The Interplay between cGMP and Calcium Signaling in Alzheimer’s Disease
Abstract
1. Introduction
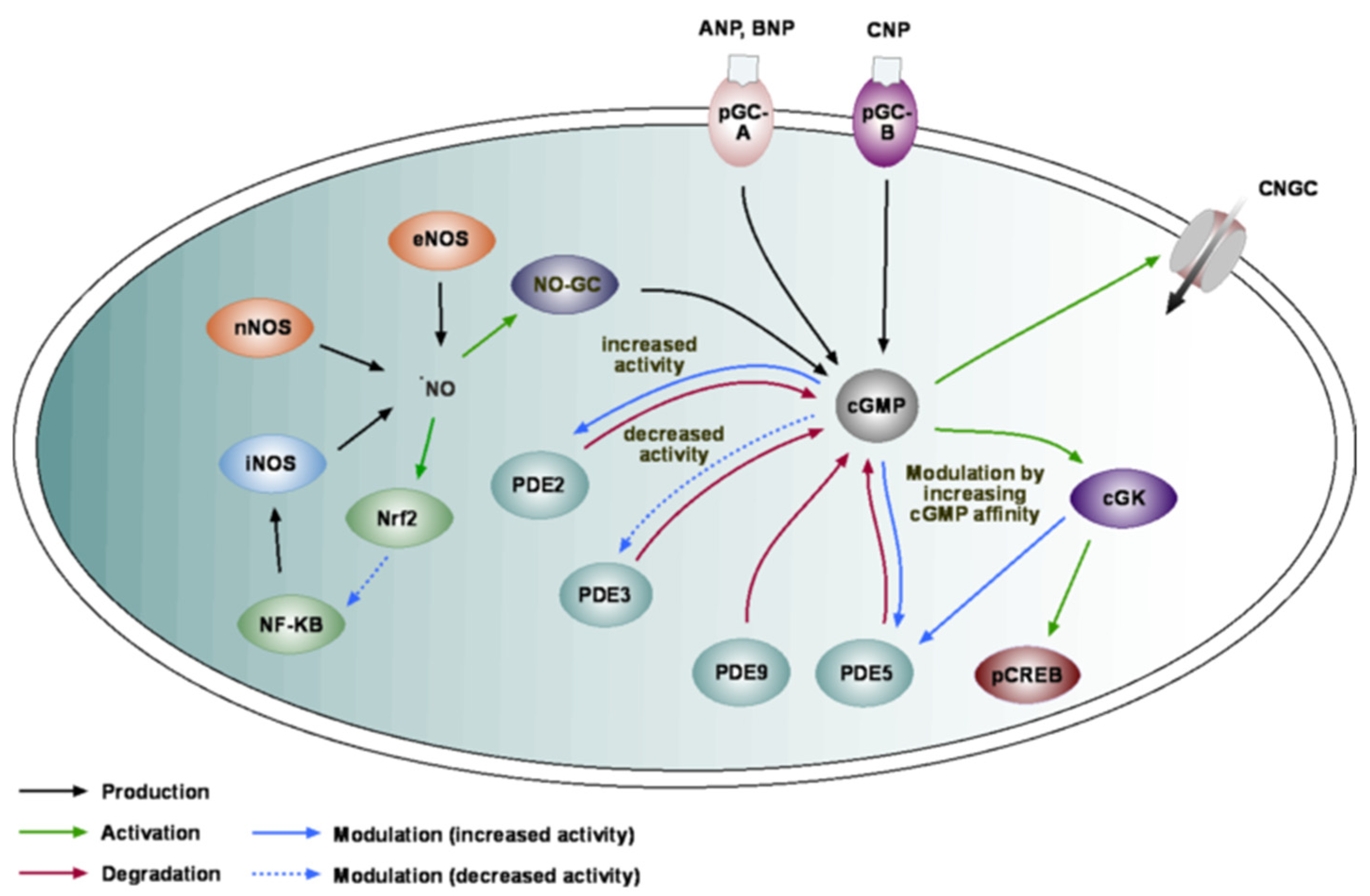
2. cGMP Toolkit in the Brain
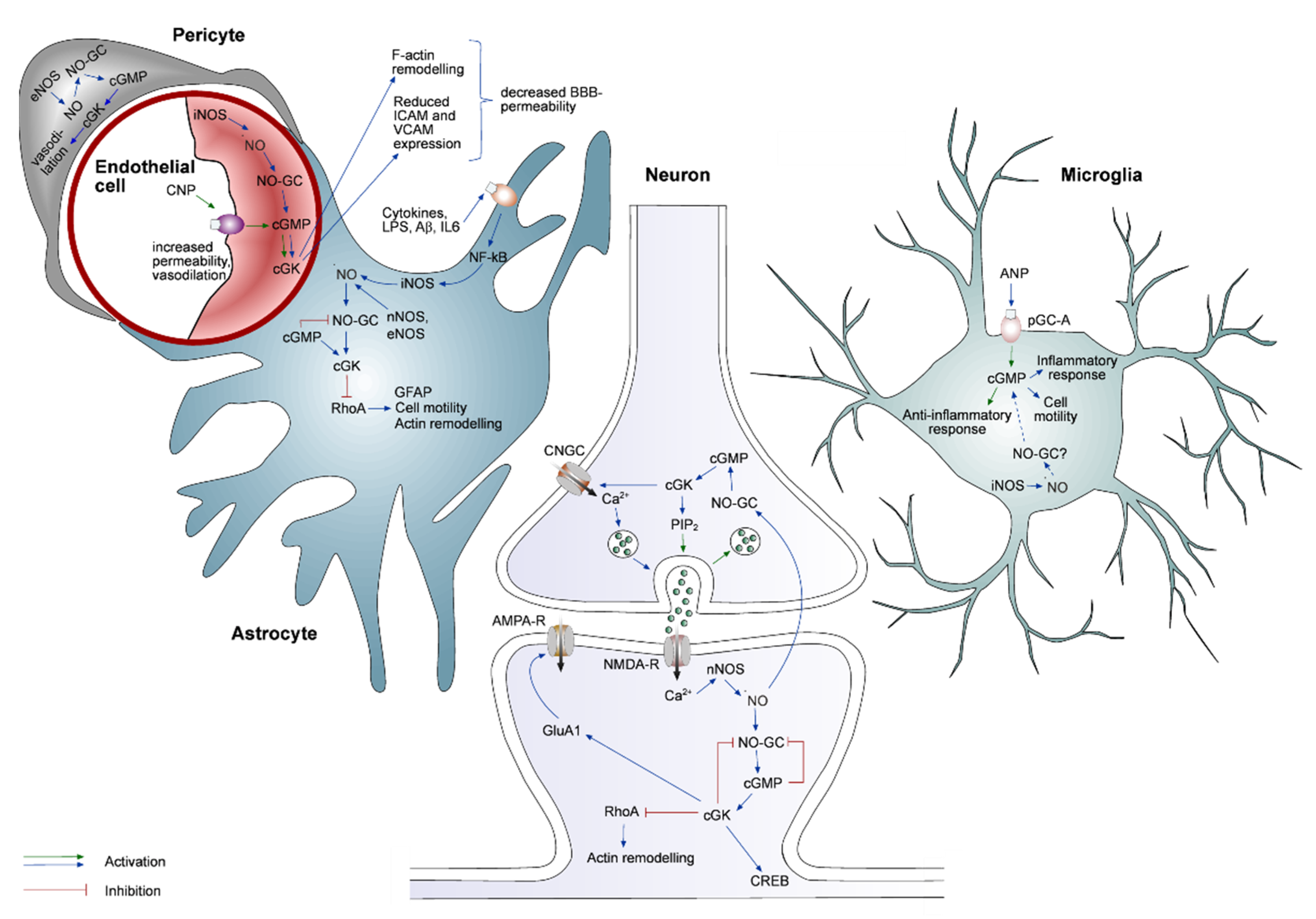
3. Cell Type Specificity of the cGMP Pathway
3.1. cGMP Signaling in Neurons
3.2. cGMP Signaling in Astrocytes
3.3. cGMP Signaling in Microglia
3.4. cGMP Signaling in Endothelial Cells and Pericytes
4. Interplay between Calcium and cGMP Signaling
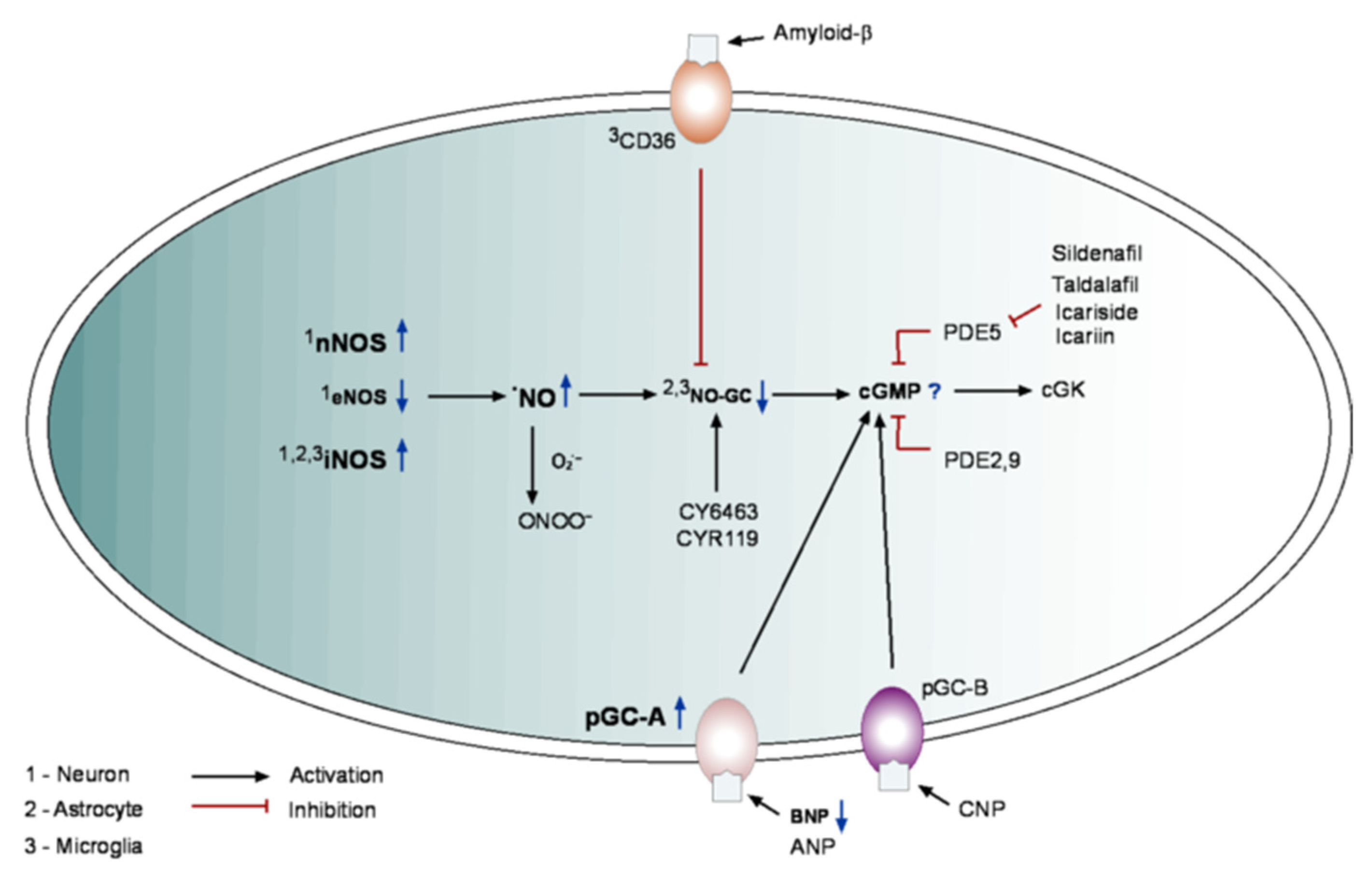
5. cGMP in Alzheimer’s Disease
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hofmann, F. The CGMP System: Components and Function. Biol. Chem. 2020, 401, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Burnett, A.L.; Rosen, R.C.; Park, P.W.; Stecher, V.J. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sex. Med. Rev. 2019, 7, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Rutten, K.; Van Donkelaar, E.L.; Ferrington, L.; Blokland, A.; Bollen, E.; Steinbusch, H.W.M.; Kelly, P.A.T.; Prickaerts, J.H. Phosphodiesterase Inhibitors Enhance Object Memory Independent of Cerebral Blood Flow and Glucose Utilization in Rats. Neuropsychopharmacology 2009, 34, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Iadecola, C. Activation of Cerebellar Climbing Fibers Increases Cerebellar Blood Flow: Role of Glutamate Receptors, Nitric Oxide, and CGMP. Stroke 1998, 29, 499–508. [Google Scholar] [CrossRef]
- Garthwaite, J.; Charles, S.L.; Chess-Williams, R. Endothelium-Derived Relaxing Factor Release on Activation of NMDA Receptors Suggests Role as Intercellular Messenger in the Brain. Nature 1988, 336, 385–388. [Google Scholar] [CrossRef]
- Weissman, B.A.; Jones, C.L.; Liu, Q.; Gross, S.S. Activation and Inactivation of Neuronal Nitric Oxide Synthase: Characterization of Ca2+-Dependent [125I]Calmodulin Binding. Eur. J. Pharmacol. 2002, 435, 9–18. [Google Scholar] [CrossRef][Green Version]
- Palop, J.J.; Mucke, L. Network Abnormalities and Interneuron Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.-H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of Hyperactive Neurons near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Science 2008, 321, 1686–1689. [Google Scholar] [CrossRef]
- Lerdkrai, C.; Asavapanumas, N.; Brawek, B.; Kovalchuk, Y.; Mojtahedi, N.; Del Moral, M.O.; Garaschuk, O. Intracellular Ca 2+ Stores Control in Vivo Neuronal Hyperactivity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2018, 115, E1279–E1288. [Google Scholar] [CrossRef]
- Brawek, B.; Garaschuk, O. Network-Wide Dysregulation of Calcium Homeostasis in Alzheimer’s Disease. Cell Tissue Res. 2014, 357, 427–438. [Google Scholar] [CrossRef]
- Brawek, B.; Schwendele, B.; Riester, K.; Kohsaka, S.; Lerdkrai, C.; Liang, Y.; Garaschuk, O. Impairment of in Vivo Calcium Signaling in Amyloid Plaque-Associated Microglia. Acta Neuropathol. 2014, 127, 495–505. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous Hyperactivity and Intercellular Calcium Waves in Astrocytes in Alzheimer Mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s Disease and Other Dementias: A Priority for European Science and Society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Li, Q.; Melino, S.; Melino, G.; Shi, Y. Can COVID-19 Pandemic Boost the Epidemic of Neurodegenerative Diseases? Biol. Direct 2020, 15, 28. [Google Scholar] [CrossRef]
- Srinivasan, G.; Brafman, D.A. The Emergence of Model Systems to Investigate the Link Between Traumatic Brain Injury and Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 813544. [Google Scholar] [CrossRef]
- Chakroborty, S.; Kim, J.; Schneider, C.; West, A.R.; Stutzmann, G.E. Nitric Oxide Signaling Is Recruited As a Compensatory Mechanism for Sustaining Synaptic Plasticity in Alzheimer’s Disease Mice. J. Neurosci. 2015, 35, 6893–6902. [Google Scholar] [CrossRef]
- Gabbott, P.L.A.; Bacon, S.J. Localisation of NADPH Diaphorase Activity and NOS Immunoreactivity in Astroglia in Normal Adult Rat Brain. Brain Res. 1996, 714, 135–144. [Google Scholar] [CrossRef]
- Lüth, H.J.; Holzer, M.; Gärtner, U.; Staufenbiel, M.; Arendt, T. Expression of Endothelial and Inducible NOS-Isoforms Is Increased in Alzheimer’s Disease, in APP23 Transgenic Mice and after Experimental Brain Lesion in Rat: Evidence for an Induction by Amyloid Pathology. Brain Res. 2001, 913, 57–67. [Google Scholar] [CrossRef]
- Wiencken, A.E.; Casagrande, V.A. Endothelial Nitric Oxide Synthetase (ENOS) in Astrocytes: Another Source of Nitric Oxide in Neocortex. Glia 1999, 26, 280–290. [Google Scholar] [CrossRef]
- Abu-Soud, H.M.; Stuehr, D.J. Nitric Oxide Synthases Reveal a Role for Calmodulin in Controlling Electron Transfer. Proc. Natl. Acad. Sci. USA 1993, 90, 10769. [Google Scholar] [CrossRef]
- Busse, R.; Mülsch, A. Calcium-Dependent Nitric Oxide Synthesis in Endothelial Cytosol Is Mediated by Calmodulin. FEBS Lett. 1990, 265, 133–136. [Google Scholar] [CrossRef]
- Sheng, W.; Zong, Y.; Mohammad, A.; Ajit, D.; Cui, J.; Han, D.; Hamilton, J.L.; Simonyi, A.; Sun, A.Y.; Gu, Z.; et al. Pro-Inflammatory Cytokines and Lipopolysaccharide Induce Changes in Cell Morphology, and Upregulation of ERK1/2, INOS and SPLA2-IIA Expression in Astrocytes and Microglia. J. Neuroinflamm. 2011, 8, 121. [Google Scholar] [CrossRef]
- Heneka, M.T.; Feinstein, D.L. Expression and Function of Inducible Nitric Oxide Synthase in Neurons. J. Neuroimmunol. 2001, 114, 8–18. [Google Scholar] [CrossRef]
- Colasanti, M.; Persichini, T.; Di Pucchio, T.; Gremo, F.; Lauro, G.M. Human Ramified Microglial Cells Produce Nitric Oxide upon Escherichia Coli Lipopolysaccharide and Tumor Necrosis Factor α Stimulation. Neurosci. Lett. 1995, 200, 144–146. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Duszenko, M.; Gospodaryov, D.V.; Garaschuk, O. Oxidative Stress and Energy Metabolism in the Brain: Midlife as a Turning Point. Antioxidants 2021, 10, 1715. [Google Scholar] [CrossRef]
- Kleinert, H.; Euchenhofer, C.; Ihrig-Biedert, I.; Förstermann, U. In Murine 3T3 Fibroblasts, Different Second Messenger Pathways Resulting in the Induction of NO Synthase II (INOS) Converge in the Activation of Transcription Factor NF-ΚB. J. Biol. Chem. 1996, 271, 6039–6044. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Y.; Zhang, X.; Liu, X.; Qi, J. Regulation of INOS Expression by NF-ΚB in Human Lens Epithelial Cells Treated with High Levels of Glucose. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5070–5077. [Google Scholar] [CrossRef]
- Um, H.C.; Jang, J.H.; Kim, D.H.; Lee, C.; Surh, Y.J. Nitric Oxide Activates Nrf2 through S-Nitrosylation of Keap1 in PC12 Cells. Nitric Oxide 2011, 25, 161–168. [Google Scholar] [CrossRef]
