Abstract
Fusarium head blight (Fhb), powdery mildew, and stripe rust are major wheat diseases globally. Aegilops geniculata Roth (UgUgMgMg, 2n = 4x = 28), a wild relative of common wheat, is valuable germplasm of disease resistance for wheat improvement and breeding. Here, we report the development and characterization of two substitution accessions with high resistance to powdery mildew, stripe rust and Fhb (W623 and W637) derived from hybrid progenies between Ae. geniculata and hexaploid wheat Chinese Spring (CS). Fluorescence in situ hybridization (FISH), Genomic in situ hybridizations (GISH), and sequential FISH-GISH studies indicated that the two substitution lines possess 40 wheat chromosomes and 2 Ae. geniculata chromosomes. Furthermore, compared that the wheat addition line parent W166, the 2 alien chromosomes from W623 and W637 belong to the 7Mg chromosomes of Ae. geniculata via sequential FISH-GISH and molecular marker analysis. Nullisomic-tetrasomic analysis for homoeologous group-7 of wheat and FISH revealed that the common wheat chromosomes 7A and 7B were replaced in W623 and W637, respectively. Consequently, lines W623, in which wheat chromosomes 7A were replaced by a pair of Ae. geniculata 7Mg chromosomes, and W637, which chromosomes 7B were substituted by chromosomes 7Mg, with resistance to Fhb, powdery mildew, and stripe rust. This study has determined that the chromosome 7Mg from Ae. geniculata exists genes resistant to Fhb and powdery mildew.
1. Introduction
Fusarium head blight (Fhb), mainly caused by the fungus Fusarium graminearum Schwabe [1], is one of the most destructive diseases of wheat, barley (Hordeum vulgare), and other-grain cereals in many areas of the world [2]. Fhb causes seriousyield losses and grain quality decreases due to Fusarium toxins contaminate cereal products, which are harmful to humans and animals [3]. In recent years, the wheat regions in the middle and lower reaches of Yangtze River and Huang-Huai wheat region in China have been frequently and suffering from Fhb due to climate change and straw returning under the wheat- maize rotation system [4]. To date, sources of Fhb resistance used wheat cultivars can be traced to few parents [5]. Resistance to Fhb in wheat is controlled by polygenes, and symptom expression is often modulated by environmental factors [6]. Currently, hundreds of QTL totally identified in a number of germplasms, which were distributed on all 21 wheat chromosomes [7,8]. Fine mapping of five major-effort QTL identified in common wheat, Fhb1 [9,10], Fhb4 [11], Fhb5 [12] and Fhb7 [13] which were widely distributed. Only a few sources of resistance are likely to cause widespread disease epidemics upon that resistance is lost. The development and characterization of new resistance sources will provide broad sources of disease resistance. Therefore, the development and identification of wild relatives of wheat-resistant materials in breeding research are quite meaningful. However, little research has been reported on the Fhb resistance of Ae. geniculata.
The genomic composition of Ae. geniculata is UgUgMgMg, in which the Ug genome was derived from the Ae. umbellulate Zhuk (2n = 2x = 14, UU), and the Mg genome originated from the Ae. comosa Sm. In Sibth. & Sm. (2n = 2x = 14, MM) [14]. Numerous researches on wild hybridization of Ae. geniculata and common wheat have been reported, and mass valuable genes have been exploited and utilized. Chromosomes 1Ug from common wheat-Ae. geniculata addition line improves the dough rheological properties [15]. thereby improving the quality of wheat, indicating that the chromosome 1Ug can advance crop quality. In addition, there are several genes involved in resistance to various diseases have been transferred from that species into common wheat. In 2007, powdery mildew resistance gene Pm29 was derived from the wheat-Ae. geniculata disomic addition line Poros wheat [16], and the stem rust resistance gene Sr53 was derived from the wheat-Ae. geniculata 5Mg translocation line [17]. Wang reported on the research of Ae. geniculata 7Mg addition and substitution lines of resistance to powdery mildew [14,18].
In the past few decades, due to the domestication and origin of common wheat along with artificial selection, the genetic diversity of wheat cultivars has been increasingly narrowed [19], which introduction of wild relatives is one of the most important ways for broadening the genetic variation. Distance hybridization is one of the most important ways for broadening the genetic variation and breeding new wheat cultivars. Ciferri et al. [20] reported the first interspecific wheat hybrid experiments in 1806. After that, the wheat breeding of distant hybridization involving wild relatives of common wheat has gradually attracted great attention and developed to a certain extent [21]. The creation of intermediate materials for wheat and wild relatives is a crucial step in wheat chromosome engineering breeding. The wheat-Thinopyrum ponticum (2n = 10x = 70) partial amphiploids were created in the 1970s via distant hybridization [22], and then, Xiaoyan 6 pedigree-related cultivars were produced [23], which made a huge contribution to Chinese varieties. In this study, we obtained two key materials substitution lines in the breeding process through distant hybridization between common wheat and Ae. geniculata, which provided important resources for chromosome engineering breeding.
Powdery mildew, which is a recurring disease caused by Blumeria graminis f. sp. tritici (Bgt) and is one of the most destructive diseases in the wheat-growing areas in the world [24]. Wheat stripe rust (or yellow rust), caused by Puccinia striiformis f. sp. tritici, is one of the considerable diseases of wheat worldwide [25]. In the meantime, stripe rust and powdery mildew have been widespread in the main wheat-producing regions of China [26]. Due to rapid climate and environmental change, it is really necessary to obtain and utilize new germplasms with resistance genes to develop resistant wheat cultivars [27]. With climate change, the physiological races of the cultivars will be easily overcome by the new races, losing the resistance [28,29], therefore, developing and deploying resistant cultivars is the most economical and safe measure.
In this study, two novel wheat-Ae. geniculata 7Mg substitution lines W623 and W637 were produced through hybridization of between the Abbondanza nullisomic lines and wheat-Ae. geniculata 7Mg disomic addition line W166. The researchers used a combination of cytogenetic characteristics, functional molecular markers, in situ hybridizations, and disease resistance to identify the chromosome composition and genetic characteristics of disease resistance of the two substitution lines, which can be used as intermediate materials for wheat chromosome engineering breeding.
