, 1063–1067. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Huang, W.S.; Ma, K.H.; Chou, T.K.; Kuo, Y.Y.; Cheng, C.Y.; Shiue, C.Y. Synthesis and comparison of 4-[18F]F-ADAM, 2-[18F]F-ADAM, N-Desmethyl-4-[18F]F-ADAM and [18F]F-AFM as serotonin transporter imaging agents. Appl. Radiat. Isot. 2012, 70, 2298–2307. [Google Scholar] [CrossRef]
- Chen, Y.A.; Huang, W.S.; Lin, Y.S.; Cheng, C.Y.; Liu, R.S.; Wang, S.J.; Li, I.H.; Huang, S.Y.; Shiue, C.Y.; Chen, C.Y.; et al. Characterization of 4-[18F]-ADAM as an imaging agent for SERT in non-human primate brain using PET: A dynamic study. Nucl. Med. Biol. 2012, 39, 279–285. [Google Scholar] [CrossRef]
- Shih, J.H.; Ma, K.H.; Chen, C.F.; Cheng, C.Y.; Pao, L.H.; Weng, S.J.; Huang, Y.S.; Shiue, C.Y.; Yeh, M.K.; Li, I.H. Evaluation of brain SERT occupancy by resveratrol against MDMA-induced neurobiological and behavioral changes in rats: A 4-[18F]-ADAM/small-animal PET study. Eur. Neuropsychopharmacol. 2016, 26, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Huang, S.Y.; Ho, P.S.; Ma, K.H.; Huang, Y.Y.; Yeh, C.B.; Liu, R.S.; Cheng, C.Y.; Shiue, C.Y. PET imaging of the brain serotonin transporters (SERT) with N,N-dimethyl-2-(2-amino-4-[18F]fluorophenylthio)benzylamine (4-[18F]-ADAM) in humans: A preliminary study. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 115–124. [Google Scholar] [CrossRef] [PubMed]
- De Win, M.M.; de Jeu, R.A.; de Bruin, K.; Habraken, J.B.; Reneman, L.; Booij, J.; den Heeten, G.J. Validity of in vivo [123I]beta-CIT SPECT in detecting MDMA-induced neurotoxicity in rats. Eur. Neuropsychopharmacol. 2004, 14, 185–189. [Google Scholar] [CrossRef]
- Klomp, A.; den Hollander, B.; de Bruin, K.; Booij, J.; Reneman, L. The effects of ecstasy (MDMA) on brain serotonin transporters are dependent on age-of-first exposure in recreational users and animals. PLoS ONE 2012, 7, e47524. [Google Scholar] [CrossRef] [Green Version]
- Laabbar, W.; Elgot, A.; Kissani, N.; Gamrani, H. Chronic aluminum intoxication in rat induced both serotonin changes in the dorsal raphe nucleus and alteration of glycoprotein secretion in the subcommissural organ: Immunohistochemical study. NeuroSci. Lett. 2014, 577, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Wang, X.; Rothman, R.B. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: A reappraisal of past and present findings. Psychopharmacology 2007, 189, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Boot, B.P.; Mechan, A.O.; McCann, U.D.; Ricaurte, G.A. MDMA- and p-chlorophenylalanine-induced reduction in 5-HT concentrations: Effects on serotonin transporter densities. Eur. J. Pharmacol. 2002, 453, 239–244. [Google Scholar] [CrossRef]
- Harkin, A.; Connor, T.J.; Mulrooney, J.; Kelly, J.P.; Leonard, B.E. Prior exposure to methylenedioxyamphetamine (MDA) induces serotonergic loss and changes in spontaneous exploratory and amphetamine-induced behaviors in rats. Life Sci. 2001, 68, 1367–1382. [Google Scholar] [CrossRef]
- Lew, R.; Sabol, K.E.; Chou, C.; Vosmer, G.L.; Richards, J.; Seiden, L.S. Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part II: Radioligand binding and autoradiography studies. J. Pharmacol. Exp. Ther. 1996, 276, 855–865. [Google Scholar]
- Scheffel, U.; Szabo, Z.; Mathews, W.B.; Finley, P.A.; Dannals, R.F.; Ravert, H.T.; Szabo, K.; Yuan, J.; Ricaurte, G.A. In vivo detection of short- and long-term MDMA neurotoxicity—A positron emission tomography study in the living baboon brain. Synapse 1998, 29, 183–192. [Google Scholar] [CrossRef]
- Reneman, L.; Lavalaye, J.; Schmand, B.; de Wolff, F.A.; van den Brink, W.; den Heeten, G.J.; Booij, J. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”): Preliminary findings. Arch. Gen. Psychiatry 2001, 58, 901–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchert, R.; Thomasius, R.; Wilke, F.; Petersen, K.; Nebeling, B.; Obrocki, J.; Schulze, O.; Schmidt, U.; Clausen, M. A voxel-based PET investigation of the long-term effects of “Ecstasy” consumption on brain serotonin transporters. Am. J. Psychiatry 2004, 161, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraj, S.; Hoshi, R.; Bhagwagar, Z.; Murthy, N.V.; Hinz, R.; Cowen, P.; Curran, H.V.; Grasby, P. Brain serotonin transporter binding in former users of MDMA (‘ecstasy’). Br. J. Psychiatry 2009, 194, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.D.; Adori, C.; Kirilly, E.; Molnar, E.; Kovacs, G.G.; Ferrington, L.; Kelly, P.A.; Bagdy, G. Acute SSRI-induced anxiogenic and brain metabolic effects are attenuated 6 months after initial MDMA-induced depletion. Behav. Brain Res. 2010, 207, 280–289. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Bakar, N.H.A.; Mohamad, N.; Adnan, L.H.M.; Fauzi, N.; Thoarlim, A.; Omar, S.H.S.; Hamzah, M.S.; Yusoff, Z.; Jufri, M.; et al. MDMA and the Brain: A Short Review on the Role of Neurotransmitters in Neurotoxicity. Basic Clin. NeuroSci. 2020, 11, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolla, N.; Wei, Z.; Richardson, J.S.; Li, X.M. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J. Psychiatry NeuroSci. 2005, 30, 196–201. [Google Scholar]
- Green, A.R.; Mechan, A.O.; Elliott, J.M.; O’Shea, E.; Colado, M.I. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol. Rev. 2003, 55, 463–508. [Google Scholar] [CrossRef] [Green Version]
- Kirilly, E.; Molnar, E.; Balogh, B.; Kantor, S.; Hansson, S.R.; Palkovits, M.; Bagdy, G. Decrease in REM latency and changes in sleep quality parallel serotonergic damage and recovery after MDMA: A longitudinal study over 180 days. Int. J. NeuropsychoPharmacol. 2008, 11, 795–809. [Google Scholar] [CrossRef] [Green Version]
- Walker, Q.D.; Williams, C.N.; Jotwani, R.P.; Waller, S.T.; Francis, R.; Kuhn, C.M. Sex differences in the neurochemical and functional effects of MDMA in Sprague-Dawley rats. Psychopharmacology 2007, 189, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Soleimani Asl, S.; Mehdizadeh, M.; Hamedi Shahraki, S.; Artimani, T.; Joghataei, M.T. Sex differences in MDMA-induced toxicity in Sprague-Dawley rats. Funct. Neurol. 2015, 30, 131–137. [Google Scholar] [CrossRef]
- Chiu, C.H.; Siow, T.Y.; Weng, S.J.; Hsu, Y.H.; Huang, Y.S.; Chang, K.W.; Cheng, C.Y.; Ma, K.H. Effect of MDMA-Induced Axotomy on the Dorsal Raphe Forebrain Tract in Rats: An In Vivo Manganese-Enhanced Magnetic Resonance Imaging Study. PLoS ONE 2015, 10, e0138431. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.H.; Chiu, C.H.; Ma, K.H.; Huang, Y.S.; Shiue, C.Y.; Yeh, T.Y.; Kao, L.T.; Lin, Y.Y.; Li, I.H. Autophagy inhibition plays a protective role against 3, 4-methylenedioxymethamphetamine (MDMA)-induced loss of serotonin transporters and depressive-like behaviors in rats. Pharmacol. Res. 2019, 142, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.H.-H.; Chiu, C.-H.; Kuo, Y.-Y.; Tsai, C.-J.; Yu, T.-H.; Huang, W.-S.; Ma, K.-H. Amitriptyline Accelerates SERT Binding Recovery Rate in MDMA-Induced Rat Model: In Vivo 4-[18F]-ADAM PET Imaging. Res. Sq. 2021. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-J.; Chiu, C.-H.; Kuo, Y.-Y.; Huang, W.-S.; Yu, T.-H.; Flores, L.G., II; Yeh, S.H.-H.; Ma, K.-H. Amitriptyline Accelerates SERT Binding Recovery in a Rat 3,4-Methylenedioxymethamphetamine (MDMA) Model: In Vivo 4-[18F]-ADAM PET Imaging. Int. J. Mol. Sci. 2022, 23, 7035. https://doi.org/10.3390/ijms23137035
Tsai C-J, Chiu C-H, Kuo Y-Y, Huang W-S, Yu T-H, Flores LG II, Yeh SH-H, Ma K-H. Amitriptyline Accelerates SERT Binding Recovery in a Rat 3,4-Methylenedioxymethamphetamine (MDMA) Model: In Vivo 4-[18F]-ADAM PET Imaging. International Journal of Molecular Sciences. 2022; 23(13):7035. https://doi.org/10.3390/ijms23137035
Chicago/Turabian StyleTsai, Chi-Jung, Chuang-Hsin Chiu, Yu-Yeh Kuo, Wen-Sheng Huang, Tsung-Hsun Yu, Leo Garcia Flores, II, Skye Hsin-Hsien Yeh, and Kuo-Hsing Ma. 2022. "Amitriptyline Accelerates SERT Binding Recovery in a Rat 3,4-Methylenedioxymethamphetamine (MDMA) Model: In Vivo 4-[18F]-ADAM PET Imaging" International Journal of Molecular Sciences 23, no. 13: 7035. https://doi.org/10.3390/ijms23137035
APA StyleTsai, C.-J., Chiu, C.-H., Kuo, Y.-Y., Huang, W.-S., Yu, T.-H., Flores, L. G., II, Yeh, S. H.-H., & Ma, K.-H. (2022). Amitriptyline Accelerates SERT Binding Recovery in a Rat 3,4-Methylenedioxymethamphetamine (MDMA) Model: In Vivo 4-[18F]-ADAM PET Imaging. International Journal of Molecular Sciences, 23(13), 7035. https://doi.org/10.3390/ijms23137035