- Iizumi, T.; Takahashi, S.; Mashima, K.; Minami, K.; Izawa, Y.; Abe, T.; Hishiki, T.; Suematsu, M.; Kajimura, M.; Suzuki, N. A Possible Role of Microglia-Derived Nitric Oxide by Lipopolysaccharide in Activation of Astroglial Pentose-Phosphate Pathway via the Keap1/Nrf2 System. J. Neuroinflamm. 2016, 13, 99. [Google Scholar] [CrossRef]
- Russwurm, M.; Wittau, N.; Koesling, D. Guanylyl Cyclase/PSD-95 Interaction: Targeting of the Nitric Oxide-Sensitive A2β1 Guanylyl Cyclase to Synaptic Membranes. J. Biol. Chem. 2001, 276, 44647–44652. [Google Scholar] [CrossRef]
- Zabel, U.; Häusler, C.; Weeger, M.; Schmidt, H.H.H.W. Homodimerization of Soluble Guanylyl Cyclase Subunits: Dimerization analysis using a glutathiones-transferase affinity tag. J. Biol. Chem. 1999, 274, 18149–18152. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.R.; Marletta, M.A. Structure and Regulation of Soluble Guanylate Cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Weichsel, A.; Montfort, W.R. Crystal Structure of the Alpha Subunit PAS Domain from Soluble Guanylyl Cyclase. Protein Sci. 2013, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Allerston, C.K.; von Delft, F.; Gileadi, O. Crystal Structures of the Catalytic Domain of Human Soluble Guanylate Cyclase. PLoS ONE 2013, 8, e57644. [Google Scholar] [CrossRef]
- Ma, X.; Beuve, A.; Van Den Akker, F. Crystal Structure of the Signaling Helix Coiled-Coil Domain of the Beta1 Subunit of the Soluble Guanylyl Cyclase. BMC Struct. Biol. 2010, 10, 2. [Google Scholar] [CrossRef]
- Pan, J.; Yuan, H.; Zhang, X.; Zhang, H.; Xu, Q.; Zhou, Y.; Tan, L.; Nagawa, S.; Huang, Z.X.; Tan, X. Probing the Molecular Mechanism of Human Soluble Guanylate Cyclase Activation by NO in Vitro and in Vivo. Sci. Rep. 2017, 7, 43112. [Google Scholar] [CrossRef]
- Mergia, E.; Russwurm, M.; Zoidl, G.; Koesling, D. Major Occurrence of the New A2β1 Isoform of NO-Sensitive Guanylyl Cyclase in Brain. Cell. Signal. 2003, 15, 189–195. [Google Scholar] [CrossRef]
- Mahinrad, S.; Bulk, M.; Van Der Velpen, I.; Mahfouz, A.; Van Roon-Mom, W.; Fedarko, N.; Yasar, S.; Sabayan, B.; Van Heemst, D.; Van Der Weerd, L. Natriuretic Peptides in Post-Mortem Brain Tissue and Cerebrospinal Fluid of Non-Demented Humans and Alzheimer’s Disease Patients. Front. Neurosci. 2018, 12, 864. [Google Scholar] [CrossRef]
- Maack, T.; Suzuki, M.; Almeida, F.A.; Nussenzveig, D.; Scarborough, R.M.; Mcenroe, G.A.; Lewicki, J.A. Physiological Role of Silent Receptors of Atrial Natriuretic Factor. Science 1987, 238, 675–678. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, 1091–1097. [Google Scholar] [CrossRef]
- Ueda, S.; Minamino, N.; Sudoh, T.; Kangawa, K.; Matsuo, H. Regional Distribution of Immunoreactive Brain Natriuretic Peptide in Porcine Brain and Spinal Cord. Biochem. Biophys. Res. Commun. 1988, 155, 733–739. [Google Scholar] [CrossRef]
- Takahashi, K.; Totsune, K.; Sone, M.; Ohneda, M.; Murakami, O.; Itoi, K.; Mouri, T. Human Brain Natriuretic Peptide-like Immunoreactivity in Human Brain. Peptides 1992, 13, 121–123. [Google Scholar] [CrossRef]
- Zamir, N.; Skofitsch, G.; Eskay, R.L.; Jacobowitz, D.M. Distribution of Immunoreactive Atrial Natriuretic Peptides in the Central Nervous System of the Rat. Brain Res. 1986, 365, 105–111. [Google Scholar] [CrossRef]
- Hösli, E.; Hösli, L. Autoradiographic Localization of Binding Sites for Arginine Vasopressin and Atrial Natriuretic Peptide on Astrocytes and Neurons of Cultured Rat Central Nervous System. Neuroscience 1992, 51, 159–166. [Google Scholar] [CrossRef]
- Teunissen, C.; Steinbusch, H.; Markerink-van Ittersum, M.; Koesling, D.; De Vente, J. Presence of Soluble and Particulate Guanylyl Cyclase in the Same Hippocampal Astrocytes. Brain Res. 2001, 891, 206–212. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nakao, K.; Nakagawa, O.; Komatsu, Y.; Hosoda, K.; Suga, S.I.; Arai, H.; Nagata, K.; Yoshida, N.; Imura, H. Human C-Type Natriuretic Peptide. Characterization of the Gene and Peptide. Hypertension 1992, 19, 809–813. [Google Scholar] [CrossRef]
- De Vente, J.; Asan, E.; Gambaryan, S.; Markerink-van Ittersum, M.; Axer, H.; Gallatz, K.; Lohmann, S.M.; Palkovits, M. Localization of CGMP-Dependent Protein Kinase Type II in Rat Brain. Neuroscience 2001, 108, 27–49. [Google Scholar] [CrossRef]
- Hofmann, F. The Biology of Cyclic GMP-Dependent Protein Kinases. J. Biol. Chem. 2005, 280, 1–4. [Google Scholar] [CrossRef]
- El-Husseini, A.E.; Bladen, C.; Vincent, S.R. Molecular Characterization of a Type II Cyclic GMP-Dependent Protein Kinase Expressed in the Rat Brain. J. Neurochem. 1995, 64, 2814–2817. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dills, W.L.; Corbin, J.D. Purification and Subunit Composition of Guanosine 3′:5′-Monophosphate-Dependent Protein Kinase from Bovine Lung. J. Biol. Chem. 1977, 252, 4269–4275. [Google Scholar] [CrossRef]
- Wolfe, L.; Corbin, J.D.; Francis, S.H. Characterization of a Novel Isozyme of CGMP-Dependent Protein Kinase from Bovine Aorta. J. Biol. Chem. 1989, 264, 7734–7741. [Google Scholar] [CrossRef]
- Glass, D.B.; Smith, S.B. Phosphorylation by Cyclic GMP-Dependent Protein Kinase of a Synthetic Peptide Corresponding to the Autophosphorylation Site in the Enzyme. J. Biol. Chem. 1983, 258, 14797–14803. [Google Scholar] [CrossRef]
- Chu, D.-M.; Francis, S.H.; Thomas, J.W.; Maksymovitch, E.A.; Fosler, M.; Corbin, J.D. Activation by Autophosphorylation or CGMP Binding Produces a Similar Apparent Conformational Change in CGMP-Dependent Protein Kinase. J. Biol. Chem. 1998, 273, 14649–14656. [Google Scholar] [CrossRef]
- Werner, C.; Raivich, G.; Cowen, M.; Strekalova, T.; Sillaber, I.; Buters, J.T.; Spanagel, R.; Hofmann, F. Importance of NO⁄cGMP Signalling via CGMP-Dependent Protein Kinase II for Controlling Emotionality and Neurobehavioural Effects of Alcohol. Eur. J. Neurosci. 2004, 20, 3498–3506. [Google Scholar] [CrossRef]
- Paul, C.; Schöberl, F.; Weinmeister, P.; Micale, V.; Wotjak, C.T.; Hofmann, F.; Kleppisch, T. Signaling through CGMP-Dependent Protein Kinase I in the Amygdala Is Critical for Auditory-Cued Fear Memory and Long-Term Potentiation. J. Neurosci. 2008, 28, 14202–14212. [Google Scholar] [CrossRef]
- Tischkau, S.A.; Mitchell, J.W.; Pace, L.A.; Barnes, J.W.; Barnes, J.A.; Gillette, M.U. Protein Kinase G Type II Is Required for Night-to-Day Progression of the Mammalian Circadian Clock. Neuron 2004, 43, 539–549. [Google Scholar] [CrossRef]
- Son, H.; Lu, Y.F.; Zhuo, M.; Arancio, O.; Kandel, E.R.; Hawkins, R.D. The Specific Role of CGMP in Hippocampal LTP. Learn. Mem. 1998, 5, 231–245. [Google Scholar] [CrossRef]