2. Results
2.1. Cytogenetic Analysis of W623 and W637
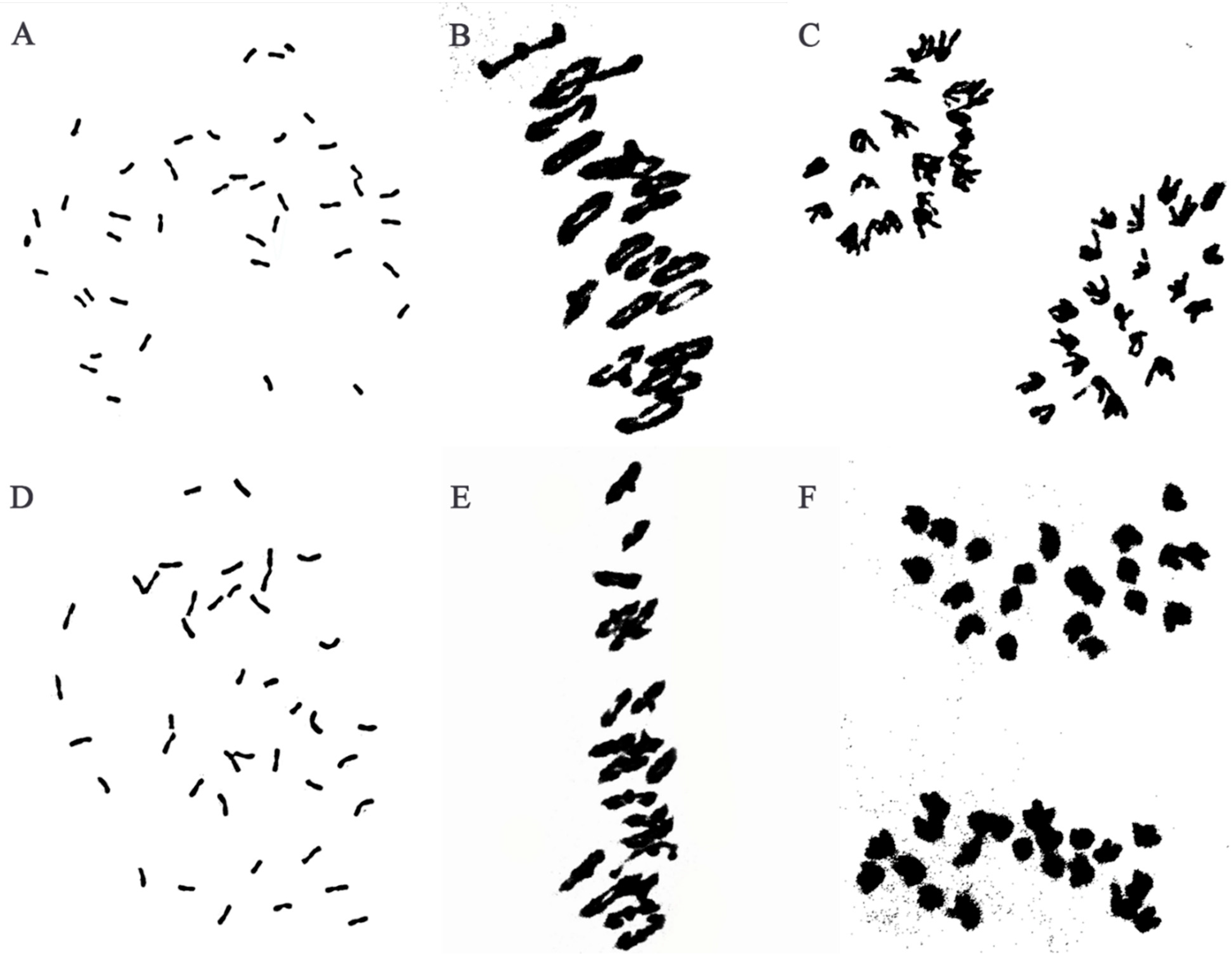
Cytogenetic analysis revealed that about 97% of metaphase mitotic root tip cells (RTCs) of W623 (Figure 1A) and W637 (Figure 1D) indicated 42 chromosomes from different plants. During the meiotic metaphase and anaphase in PMCs of W623 (Figure 1B) and W637 (Figure 1E) had a chromosome configuration of 2n = 21II, univalent, trivalent, or quadrivalent chromosomes were not observed. Meanwhile, all chromosomes were evenly distributed on both sides of the equatorial plate, no chromosome was legged at meiotic anaphase I of two lines (Figure 1C,F). Therefore, all results that line W623 and W637 exhibited highly cytological stability.
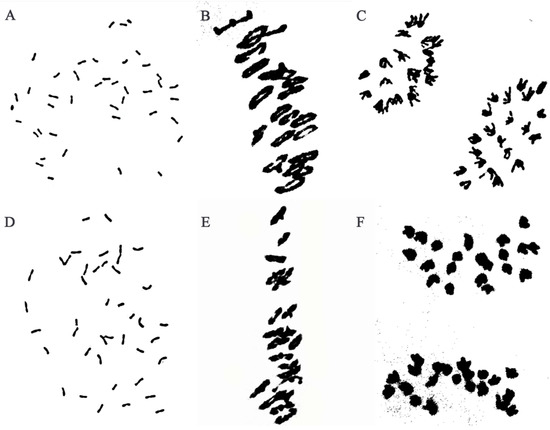
Figure 1.
Cytogenetic analysis of W623 and W637. Analysis of root tip cells in W623 (A) and W637 (D) at mitotic metaphase: 2n = 42. Pollen mother cell chromosomal configurations of W623 (B) and W637 (E) at meiotic metaphase I: 2n = 21II. Pollen mother cell chromosomal configurations of W623 (C) and W637 (F) at anaphase I: 2n = 21I + 21I.
2.2. Sequential FISH-GISH Analysis
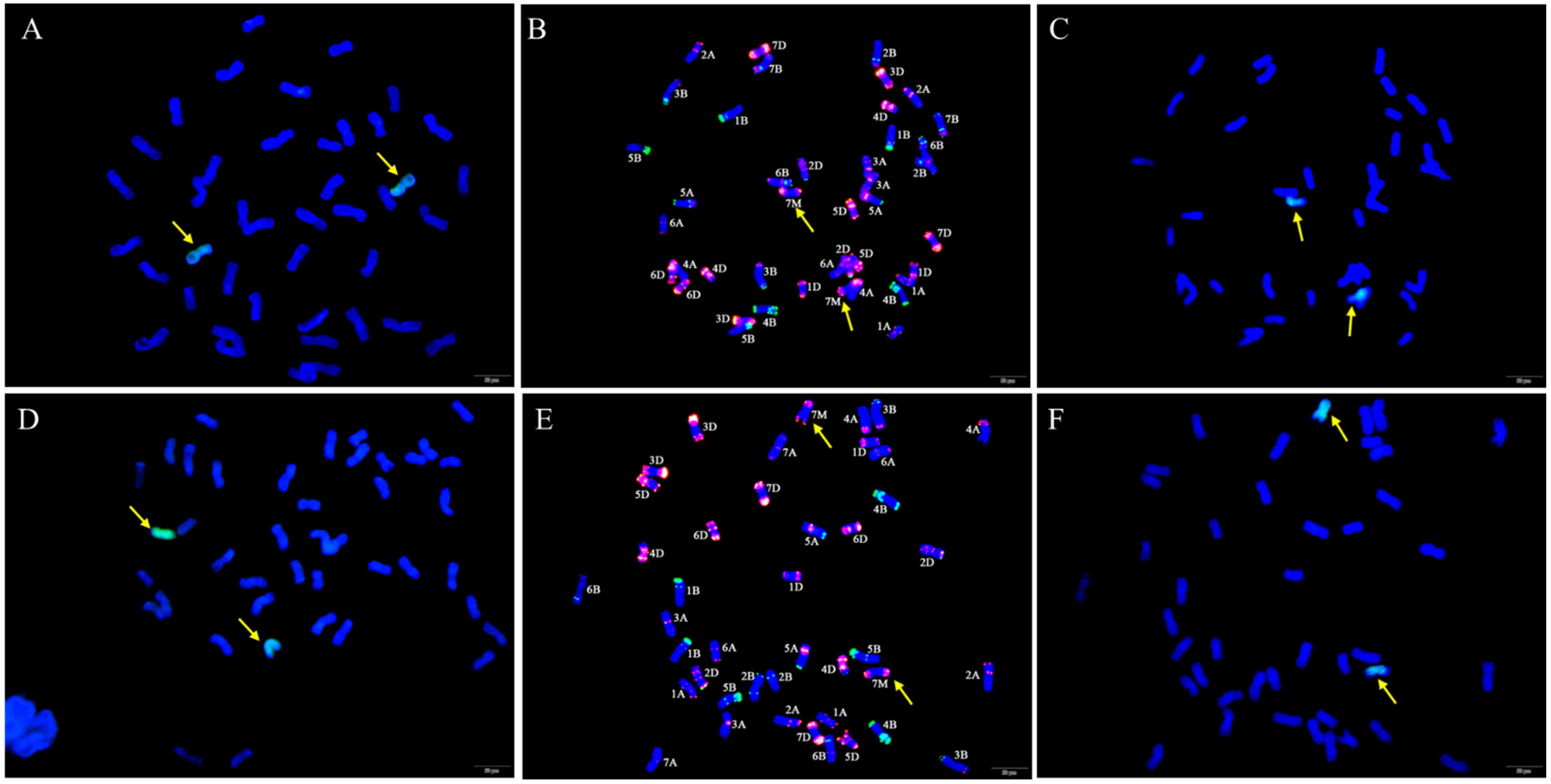
GISH and FISH analyses were performed to examine the chromosome composition of W623 and W637 (Figure 2). When Ae. geniculata total genomic DNA was used as the labeled probe and sheared CS genomic DNA as the blocker, we observed 40 wheat chromosomes plus two Ae. geniculata chromosomes in both W623 and W637 (Figure 2A,D). To further determine the identities of the wheat chromosomes involved in the substitutions, sequential FISH-GISH analysis was performed on the same slide. FISH analysis was primarily performed using two repetitive oligonucleotide probes, Oligo-pSc119.2 (green) and Oligo-pTa535 (red) (Figure 2B,E), and then GISH analysis was carried out (Figure 2C,F). The sequential FISH-GISH screening of mitotic cell divisions showed that W623 contained 40 chromosomes of wheat, two chromosomes, and lost two 7A chromosomes of wheat (Figure 2B). As well as, the sequential FISH-GISH results indicated that W637 also completed 40 chromosomes of wheat, plus two Ae. geniculata chromosomes, but lost two 7B chromosomes of wheat (Figure 2E).
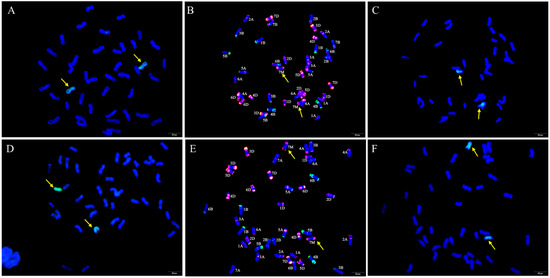
Figure 2.
GISH and sequential FISH-GISH analyses of W623 and W637. GISH of a mitotic metaphase cell of W623 (A) and W637 (D) Ae. geniculata genomic DNA (green) as probe and CS genomic DNA as a blocker. FISH of the mitotic metaphase cells of W623 (B) and W637 (E) using Oligo-pSc119.2 (green) and Oligo-pTa535 (red) were used as probes. GISH in sequential FISH-GISH of W623 (C) and W637 (F). The yellow arrows indicate Ae. geniculata chromosomes in W623 and W637.
To further determine whether the identity of the alien chromosomes of W623 and W637 were 7Mg, FISH of the wheat-Ae. geniculata 7Mg addition line W166 (2n = 44) was performed using Oligo-pSc119.2 and Oligo-pTa535 probes, and the special GISH of W623 using was also performed using Ae. geniculata total genomic DNA and Oligo-pTa535 as probes (Figure 3). That result showed that the red signal of pTa535 appeared at the end of two 7Mg chromosomes arms, which was the same as the FISH pattern of alien chromosomes in W623 and W637.