- Monfort, P.; Gomez-Gimenez, B.; Llansola, M.; Felipo, V. Gender Differences in Spatial Learning, Synaptic Activity, and Long-Term Potentiation in the Hippocampus in Rats: Molecular Mechanisms. ACS Chem. Neurosci. 2015, 6, 1420–1427. [Google Scholar] [CrossRef]
- Biel, M.; Zong, X.; Hofmann, F. Molecular Diversity of Cyclic Nucleotide-Gated Cation Channels. Naunyn. Schmiedebergs Arch. Pharmacol. 1995, 353, 1–10. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Kolesnikov, S.S.; Lyubarsky, A.L. Induction by Cyclic GMP of Cationic Conductance in Plasma Membrane of Retinal Rod Outer Segment. Nature 1985, 313, 310–313. [Google Scholar] [CrossRef]
- Barnstable, C.J.; Wei, J.Y.; Han, M.H. Modulation of Synaptic Function by CGMP and CGMP-Gated Cation Channels. Neurochem. Int. 2004, 45, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Irisarri, E.; Markerink-Van Ittersum, M.; Mengod, G.; De Vente, J. Expression of the CGMP-Specific Phosphodiesterases 2 and 9 in Normal and Alzheimer’s Disease Human Brains. Eur. J. Neurosci. 2007, 25, 3332–3338. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, S.G.; Dikkes, P.; Epstein, P.M.; Rosenberg, P.A. Expression of CGMP-Specific Phosphodiesterase 9A MRNA in the Rat Brain. J. Neurosci. 2001, 21, 9068–9076. [Google Scholar] [CrossRef] [PubMed]
- Surapisitchat, J.; Jeon, K.I.; Yan, C.; Beavo, J.A. Differential Regulation of Endothelial Cell Permeability by CGMP via Phosphodiesterases 2 and 3. Circ. Res. 2007, 101, 811–818. [Google Scholar] [CrossRef]
- Rybalkin, S.D.; Rybalkina, I.G.; Fei, R.; Hofmann, F.; Beavo, J.A. Regulation of CGMP-Specific Phosphodiesterase (PDE5) Phosphorylation in Smooth Muscle Cells. J. Biol. Chem. 2002, 277, 3310–3317. [Google Scholar] [CrossRef]
- Lee, D.I.; Zhu, G.; Sasaki, T.; Cho, G.S.; Hamdani, N.; Holewinski, R.; Jo, S.H.; Danner, T.; Zhang, M.; Rainer, P.P.; et al. Phosphodiesterase 9A Controls Nitric-Oxide Independent CGMP and Hypertrophic Heart Disease. Nature 2015, 519, 472. [Google Scholar] [CrossRef]
- Murthy, K.S. Activation of Phosphodiesterase 5 and Inhibition of Guanylate Cyclase by CGMP-Dependent Protein Kinase in Smooth Muscle. Biochem. J. 2001, 360, 199. [Google Scholar] [CrossRef]
- Zhou, Z.; Sayed, N.; Pyriochou, A.; Roussos, C.; Fulton, D.; Beuve, A.; Papapetropoulos, A. Protein Kinase G Phosphorylates Soluble Guanylyl Cyclase on Serine 64 and Inhibits Its Activity. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1803. [Google Scholar] [CrossRef]
- Anthony Altar, C.; Boyar, W.C.; Kim, H.S. Discriminatory Roles for D1 and D2 Dopamine Receptor Subtypes in the in Vivo Control of Neostriatal Cyclic GMP. Eur. J. Pharmacol. 1990, 181, 17–21. [Google Scholar] [CrossRef]
- Mathes, C.; Thompson, S.H. The Nitric Oxide/CGMP Pathway Couples Muscarinic Receptors to the Activation of Ca2+ Influx. J. Neurosci. 1996, 76, 1702–1709. [Google Scholar] [CrossRef]
- De Vente, J.; Markerink-Van Ittersum, M.; Axer, H.; Steinbusch, H.W.M. Nitric-Oxide-Induced CGMP Synthesis in Cholinergic Neurons in the Rat Brain. Exp. Brain Res. 2001, 136, 480–491. [Google Scholar] [CrossRef]
- Piedrafita, B.; Cauli, O.; Montoliu, C.; Felipo, V. The Function of the Glutamate-Nitric Oxide-CGMP Pathway in Brain in Vivo and Learning Ability Decrease in Parallel in Mature Compared with Young Rats. Learn. Mem. 2007, 14, 254–258. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Hillier, B.J.; Lim, W.A.; Bredt, D.S. PSD-95 Assembles a Ternary Complex with the N-Methyl-D-Aspartic Acid Receptor and a Bivalent Neuronal NO Synthase PDZ Domain. J. Biol. Chem. 1999, 274, 27467–27473. [Google Scholar] [CrossRef]
- Gudi, T.; Lohmann, S.M.; Pilz, R.B. Regulation of Gene Expression by Cyclic GMP-Dependent Protein Kinase Requires Nuclear Translocation of the Kinase: Identification of a Nuclear Localization Signal. Mol. Cell. Biol. 1997, 17, 5244–5254. [Google Scholar] [CrossRef]
- Gudi, T.; Casteel, D.E.; Vinson, C.; Boss, G.R.; Pilz, R.B. NO Activation of Fos Promoter Elements Requires Nuclear Translocation of G-Kinase I and CREB Phosphorylation but Is Independent of MAP Kinase Activation. Oncogene 2000, 19, 6324–6333. [Google Scholar] [CrossRef]
- Casteel, D.E.; Zhang, T.; Zhuang, S.; Pilz, R.B. CGMP-Dependent Protein Kinase Anchoring by IRAG Regulates Its Nuclear Translocation and Transcriptional Activity. Cell. Signal. 2008, 20, 1392–1399. [Google Scholar] [CrossRef]
- Seo, S.Y.; Oh, J.H.; Choe, E.S. Protein Kinase G Increases AMPA Receptor GluR1 Phosphorylation at Serine 845 after Repeated Cocaine Administration in the Rat Nucleus Accumbens. Neurosci. Lett. 2013, 544, 147–151. [Google Scholar] [CrossRef]
- Serulle, Y.; Zhang, S.; Ninan, I.; Puzzo, D.; Mccarthy, M.; Khatri, L.; Arancio, O.; Ziff, E.B. A GluR1-CGKII Interaction Regulates AMPA Receptor Trafficking. Neuron 2007, 56, 670–688. [Google Scholar] [CrossRef]
- Burette, A.; Zabel, U.; Weinberg, R.J.; Schmidt, H.H.H.W.; Valtschanoff, J.G. Synaptic Localization of Nitric Oxide Synthase and Soluble Guanylyl Cyclase in the Hippocampus. J. Neurosci. 2002, 22, 8961–8970. [Google Scholar] [CrossRef]
- Ogawa, H.; Mizusawa, A.; Kikuchi, Y.; Hida, W.; Miki, H.; Shirato, K. Nitric Oxide as a Retrograde Messenger in the Nucleus Tractus Solitarii of Rats during Hypoxia. J. Physiol. 1995, 486, 495–504. [Google Scholar] [CrossRef]
- O’Dell, T.J.; Hawkins, R.D.; Kandel, E.R.; Arancio, O. Tests of the Roles of Two Diffusible Substances in Long-Term Potentiation: Evidence for Nitric Oxide as a Possible Early Retrograde Messenger. Proc. Natl. Acad. Sci. USA 1991, 88, 11285. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O.; Lev-Ram, V.; Tsien, R.Y.; Kandel, E.R.; Hawkins, R.D. Nitric Oxide Acts as a Retrograde Messenger during Long-Term Potentiation in Cultured Hippocampal Neurons. J. Physiol. 1996, 90, 321–322. [Google Scholar] [CrossRef]
- Neitz, A.; Mergia, E.; Eysel, U.T.; Koesling, D.; Mittmann, T. Presynaptic Nitric Oxide/CGMP Facilitates Glutamate Release via Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels in the Hippocampus. Eur. J. Neurosci. 2011, 33, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Speigel, I.; Osman, V.; Hemmings, H.C. Volatile Anesthetics Inhibit Presynaptic CGMP Signaling to Depress Presynaptic Excitability in Rat Hippocampal Neurons. BioRxiv 2022. [Google Scholar] [CrossRef]
- Caviedes, A.; Varas-Godoy, M.; Lafourcade, C.; Sandoval, S.; Bravo-Alegria, J.; Kaehne, T.; Massmann, A.; Figueroa, J.P.; Nualart, F.; Wyneken, U. Endothelial Nitric Oxide Synthase Is Present in Dendritic Spines of Neurons in Primary Cultures. Front. Cell. Neurosci. 2017, 11, 180. [Google Scholar] [CrossRef]