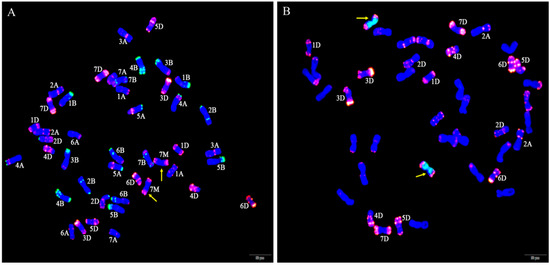
Figure 3.
FISH and special GISH analysis. (A) Oligo-pSc119.2 (green) and Oligo-pTa535 (red) as probes used in W166. (B) Ae. geniculata genomic DNA (green), Oligo-pTa535 (red) as probes, and shared CS genomic DNA as a blocker in W623. The yellow arrows indicate Ae. geniculata chromosomes.
2.3. Molecular Markers Analysis
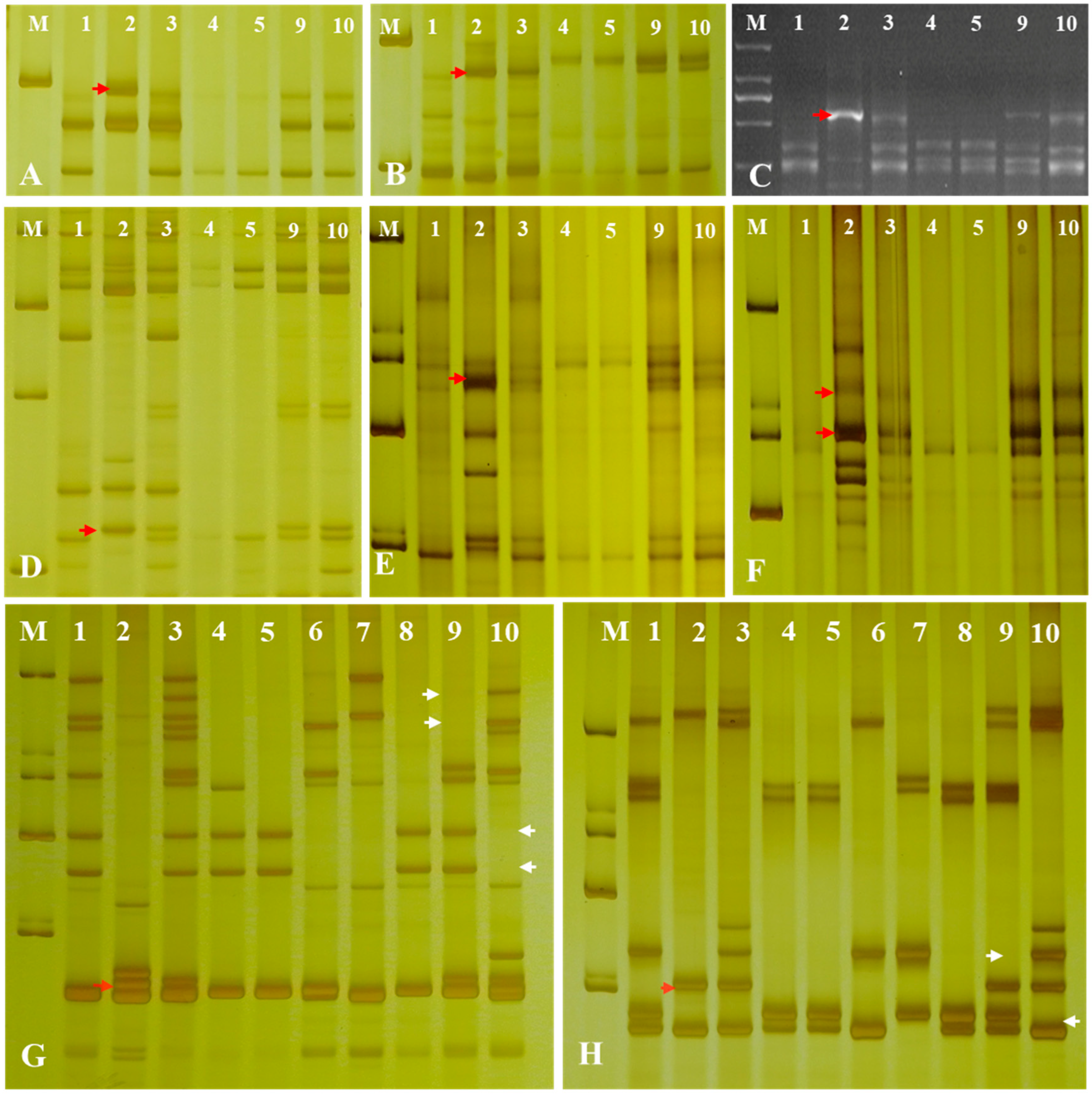
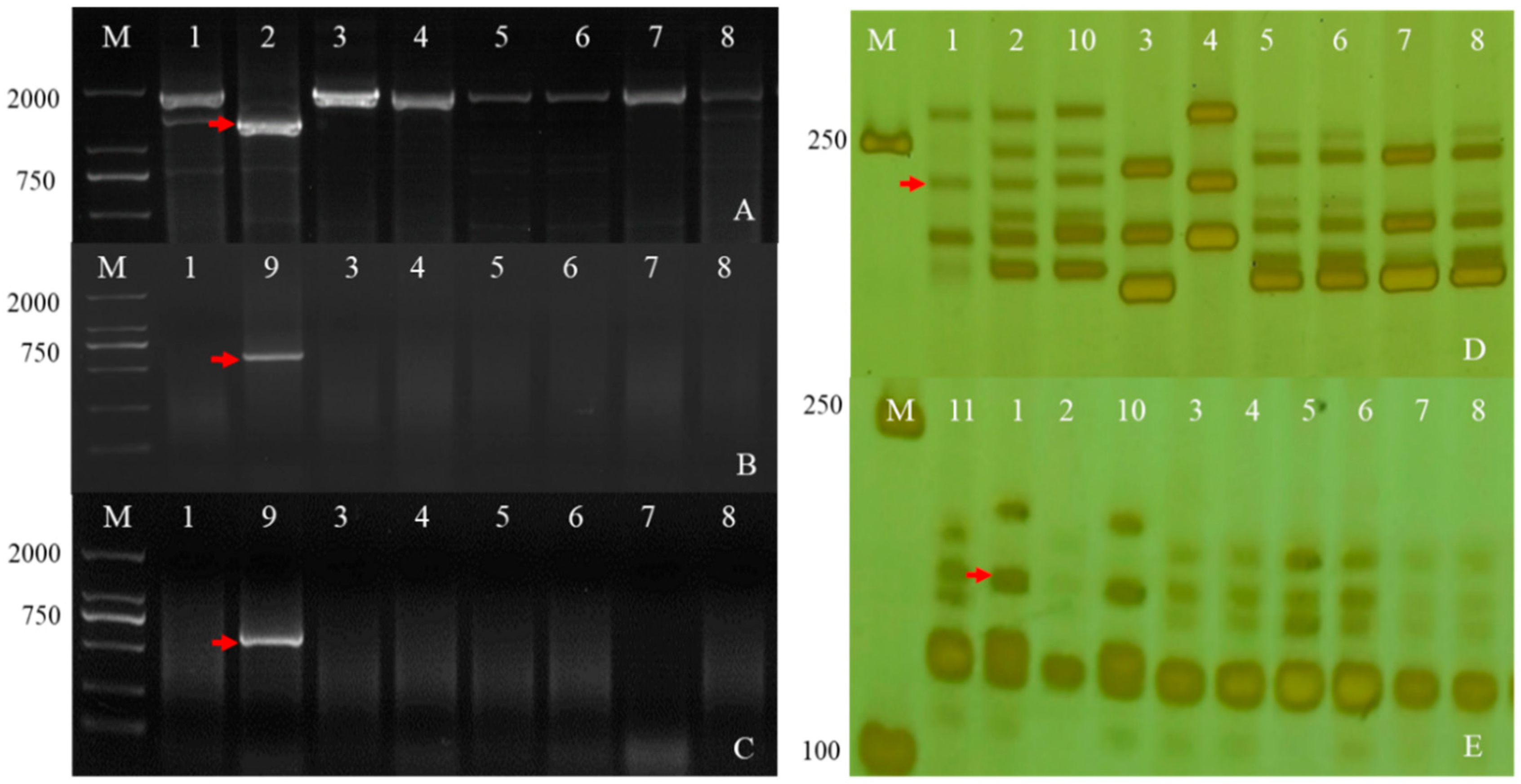
To further determine the chromosomal composition of the two substitution lines, molecular markers analysis was performed. Two substitution lines were analyzed by employing 72 EST-STS markers and 135 PLUG markers on seven different homologous groups in wheat to clarify the homologous group relationships of the alien chromosomes (Figure 4). In the present study, five PLUG markers (TNAC1782-TaqI, TNAC1845-TaqI, TNAC1929-TaqI/HaeIII, TNAC1888-HaeIII, and TNAC1811-HaeIII) (Table 1) on wheat homoeologous group 7 chromosomes generated Ae. geniculata specific bands in four Ae. geniculata, the wheat-Ae. geniculata addition line W166, W623, and W637. In contrast, no specific fragment from CS, as well as Abbondanza nullisomic line 7A and Abbondanza nullisomic line 7B (Figure 4A–F). This indicated that W623 and W637 had a specific fragment of Ae. geniculata.
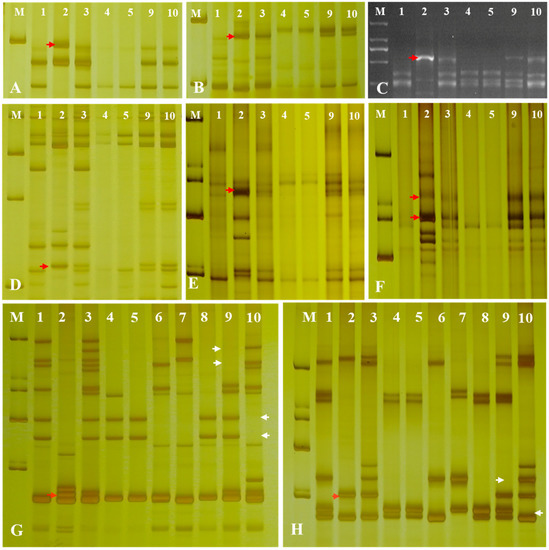
Figure 4.
Molecular markers analysis of W623 and W637. M, marker; 1, Triticum aestivum cv. Chinese Spring; 2, Ae. geniculata; 3, W166; 4, Abbondanza nullisomic line 7A; Lane 5, Abbondanza nullisomic line 7B; 6, CSN7AT7D; 7, CSN7DT7B; 8, CSN7BT7A; 9, W623; 10, W637; (A) TANC1782-TaqI. (B) TANC1845-TaqI. (C) TANC1811-HaeIII. (D) TANC1888- HaeIII. (E) TANC1929-TaqI. (F) TANC1929- HaeIII. (G) BE426692. (H) BE637663. The red arrow indicated an Ae. geniculata-specific bands and the white arrow directed CS and the nulli-tetrasomic specific bands.

Table 1.
EST-STS and PLUG markers used in this study of W623 and W637.
Nulli-tetrasomic analysis was implemented to analyze the lost pair of chromosomes in W623 and W637 andCS nulli-tetrasomic lines N7AT7B, N7BT7D, and N7DT7A. As shown in Figure 4F,G, two EST-STS markers (BE426692 and BE637663), which could specifically amplify fragments from Ae. geniculata, W166, W623, and W637, did not amplify specific bands from other lines, as the white arrow indicated. In addition, compared with the bands of CS and nulli-tetrasomic lines, it was shown that the chromosomes 7A and 7B specific bands were significantly absent in W623 and W637, respectively, as the red arrow indicated. Molecular markers and nulli-tetrasomic analyses showed that the two substitution lines were missing a pair of 7A and 7B chromosomes, respectively, and added a pair of chromosomes 7Mg in Ae. geniculata.
2.4. Resistance against F. graminearum
To determine its function in resistance against F. graminearum, two substitution lines W623 and W637 as well as their parents Ae. geniculata, wheat-Ae. geniculata 7Mg addition line W166, and CS were evaluated in the field (Figure 5A and Figure S2). W623, W637, W166, and Ae. geniculata showed a lower Fhb infected spike ratio due to only one spikelet infection compared with their parent CS, indicating that the resistance gene of two substitution lines to Fhb was derived from the ancestral parent Ae. geniculata. On the contrary, another ancestral parent, Chinese Spring, was not resistant to Fhb at all because all spikelets were infected and died (Figure 5A1).