- Petrov, A.M.; Giniatullin, A.R.; Sitdikova, G.F.; Zefirov, A.L. The Role of CGMP-Dependent Signaling Pathway in Synaptic Vesicle Cycle at the Frog Motor Nerve Terminals. J. Neurosci. 2008, 28, 13216. [Google Scholar] [CrossRef]
- Collado-Alsina, A.; Ramírez-Franco, J.; Sánchez-Prieto, J.; Torres, M. The Regulation of Synaptic Vesicle Recycling by CGMP-Dependent Protein Kinase Type II in Cerebellar Granule Cells under Strong and Sustained Stimulation. J. Neurosci. 2014, 34, 8788. [Google Scholar] [CrossRef]
- Eguchi, K.; Nakanishi, S.; Takagi, H.; Taoufiq, Z.; Takahashi, T. Maturation of a PKG-Dependent Retrograde Mechanism for Exoendocytic Coupling of Synaptic Vesicles. Neuron 2012, 74, 517–529. [Google Scholar] [CrossRef]
- Benz, P.M.; Blume, C.; Seifert, S.; Wilhelm, S.; Waschke, J.; Schuh, K.; Gertler, F.; Münzel, T.; Renné, T. Differential VASP Phosphorylation Controls Remodeling of the Actin Cytoskeleton. J. Cell Sci. 2009, 122, 3954–3965. [Google Scholar] [CrossRef]
- Lin, W.H.; Nebhan, C.A.; Anderson, B.R.; Webb, D.J. Vasodilator-Stimulated Phosphoprotein (VASP) Induces Actin Assembly in Dendritic Spines to Promote Their Development and Potentiate Synaptic Strength. J. Biol. Chem. 2010, 285, 36010–36020. [Google Scholar] [CrossRef]
- Wang, H.G.; Lu, F.M.; Jin, I.; Udo, H.; Kandel, E.R.; De Vente, J.; Walter, U.; Lohmann, S.M.; Hawkins, R.D.; Antonova, I. Presynaptic and Postsynaptic Roles of NO, CGK, and RhoA in Long-Lasting Potentiation and Aggregation of Synaptic Proteins. Neuron 2005, 45, 389–403. [Google Scholar] [CrossRef]
- Petratos, S.; Li, Q.X.; George, A.J.; Hou, X.; Kerr, M.L.; Unabia, S.E.; Hatzinisiriou, I.; Maksel, D.; Aguilar, M.I.; Small, D.H. The Beta-Amyloid Protein of Alzheimer’s Disease Increases Neuronal CRMP-2 Phosphorylation by a Rho-GTP Mechanism. Brain 2008, 131, 90–108. [Google Scholar] [CrossRef]
- Sunico, C.R.; González-Forero, D.; Domínguez, G.; Manuel García-Verdugo, J.; Moreno-López, B. Cellular/Molecular Nitric Oxide Induces Pathological Synapse Loss by a Protein Kinase G-, Rho Kinase-Dependent Mechanism Preceded by Myosin Light Chain Phosphorylation. J. Neurosci. 2010, 30, 973–984. [Google Scholar] [CrossRef]
- Arbonés, M.L.; Ribera, J.; Agullo, L.; Baltrons, M.A.; Casanovas, A.; Riveros-Moreno, V.; García, A. Characteristics of Nitric Oxide Synthase Type I of Rat Cerebellar Astrocytes. Glia 1996, 18, 224–232. [Google Scholar] [CrossRef]
- Hua, L.L.; Zhao, M.L.; Cosenza, M.; Kim, M.O.; Huang, H.; Tanowitz, H.B.; Brosnan, C.F.; Lee, S.C. Role of Mitogen-Activated Protein Kinases in Inducible Nitric Oxide Synthase and TNFα Expression in Human Fetal Astrocytes. J. Neuroimmunol. 2002, 126, 180–189. [Google Scholar] [CrossRef]
- Auch, C.J.; Saha, R.N.; Sheikh, F.G.; Liu, X.; Jacobs, B.L.; Pahan, K. Role of Protein Kinase R in Double-Stranded RNA-Induced Expression of Nitric Oxide Synthase in Human Astroglia. FEBS Lett. 2004, 563, 223–228. [Google Scholar] [CrossRef]
- Jana, M.; Anderson, J.A.; Saha, R.N.; Liu, X.; Pahan, K. Regulation of Inducible Nitric Oxide Synthase in Proinflammatory Cytokine-Stimulated Human Primary Astrocytes. Free Radic. Biol. Med. 2005, 38, 655–664. [Google Scholar] [CrossRef]
- Ding, J.D.; Burette, A.; Nedvetsky, P.I.; Schmidt, H.H.H.W.; Weinberg, R.J. Distribution of Soluble Guanylyl Cyclase in the Rat Brain. J. Comp. Neurol. 2004, 472, 437–448. [Google Scholar] [CrossRef]
- Rapôso, C.; Luna, R.L.d.A.; Nunes, A.K.S.; Thomé, R.; Peixoto, C.A. Role of INOS-NO-CGMP Signaling in Modulation of Inflammatory and Myelination Processes. Brain Res. Bull. 2014, 104, 60–73. [Google Scholar] [CrossRef]
- Brahmachari, S.; Fung, Y.K.; Pahan, K. Induction of Glial Fibrillary Acidic Protein Expression in Astrocytes by Nitric Oxide. Neurobiol. Dis. 2006, 26, 4930–4939. [Google Scholar] [CrossRef]
- Borán, M.S.; García, A. The Cyclic GMP-Protein Kinase G Pathway Regulates Cytoskeleton Dynamics and Motility in Astrocytes. J. Neurochem. 2007, 102, 216–230. [Google Scholar] [CrossRef]
- Pifarré, P.; Prado, J.; Giralt, M.; Molinero, A.; Hidalgo, J.; Garcia, A. Cyclic GMP Phosphodiesterase Inhibition Alters the Glial Inflammatory Response, Reduces Oxidative Stress and Cell Death and Increases Angiogenesis Following Focal Brain Injury. J. Neurochem. 2010, 112, 807–817. [Google Scholar] [CrossRef]
- Correia, S.S.; Liu, G.; Jacobson, S.; Bernier, S.G.; Tobin, J.V.; Schwartzkopf, C.D.; Atwater, E.; Lonie, E.; Rivers, S.; Carvalho, A.; et al. The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CYR119 Attenuates Markers of Inflammation in the Central Nervous System. J. Neuroinflamm. 2021, 18, 213. [Google Scholar] [CrossRef]
- Murphy, S.; Simmons, M.L.; Agullo, L.; Garcia, A.; Feinstein, D.L.; Galea, E.; Reis, D.J.; Minc-Golomb, D.; Schwartz, J.P. Synthesis of Nitric Oxide in CNS Glial Cells. Trends Neurosci. 1993, 16, 323–328. [Google Scholar] [CrossRef]
- Marques, C.P.; Cheeran, M.C.J.; Palmquist, J.M.; Hu, S.; Lokensgard, J.R. Microglia Are the Major Cellular Source of INOS during Experimental Herpes Encephalitis. J. Neurovirol. 2008, 14, 229. [Google Scholar] [CrossRef]
- Garaschuk, O. The Role of NLRP3 Inflammasome for Microglial Response to Peripheral Inflammation. Neural Regen. Res. 2021, 16, 294–295. [Google Scholar] [CrossRef]
- Agulló, L.; Baltrons, M.A.; García, A. Calcium-Dependent Nitric Oxide Formation in Glial Cells. Brain Res. 1995, 686, 160–168. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Steinbusch, H.W.M.; Markerink-van Ittersum, M.; De Bruijn, C.; Axer, H.; De Vente, J. Whole Brain Spheroid Cultures as a Model to Study the Development of Nitric Oxide Synthase-Guanylate Cyclase Signal Transduction. Brain Res. Dev. Brain Res. 2000, 125, 99–115. [Google Scholar] [CrossRef]
- Kiprianova, I.; Schwab, S.; Fandrey, J.; Spranger, M. Suppression of the Oxidative Burst in Murine Microglia by Nitric Oxide. Neurosci. Lett. 1997, 226, 75–78. [Google Scholar] [CrossRef]