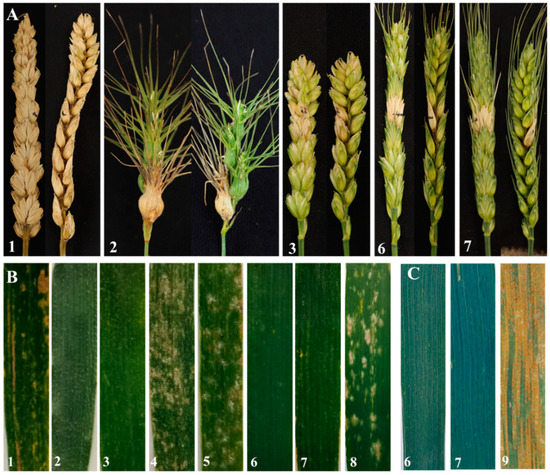
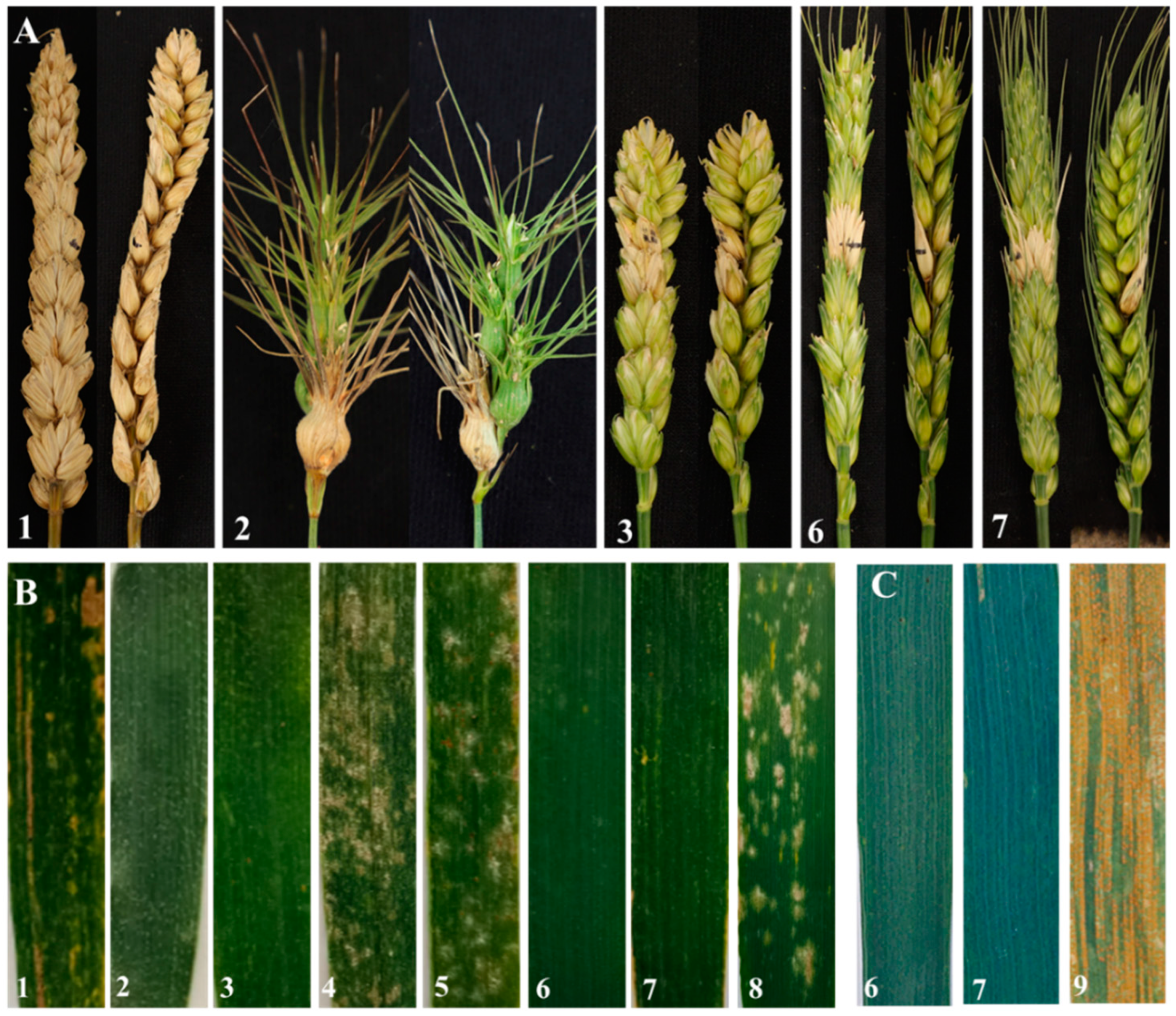
Figure 5.
Disease reaction of two lines and their parents. (A) Spikes infected with Fusarium graminearum. (B) Leaves infected with Blumeria graminis at the adult stage. (C) Leaves infected with Puccinia striiformis at the adult stage. 1, Triticum aestivum cv. Chinese Spring; 2, Ae. geniculata; 3, wheat-Ae. geniculata 7Mg addition line W166; 4, Abbondanza nullisomic line 7A; 5, Abbondanza nullisomic line 7B; 6, W623; 7, W637; 8, Shaanyou 225; 9, Huixianhong.
Because of the dependence of QTL effects on the environment, verification and marker saturation of detected QTL are always necessary for breeding and disease resistance research. To achieve this goal, we used functional markers or linkage markers with Fhb resistance gene or QTLs Fhb1, Fhb4, Fhb5, and Fhb7 of two lines, and their parents were detected (Figure 6), for example, an Fhb gene linked to Xgwm149 on chromosome 4BS. As shown in Figure 7, Ae. geniculata and its derived offspring don’t carry the above Table 2 resistance QTL. In order to validate whether the GST gene exists in the wheat background, primers (F7-1 and F7-7) were designed using its specific fragment and its function was clarified. The sequence alignment results are shown in Figure S3. The above results show that the plant material containing the 7Mg chromosome has the characteristics of resistance to Fhb and does not contain the QTLs Fhb1, Fhb4, Fhb5, and Fhb7, suggesting that there may be a new resistance QTL of Fhb in these materials, which was derived from the 7Mg chromosome of Ae. geniculata.