- Von Knethen, A.; Brüne, B. Activation of Peroxisome Proliferator-Activated Receptor γ by Nitric Oxide in Monocytes/Macrophages Down-Regulates P47phox and Attenuates the Respiratory Burst. J. Immunol. 2002, 169, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Tantucci, M.; Mazzocchetti, P.; de Iure, A.; Durante, V.; Macchioni, L.; Giampà, C.; Alvino, A.; Gaetani, L.; Costa, C.; et al. Microglial Activation and the Nitric Oxide/CGMP/PKG Pathway Underlie Enhanced Neuronal Vulnerability to Mitochondrial Dysfunction in Experimental Multiple Sclerosis. Neurobiol. Dis. 2018, 113, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, W.; Ren, Q.; Hu, J.; Yang, S.; Han, W.; Wang, J.; Wang, X.; Wang, H. Serum IgG-Induced Microglial Activation Enhances Neuronal Cytolysis via the NO/SGC/PKG Pathway in Children with Opsoclonus-Myoclonus Syndrome and Neuroblastoma. J. Neuroinflamm. 2020, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Arandarcikaite, O.; Jokubka, R.; Borutaite, V. Neuroprotective Effects of Nitric Oxide Donor NOC-18 against Brain Ischemia-Induced Mitochondrial Damages: Role of PKG and PKC. Neurosci. Lett. 2015, 586, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, N.; Taniguchi, M.; Miyano, K.; Miyoshi, M.; Watanabe, T. ANP Inhibits LPS-Induced Stimulation of Rat Microglial Cells by Suppressing NF-ΚB and AP-1 Activations. Biochem. Biophys. Res. Commun. 2006, 350, 322–328. [Google Scholar] [CrossRef]
- Miller, T.W.; Isenberg, J.S.; Shih, H.B.; Wang, Y.; Roberts, D.D. Amyloid-β Inhibits No-CGMP Signaling in a CD36- and CD47-Dependent Manner. PLoS ONE 2010, 5, e15686. [Google Scholar] [CrossRef]
- Duan, Y.; Sahley, C.L.; Muller, K.J. ATP and NO Dually Control Migration of Microglia to Nerve Lesions. Dev. Neurobiol. 2009, 69, 60–72. [Google Scholar] [CrossRef]
- Dibaj, P.; Nadrigny, F.; Steffens, H.; Scheller, A.; Hirrlinger, J.; Schomburg, E.D.; Neusch, C.; Kirchhoff, F. NO Mediates Microglial Response to Acute Spinal Cord Injury under ATP Control in Vivo. Glia 2010, 58, 1133–1144. [Google Scholar] [CrossRef]
- Scheiblich, H.; Roloff, F.; Singh, V.; Stangel, M.; Stern, M.; Bicker, G. Nitric Oxide/Cyclic GMP Signaling Regulates Motility of a Microglial Cell Line and Primary Microglia in Vitro. Brain Res. 2014, 1564, 9–21. [Google Scholar] [CrossRef]
- Scheiblich, H.; Bicker, G. Nitric Oxide Regulates Antagonistically Phagocytic and Neurite Outgrowth Inhibiting Capacities of Microglia. Dev. Neurobiol. 2016, 76, 566–584. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, D.H.; Song, K.S.; Shin, K.H.; Chun, B.G. Zaprinast, an Inhibitor of CGMP-Selective Phosphodiesterases, Enhances the Secretion of TNF-α and IL-1β and the Expression of INOS and MHC Class II Molecules in Rat Microglial Cells. J. Neurosci. Res. 2002, 67, 411–421. [Google Scholar] [CrossRef]
- Roy, A.; Fung, Y.K.; Liu, X.; Pahan, K. Up-Regulation of Microglial CD11b Expression by Nitric Oxide. J. Biol. Chem. 2006, 281, 14971–14980. [Google Scholar] [CrossRef]
- Kalla, R.; Bohatschek, M.; Kloss, C.U.A.; Krol, J.; Von Maltzan, X.; Raivich, G. Loss of Microglial Ramification in Microglia-Astrocyte Cocultures: Involvement of Adenylate Cyclase, Calcium, Phosphatase, and Gi-Protein Systems. Glia 2003, 41, 50–63. [Google Scholar] [CrossRef]
- Tsai, E.J.; Kass, D.A. Cyclic GMP Signaling in Cardiovascular Pathophysiology and Therapeutics. Pharmacol. Ther. 2009, 122, 216. [Google Scholar] [CrossRef]
- Joyce, N.C.; DeCamilli, P.; Boyles, J. Pericytes, like Vascular Smooth Muscle Cells, Are Immunocytochemically Positive for Cyclic GMP-Dependent Protein Kinase. Microvasc. Res. 1984, 28, 206–219. [Google Scholar] [CrossRef]
- Mori, Y.; Takayasu, M.; Suzuki, Y.; Shibuya, M.; Yoshida, J.; Hidaka, H. Vasodilator Effects of C-Type Natriuretic Peptide on Cerebral Arterioles in Rats. Eur. J. Pharmacol. 1997, 320, 183–186. [Google Scholar] [CrossRef]
- Bohara, M.; Kambe, Y.; Nagayama, T.; Tokimura, H.; Arita, K.; Miyata, A. C-Type Natriuretic Peptide Modulates Permeability of the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2014, 34, 589–596. [Google Scholar] [CrossRef]
- Steiner, O.; Coisne, C.; Cecchelli, R.; Boscacci, R.; Deutsch, U.; Engelhardt, B.; Lyck, R. Differential Roles for Endothelial ICAM-1, ICAM-2, and VCAM-1 in Shear-Resistant T Cell Arrest, Polarization, and Directed Crawling on Blood-Brain Barrier Endothelium. J. Immunol. 2010, 185, 4846–4855. [Google Scholar] [CrossRef]
- Wong, D.; Dorovini-Zis, K.; Vincent, S.R. Cytokines, Nitric Oxide, and CGMP Modulate the Permeability of an in Vitro Model of the Human Blood–Brain Barrier. Exp. Neurol. 2004, 190, 446–455. [Google Scholar] [CrossRef]
- Liu, S.M.; Sundqvist, T. Nitric Oxide and CGMP Regulate Endothelial Permeability and F-Actin Distribution in Hydrogen Peroxide-Treated Endothelial Cells. Exp. Cell Res. 1997, 235, 238–244. [Google Scholar] [CrossRef]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What Is a Pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451. [Google Scholar] [CrossRef]
- Sakagami, K.; Kawamura, H.; Wu, D.M.; Puro, D.G. Nitric Oxide/CGMP-Induced Inhibition of Calcium and Chloride Currents in Retinal Pericytes. Microvasc. Res. 2001, 62, 196–203. [Google Scholar] [CrossRef]
- Fessenden, J.D.; Schacht, J. Localization of Soluble Guanylate Cyclase Activity in the Guinea Pig Cochlea Suggests Involvement in Regulation of Blood Flow and Supporting Cell Physiology. J. Histochem. Cytochem. 1997, 45, 1401–1408. [Google Scholar] [CrossRef]
- Tian, F.; Fessenden, J.D.; Schacht, J. Cyclic GMP-Dependent Protein Kinase-I in the Guinea Pig Cochlea. Hear. Res. 1999, 131, 63–70. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Harbison, R.G.; Wood, K.S.; Kadowitz, P.J. Activation of Purified Soluble Guanylate Cyclase by Endothelium-Derived Relaxing Factor from Intrapulmonary Artery and Vein: Stimulation by Acetylcholine, Bradykinin and Arachidonic Acid. J. Pharmacol. Exp. Ther. 1986, 237, 893–900. [Google Scholar]
- Zambach, S.A.; Cai, C.; Helms, H.C.C.; Hald, B.O.; Dong, Y.; Fordsmann, J.C.; Nielsen, R.M.; Hu, J.; Lønstrup, M.; Brodin, B.; et al. Precapillary Sphincters and Pericytes at First-Order Capillaries as Key Regulators for Brain Capillary Perfusion. Proc. Natl. Acad. Sci. USA 2021, 118, e2023749118. [Google Scholar] [CrossRef]
- Dehouck, M.P.; Vigne, P.; Torpier, G.; Breittmayer, J.P.; Cecchelli, R.; Frelin, C. Endothelin-1 as a Mediator of Endothelial Cell-Pericyte Interactions in Bovine Brain Capillaries. J. Cereb. Blood Flow Metab. 1997, 17, 464–469. [Google Scholar] [CrossRef]
- Rapoport, R.M. Acute Nitric Oxide Synthase Inhibition and Endothelin-1-Dependent Arterial Pressure Elevation. Front. Pharmacol. 2014, 5, 57. [Google Scholar] [CrossRef]
- Bourque, S.L.; Davidge, S.T.; Adams, M.A. The Interaction between Endothelin-1 and Nitric Oxide in the Vasculature: New Perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1288–1295. [Google Scholar] [CrossRef]
- Komeima, K.; Hayashi, Y.; Naito, Y.; Watanabe, Y. Inhibition of Neuronal Nitric-Oxide Synthase by Calcium/ Calmodulin-Dependent Protein Kinase IIα through Ser847 Phosphorylation in NG108-15 Neuronal Cells. J. Biol. Chem. 2000, 275, 28139–28143. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nishio, M.; Naito, Y.; Yokokura, H.; Nimura, Y.; Hidaka, H.; Watanabe, Y. Regulation of Neuronal Nitric-Oxide Synthase by Calmodulin Kinases. J. Biol. Chem. 1999, 274, 20597–20602. [Google Scholar] [CrossRef]
- Peunova, N.; Enikolopov, G. Amplification of Calcium-Induced Gene Transcription by Nitric Oxide in Neuronal Cells. Nature 1993, 364, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Bae, H.R.; Park, J.K.; Kang, E.K.; Bae, K.W.; Park, H.T. Calmodulin-Dependent Activation of P38 and P42/44 Mitogen-Activated Protein Kinases Contributes to c-Fos Expression by Calcium in PC12 Cells: Modulation by Nitric Oxide. Brain Res. Mol. Brain Res. 2000, 75, 16–24. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and Their Regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Zolle, O.; Lawrie, A.M.; Simpson, A.W.M. Activation of the Particulate and Not the Soluble Guanylate Cyclase Leads to the Inhibition of Ca2+ Extrusion through Localized Elevation of CGMP*. J. Biol. Chem. 2000, 275, 25892–25899. [Google Scholar] [CrossRef]
- Konopacka, A.; Zielińska, M.; Albrecht, J. Ammonia Inhibits the C-Type Natriuretic Peptide-Dependent Cyclic GMP Synthesis and Calcium Accumulation in a Rat Brain Endothelial Cell Line. Neurochem. Int. 2008, 52, 1160–1166. [Google Scholar] [CrossRef]
- Lu, Y.F.; Hawkins, R.D. Ryanodine Receptors Contribute to CGMP-Induced Late-Phase LTP and CREB Phosphorylation in the Hippocampus. J. Neurophysiol. 2002, 88, 1270–1278. [Google Scholar] [CrossRef]
- Verkhratsky, A. Glial Calcium Signaling in Physiology and Pathophysiology 1. Acta Pharmacol. Sin. 2006, 27, 773–780. [Google Scholar] [CrossRef]
- Willmott, N.J.; Wong, K.; Strong, A.J. A Fundamental Role for the Nitric Oxide-G-Kinase Signaling Pathway in Mediating Intercellular Ca2+ Waves in Glia. J. Neurosci. 2000, 20, 1767–1779. [Google Scholar] [CrossRef]
- Krzan, M.; Stenovec, M.; Kreft, M.; Pangršič, T.; Grilc, S.; Haydon, P.G.; Zorec, R. Calcium-Dependent Exocytosis of Atrial Natriuretic Peptide from Astrocytes. J. Neurosci. 2003, 23, 1580–1583. [Google Scholar] [CrossRef]
- Kubo, S.H.; Atlas, S.A.; Laragh, J.H.; Cody, R.J. Maintenance of Forearm Vasodilator Action of Atrial Natriuretic Factor in Congestive Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 1992, 69, 1306–1309. [Google Scholar] [CrossRef]
- McKenzie, J.C.; Juan, Y.W.; Thomas, C.R.; Berman, N.E.J.; Klein, R.M. Atrial Natriuretic Peptide-like Immunoreactivity in Neurons and Astrocytes of Human Cerebellum and Inferior Olivary Complex. J. Histochem. Cytochem. 2001, 49, 1453–1467. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The Role of Pathological Tau in Synaptic Dysfunction in Alzheimer’s Diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Yamada, K.A.; Finn, M.B.; Sloviter, R.S.; Bales, K.R.; May, P.C.; Schoepp, D.D.; Paul, S.M.; Mennerick, S.; Holtzman, D.M. Synaptic Activity Regulates Interstitial Fluid Amyloid-Beta Levels in Vivo. Neuron 2005, 48, 913–922. [Google Scholar] [CrossRef]
- Caudano, F.; Montalto, G.; Passalacqua, M.; Pronzato, M.A.; Fedele, E.; Ricciarelli, R. CGMP Favors the Interaction between APP and BACE1 by Inhibiting Rab5 GTPase Activity. Sci. Rep. 2020, 10, 1358. [Google Scholar] [CrossRef]
- Palmeri, A.; Ricciarelli, R.; Gulisano, W.; Rivera, D.; Rebosio, C.; Calcagno, E.; Tropea, M.R.; Conti, S.; Das, U.; Roy, S.; et al. Amyloid-β Peptide Is Needed for Cgmp-Induced Long-Term Potentiation and Memory. J. Neurosci. 2017, 37, 6926–6937. [Google Scholar] [CrossRef]
- Chapman, P.F.; White, G.L.; Jones, M.W.; Cooper-Blacketer, D.; Marshall, V.J.; Irizarry, M.; Younkin, L.; Good, M.A.; Bliss, T.V.P.; Hyman, B.T.; et al. Impaired Synaptic Plasticity and Learning in Aged Amyloid Precursor Protein Transgenic Mice. Nat. Neurosci. 1999, 2, 271–276. [Google Scholar] [CrossRef]
- Nalbantoglu, J.; Tirado-Santiago, G.; Lahsaïni, A.; Poirier, J.; Goncalves, O.; Verge, G.; Momoli, F.; Welner, S.A.; Massicotte, G.; Julien, J.P.; et al. Impaired Learning and LTP in Mice Expressing the Carboxy Terminus of the Alzheimer Amyloid Precursor Protein. Nature 1997, 387, 500–505. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Cavalier, A.N.; Roberts, C.M.; Lemieux, M.R.; Ramesh, P.; Garcia, M.A.; Link, C.D. Amyloid Beta Acts Synergistically as a Pro-Inflammatory Cytokine. Neurobiol. Dis. 2021, 159, 105493. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. Journals Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. The Amyloid Cascade-Inflammatory Hypothesis of Alzheimer Disease: Implications for Therapy. Acta Neuropathol. 2013, 126, 479–497. [Google Scholar] [CrossRef]
- Sǐmic, G.; Lucassen, P.J.; Krsnik, Ž.; Krušlin, B.; Kostović, I.; Winblad, B.; Bogdanović, N. NNOS Expression in Reactive Astrocytes Correlates with Increased Cell Death Related DNA Damage in the Hippocampus and Entorhinal Cortex in Alzheimer’s Disease. Exp. Neurol. 2000, 165, 12–26. [Google Scholar] [CrossRef]
- Thorns, V.; Hansen, L.; Masliah, E. NNOS Expressing Neurons in the Entorhinal Cortex and Hippocampus Are Affected in Patients with Alzheimer’s Disease. Exp. Neurol. 1998, 150, 14–20. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Bloch, K.D. Aberrant Expression of the Constitutive Endothelial Nitric Oxide Synthase Gene in Alzheimer Disease. Mol. Chem. Neuropathol. 1997, 30, 139–159. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, J.; Bekris, L.M.; Kim, Y.H.; Pieper, A.A.; Leverenz, J.B.; Cummings, J.; Cheng, F. AlzGPS: A Genome-Wide Positioning Systems Platform to Catalyze Multi-Omics for Alzheimer’s Drug Discovery. Alzheimer’s Res. Ther. 2021, 13, 24. [Google Scholar] [CrossRef]
- Haas, J.; Storch-Hagenlocher, B.; Biessmann, A.; Wildemann, B. Inducible Nitric Oxide Synthase and Argininosuccinate Synthetase: Co-Induction in Brain Tissue of Patients with Alzheimer’s Dementia and Following Stimulation with β-Amyloid 1–42 In Vitro. Neurosci. Lett. 2002, 322, 121–125. [Google Scholar] [CrossRef]
- Heneka, M.T.; Wiesinger, H.; Dumitrescu-Ozimek, L.; Riederer, P.; Feinstein, D.L.; Klockgether, T. Neuronal and Glial Coexpression of Argininosuccinate Synthetase and Inducible Nitric Oxide Synthase in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2001, 60, 906–916. [Google Scholar] [CrossRef]
- Lee, S.C.; Zhao, M.L.; Hirano, A.; Dickson, D.W. Inducible Nitric Oxide Synthase Immunoreactivity in the Alzheimer Disease Hippocampus: Association with Hirano Bodies, Neurofibrillary Tangles, and Senile Plaques. J. Neuropathol. Exp. Neurol. 1999, 58, 1163–1169. [Google Scholar] [CrossRef]