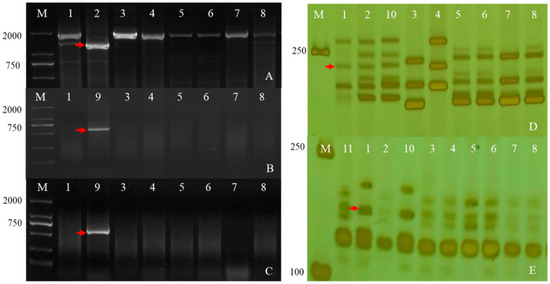
Figure 6.
Detection Fhb1 (A), Fhb7 (B,C), Fhb5 (D) and Fhb4 (E) using the markers TaHRC-STS, F7-1, F7-7, Xgwm304 and Xgwm149. The red arrows indicate Fhb-specific bands. (M). DL2000. (1). CS. (2). Sumai3. (3). SY159. (4). W166. (5). Abbondanza nullisomic line 7A. (6). Abbondanza nullisomic line 7B. (7). W623. (8). W637. (9). Th. Elongatum. (10). Wangshuibai. (11). Aikang58. (a Chinese wheat cultivar).
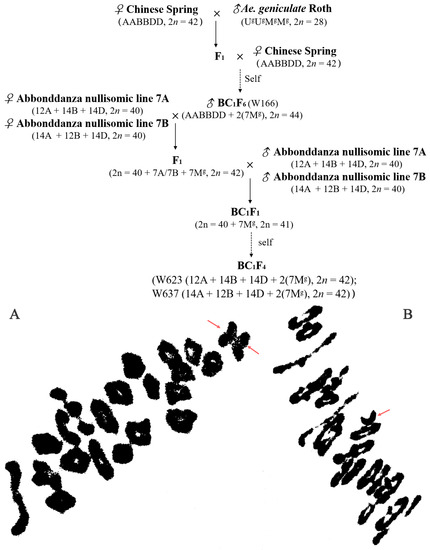
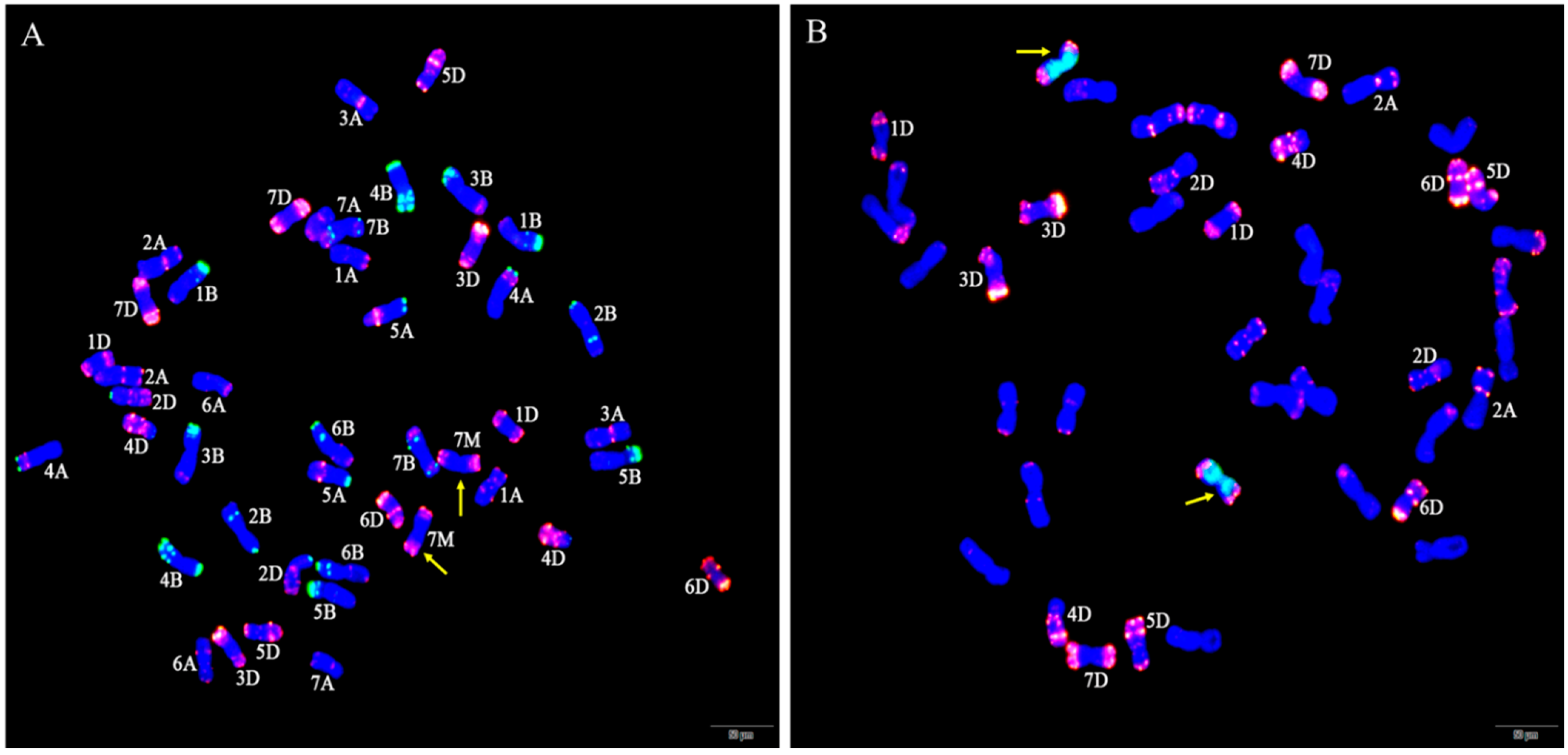
Figure 7.