- Saha, R.N.; Liu, X.; Pahan, K. Up-Regulation of BDNF in Astrocytes by TNF-α: A Case for the Neuroprotective Role of Cytokine. J. Neuroimmune Pharmacol. 2006, 1, 212. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Estévez, A.G. Tyrosine Nitration as Mediator of Cell Death. Cell. Mol. Life Sci. 2014, 71, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread Peroxynitrite-Mediated Damage in Alzheimer’s Disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Im, H.; Kang, Y.J.; Kim, Y.; Shin, J.H.; Won, W.; Lim, J.; Ju, Y.; Park, Y.M.; Kim, S.; et al. Severe Reactive Astrocytes Precipitate Pathological Hallmarks of Alzheimer’s Disease via H2O2− Production. Nat. Neurosci. 2020, 23, 1555–1566. [Google Scholar] [CrossRef]
- Lin, K.T.; Xue, J.Y.; Sun, F.F.; Wong, P.Y.K. Reactive Oxygen Species Participate in Peroxynitrite-Induced Apoptosis in HL-60 Cells. Biochem. Biophys. Res. Commun. 1997, 230, 115–119. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Ibarra, C.; Nedvetsky, P.I.; Gerlach, M.; Riederer, P.; Schmidt, H.H.H.W. Regional and Age-Dependent Expression of the Nitric Oxide Receptor, Soluble Guanylyl Cyclase, in the Human Brain. Brain Res. 2001, 907, 54–60. [Google Scholar] [CrossRef]
- Bonkale, W.L.; Winblad, B.; Ravid, R.; Cowburn, R.F. Reduced Nitric Oxide Responsive Soluble Guanylyl Cyclase Activity in the Superior Temporal Cortex of Patients with Alzheimer’s Disease. Neurosci. Lett. 1995, 187, 5–8. [Google Scholar] [CrossRef]
- Baltrons, M.A.; Pedraza, C.E.; Heneka, M.T.; García, A. Β-Amyloid Peptides Decrease Soluble Guanylyl Cyclase Expression in Astroglial Cells. Neurobiol. Dis. 2002, 10, 139–149. [Google Scholar] [CrossRef]
- Pedraza, C.E.; Baltrons, M.A.; Heneka, M.T.; García, A. Interleukin-1β and Lipopolysaccharide Decrease Soluble Guanylyl Cyclase in Brain Cells: NO-Independent Destabilization of Protein and NO-Dependent Decrease of MRNA. J. Neuroimmunol. 2003, 144, 80–90. [Google Scholar] [CrossRef]
- Baltrons, M.A.; Pifarré, P.; Ferrer, I.; Carot, J.M.; García, A. Reduced Expression of NO-Sensitive Guanylyl Cyclase in Reactive Astrocytes of Alzheimer Disease, Creutzfeldt-Jakob Disease, and Multiple Sclerosis Brains. Neurobiol. Dis. 2004, 17, 462–472. [Google Scholar] [CrossRef]
- Causevic, M.; Begic, E.; Hadzidedic, S.; Kulaglic, A.; Ramic-Brkic, B.; Begic, Z. SOMAscan-Based Proteomic Measurements of Plasma Brain Natriuretic Peptide Are Decreased in Mild Cognitive Impairment and in Alzheimer’s Dementia Patients. PLoS ONE 2019, 14, e0212261. [Google Scholar] [CrossRef]
- Montalto, G.; Caudano, F.; Sturla, L.; Bruzzone, S.; Salis, A.; Damonte, G.; Prickaerts, J.; Fedele, E.; Ricciarelli, R. Protein Kinase G Phosphorylates the Alzheimer’s Disease-Associated Tau Protein at Distinct Ser/Thr Sites. BioFactors 2021, 47, 126–134. [Google Scholar] [CrossRef]
- Chalimoniuk, M.; Strosznajder, J.B. Aging Modulates Nitric Oxide Synthesis and CGMP Levels in Hippocampus and Cerebellum: Effects of Amyloid β Peptide. Mol. Chem. Neuropathol. 1998, 35, 77–95. [Google Scholar] [CrossRef]
- Ugarte, A.; Gil-Bea, F.; García-Barroso, C.; Cedazo-Minguez, Á.; Ramírez, M.J.; Franco, R.; García-Osta, A.; Oyarzabal, J.; Cuadrado-Tejedor, M. Decreased Levels of Guanosine 3′, 5′-Monophosphate (CGMP) in Cerebrospinal Fluid (CSF) Are Associated with Cognitive Decline and Amyloid Pathology in Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2015, 41, 471–482. [Google Scholar] [CrossRef]
- Hesse, R.; Lausser, L.; Gummert, P.; Schmid, F.; Wahler, A.; Schnack, C.; Kroker, K.S.; Otto, M.; Tumani, H.; Kestler, H.A.; et al. Reduced CGMP Levels in CSF of AD Patients Correlate with Severity of Dementia and Current Depression. Alzheimer’s Res. Ther. 2017, 9, 17. [Google Scholar] [CrossRef]
- Duszczyk, M.; Kuszczyk, M.; Guridi, M.; Lazarewicz, J.W.; Sadowski, M.J. In Vivo Hippocampal Microdialysis Reveals Impairment of NMDA Receptor–CGMP Signaling in APPSW and APPSW/PS1L166P Alzheimer’s Transgenic Mice. Neurochem. Int. 2012, 61, 976–980. [Google Scholar] [CrossRef]
- Rutten, K.; De Vente, J.; Şik, A.; Markerink-Van Ittersum, M.; Prickaerts, J.; Blokland, A. The Selective PDE5 Inhibitor, Sildenafil, Improves Object Memory in Swiss Mice and Increases CGMP Levels in Hippocampal Slices. Behav. Brain Res. 2005, 164, 11–16. [Google Scholar] [CrossRef]
- Rutten, K.; Basile, J.L.; Prickaerts, J.; Blokland, A.; Vivian, J.A. Selective PDE Inhibitors Rolipram and Sildenafil Improve Object Retrieval Performance in Adult Cynomolgus Macaques. Psychopharmacology 2008, 196, 643–648. [Google Scholar] [CrossRef]
- Prickaerts, J.; Şık, A.; Van Der Staay, F.J.; De Vente, J.; Blokland, A. Dissociable Effects of Acetylcholinesterase Inhibitors and Phosphodiesterase Type 5 Inhibitors on Object Recognition Memory: Acquisition versus Consolidation. Psychopharmacology 2005, 177, 381–390. [Google Scholar] [CrossRef]
- Puzzo, D.; Staniszewski, A.; XianDeng, S.; Privitera, L.; Leznik, E.; Liu, S.; Zhang, H.; Feng, Y.; Palmeri, A.; Landry, D.W.; et al. Phosphodiesterase 5 Inhibition Improves Synaptic Function, Memory, and Amyloid-β Load in an Alzheimer’s Disease Mouse Model. J. Neurosci. 2009, 29, 8075–8086. [Google Scholar] [CrossRef]
- Cuadrado-Tejedor, M.; Hervias, I.; Ricobaraza, A.; Puerta, E.; Pérez-Roldán, J.M.; García-Barroso, C.; Franco, R.; Aguirre, N.; García-Osta, A. Sildenafil Restores Cognitive Function without Affecting β-Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Br. J. Pharmacol. 2011, 164, 2029–2041. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Zhao, X.; Chen, Z.; Wang, G.; Liu, A.; Wang, Q.; Zhou, W.; Xu, Y.; Wang, C. Phosphodiesterase-5 Inhibitor Sildenafil Prevents Neuroinflamm., Lowers Beta-Amyloid Levels and Improves Cognitive Performance in APP/PS1 Transgenic Mice. Behav. Brain Res. 2013, 250, 230–237. [Google Scholar] [CrossRef]
- Nunes, A.K.S.; Rapôso, C.; Rocha, S.W.S.; Barbosa, K.P.D.S.; De Almeida Luna, R.L.; Da Cruz-Höfling, M.A.; Peixoto, C.A. Involvement of AMPK, IKβα-NFκB and ENOS in the Sildenafil Anti-Inflammatory Mechanism in a Demyelination Model. Brain Res. 2015, 1627, 119–133. [Google Scholar] [CrossRef]
- Agusti, A.; Hernández-Rabaza, V.; Balzano, T.; Taoro-Gonzalez, L.; Ibañez-Grau, A.; Cabrera-Pastor, A.; Fustero, S.; Llansola, M.; Montoliu, C.; Felipo, V. Sildenafil Reduces Neuroinflamm. in Cerebellum, Restores GABAergic Tone, and Improves Motor in-Coordination in Rats with Hepatic Encephalopathy. CNS Neurosci. Ther. 2017, 23, 386–394. [Google Scholar] [CrossRef]
- Hansson, E.; Björklund, U.; Skiöldebrand, E.; Rönnbäck, L. Anti-Inflammatory Effects Induced by Pharmaceutical Substances on Inflammatory Active Brain Astrocytes—Promising Treatment of Neuroinflammation. J. Neuroinflamm. 2018, 15, 321. [Google Scholar] [CrossRef]
- De Santana Nunes, A.K.; Rapôso, C.; Björklund, U.; da Cruz-Höfling, M.A.; Peixoto, C.A.; Hansson, E. Sildenafil (Viagra®) Prevents and Restores LPS-Induced Inflammation in Astrocytes. Neurosci. Lett. 2016, 630, 59–65. [Google Scholar] [CrossRef]
- Grass, H.; Klotz, T.; Fathian-Sabet, B.; Berghaus, G.; Engelmann, U.; Käferstein, H. Sildenafil (Viagra): Is There an Influence on Psychological Performance? Int. Urol. Nephrol. 2001, 32, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, D.; Müller, S.V.; Nager, W.; Stief, C.G.; Schlote, N.; Jonas, U.; Asvestis, C.; Johannes, S.; Münte, T.F. Central Effects of Sildenafil (Viagra) on Auditory Selective Attention and Verbal Recognition Memory in Humans: A Study with Event-Related Brain Potentials. World J. Urol. 2001, 19, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, P.; Zhou, Y.; Chiang, C.-W.; Tan, J.; Hou, Y.; Stauffer, S.; Li, L.; Pieper, A.A.; Cummings, J.; et al. Endophenotype-Based in Silico Network Medicine Discovery Combined with Insurance Record Data Mining Identifies Sildenafil as a Candidate Drug for Alzheimer’s Disease. Nat. Aging 2021, 1, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Lu, H.; Liu, P.; Li, Y.; Ravi, H.; Peng, S.L.; Diaz-Arrastia, R.; Devous, M.D.; Womack, K.B. Sildenafil Improves Vascular and Metabolic Function in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1351–1364. [Google Scholar] [CrossRef]
- Samudra, N.; Motes, M.; Lu, H.; Sheng, M.; Diaz-Arrastia, R.; Devous, M.; Hart, J.; Womack, K.B. A Pilot Study of Changes in Medial Temporal Lobe Fractional Amplitude of Low Frequency Fluctuations after Sildenafil Administration in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2019, 70, 163–170. [Google Scholar] [CrossRef]
- Thomas Forgue, S.; Patterson, B.E.; Bedding, A.W.; Payne, C.D.; Phillips, D.L.; Wrishko, R.E.; Mitchell, M.I. Tadalafil Pharmacokinetics in Healthy Subjects. Br. J. Clin. Pharmacol. 2005, 61, 280–288. [Google Scholar] [CrossRef]
- García-Barroso, C.; Ricobaraza, A.; Pascual-Lucas, M.; Unceta, N.; Rico, A.J.; Goicolea, M.A.; Sallés, J.; Lanciego, J.L.; Oyarzabal, J.; Franco, R.; et al. Tadalafil Crosses the Blood–Brain Barrier and Reverses Cognitive Dysfunction in a Mouse Model of AD. Neuropharmacology 2013, 64, 114–123. [Google Scholar] [CrossRef]
- Salem, M.A.; Budzyńska, B.; Kowalczyk, J.; El Sayed, N.S.; Mansour, S.M. Tadalafil and Bergapten Mitigate Streptozotocin-Induced Sporadic Alzheimer’s Disease in Mice via Modulating Neuroinflamm., PI3K/Akt, Wnt/β-Catenin, AMPK/MTOR Signaling Pathways. Toxicol. Appl. Pharmacol. 2021, 429, 115697. [Google Scholar] [CrossRef]
- Daily, K.P.; Amer, A. Microglial Autophagy-Mediated Clearance of Amyloid-Beta Plaques Is Dysfunctional in Alzheimer’s Disease Mice. Alzheimer’s Dement. 2020, 16, e044120. [Google Scholar] [CrossRef]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive Involvement of Autophagy in Alzheimer Disease: An Immuno-Electron Microscopy Study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef]
- Orhan, I.E.; Rauf, A.; Saleem, M.; Khalil, A.A. Natural Molecules as Talented Inhibitors of Nucleotide Pyrophosphatases/ Phosphodiesterases (PDEs). Curr. Top. Med. Chem. 2021, 22, 209–228. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Gao, J.; Liu, Y.; Shi, J.; Gong, Q. Icariside II, a Phosphodiesterase-5 Inhibitor, Attenuates Beta-Amyloid-Induced Cognitive Deficits via BDNF/TrkB/CREB Signaling. Cell. Physiol. Biochem. 2018, 49, 1010–1025. [Google Scholar] [CrossRef]
- Jin, F.; Gong, Q.-H.; Xu, Y.-S.; Wang, L.-N.; Jin, H.; Li, F.; Li, L.-S.; Ma, Y.-M.; Shi, J.-S. Icariin, a Phoshphodiesterase-5 Inhibitor, Improves Learning and Memory in APP/PS1 Transgenic Mice by Stimulation of NO/CGMP Signalling. Int. J. Neuropsychopharmacol. 2014, 17, 871–881. [Google Scholar] [CrossRef]
- Correia, S.S.; Iyengar, R.R.; Germano, P.; Tang, K.; Bernier, S.G.; Schwartzkopf, C.D.; Tobin, J.; Lee, T.W.H.; Liu, G.; Jacobson, S.; et al. The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CY6463 Reveals Its Therapeutic Potential in Neurodegenerative Diseases. Front. Pharmacol. 2021, 12, 656561. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, M.; Mullershausen, F.; Friebe, A.; Jäger, R.; Russwurm, C.; Koesling, D. Design of Fluorescence Resonance Energy Transfer (FRET)-Based CGMP Indicators: A Systematic Approach. Biochem. J. 2007, 407, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Betolngar, D.B.; Mota, É.; Fabritius, A.; Nielsen, J.; Hougaard, C.; Christoffersen, C.T.; Yang, J.; Kehler, J.; Griesbeck, O.; Castro, L.R.V.; et al. Phosphodiesterase 1 Bridges Glutamate Inputs with NO-and Dopamine-Induced Cyclic Nucleotide Signals in the Striatum. Cereb. Cortex 2019, 29, 5022–5036. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B.K.; Kerlin, A.M.; Hasseman, J.P.; Tsegaye, G.; Tsang, A.; Wong, A.; Patel, R.; et al. High-Performance Calcium Sensors for Imaging Activity in Neuronal Populations and Microcompartments. Nat. Methods 2019, 16, 649–657. [Google Scholar] [CrossRef]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical Role of Soluble Amyloid-β for Early Hippocampal Hyperactivity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef]
- Grienberger, C.; Rochefort, N.L.; Adelsberger, H.; Henning, H.A.; Hill, D.N.; Reichwald, J.; Staufenbiel, M.; Konnerth, A. Staged Decline of Neuronal Function in Vivo in an Animal Model of Alzheimer’s Disease. Nat. Commun. 2012, 3, 774. [Google Scholar] [CrossRef]
- O’Brien, J.L.; O’Keefe, K.M.; Laviolette, P.S.; Deluca, A.N.; Blacker, D.; Dickerson, B.C.; Sperling, R.A. Longitudinal FMRI in Elderly Reveals Loss of Hippocampal Activation with Clinical Decline. Neurology 2010, 74, 1969. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehle, A.; Garaschuk, O. The Interplay between cGMP and Calcium Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 7048. https://doi.org/10.3390/ijms23137048
Jehle A, Garaschuk O. The Interplay between cGMP and Calcium Signaling in Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(13):7048. https://doi.org/10.3390/ijms23137048
Chicago/Turabian StyleJehle, Aileen, and Olga Garaschuk. 2022. "The Interplay between cGMP and Calcium Signaling in Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 13: 7048. https://doi.org/10.3390/ijms23137048
APA StyleJehle, A., & Garaschuk, O. (2022). The Interplay between cGMP and Calcium Signaling in Alzheimer’s Disease. International Journal of Molecular Sciences, 23(13), 7048. https://doi.org/10.3390/ijms23137048