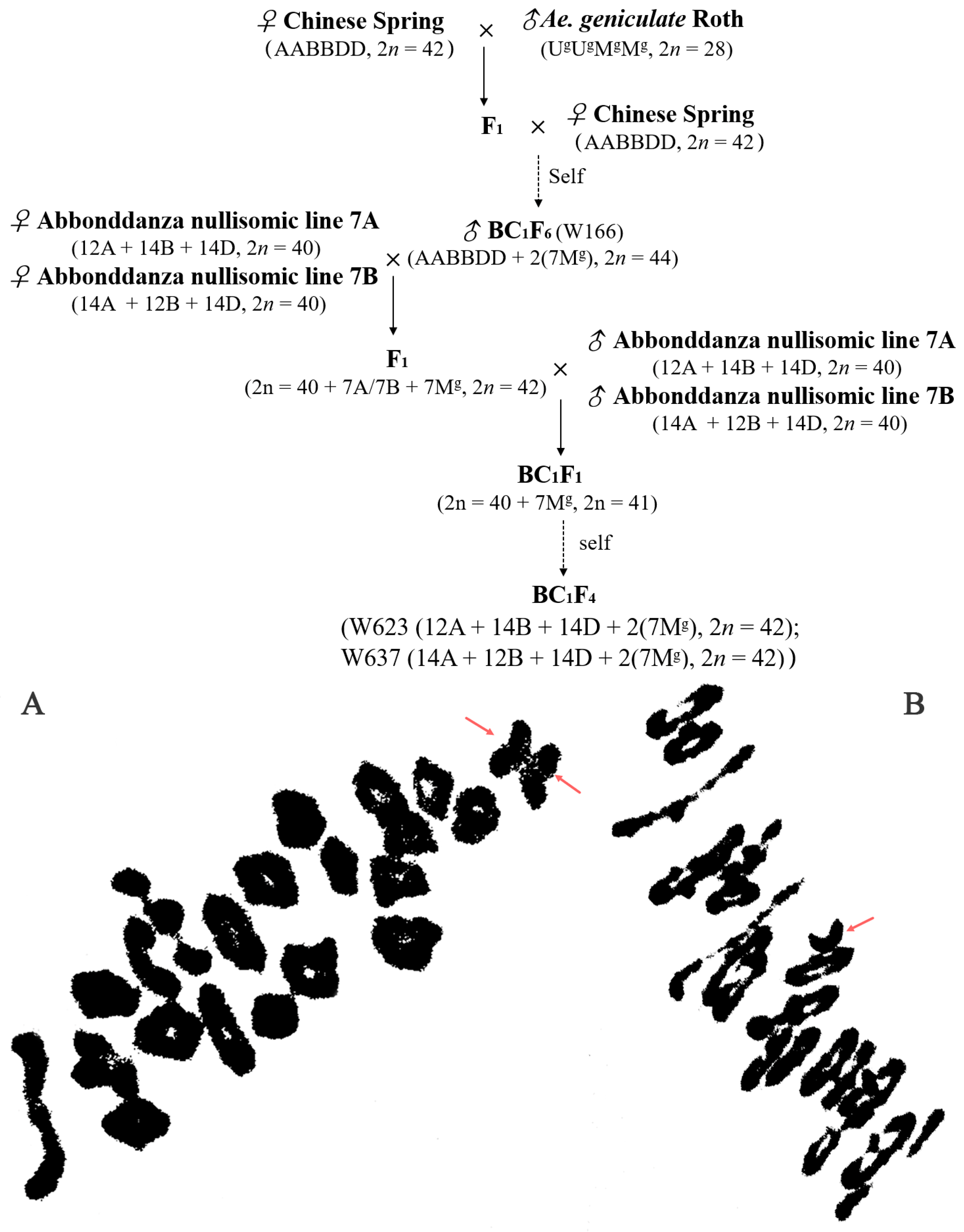
A crossing program showing strategies employed to generate wheat-Ae. geniculata substitution lines and cytogenetic analysis of interspecific hybrids. (A). pollen mother cell chromosomal configurations of F1 originated from the crossing between Abbondanza nullisomic lines and W166 at meiotic metaphase I: 2n = 20II + I + I (B). pollen mother cell chromosomal configurations of BC1F1 originated from the crossing between F1 and Abbondanza nullisomic lines at meiotic metaphase I: 2n = 20II + I. The red arrow indicated univalent chromosomes.
2.5. Powdery Mildew and Stripe Rust Resistance
The reactions to powdery mildew in W623 and W637 were evaluated at the seedling and adult plant stages using the wheat cultivar Shaanyou 225 as a control. At the seedling and the adult plant stages, Ae. geniculata, wheat-Ae. geniculata 7Mg addition line W166, and two substitution lines were immune to the powdery mildew race E09 isolate with an IT score of 0 (Figure 5B and Figure S1). In contrast, the Abbondanza nullisomic line, as well as susceptible control Shaanyou 225, were highly susceptible with an IT score of 4, and CS was moderately susceptible to E09 isolate with an IT score of 3. The results suggested that two substitution lines W623 and W637 had powdery mildew resistance in the whole stage, and these powdery mildew resistance genes were derived from the Ae. geniculata.
For testing the stripe rust reaction at the adult stage, W623, W637, and control variety Huixianhong were inoculated a mixture of Pst races CYR32, CYR33, and CYR34. In the field test, the stripe rust developed to a sufficient level to judge the disease resistance of the experimental material. Two lines W623 and W637 were nearly immune to stripe rust, meantime, the susceptible control Huixianhong was considered to be susceptible (Figure 5C).
3. Discussion
Since the mutual compensation between the alien chromosomes from wild relatives and individual chromosomes of the parents often produce the substitution lines via the distant hybridization [26]. To date, excellent phenotypic traits and genotypes between wheat and wild relatives have been successfully produced in current wheat breeding [30]. Creation of a series of tool materials such as substation lines, addition lines, and translocation lines is a critical step in introducing the excellent genes of the wild relative into cultivated wheat. After that, the exogenous fragments are fragmented by hybridization or radiation to produce the introgression lines applied to production. In this research, in situ hybridization analysis confirmed that W623 and W637 are stable wheat-Ae. geniculata substitution lines that may be useful for breeding new wheat varieties with improved disease resistance.
Research shows that Aegilops geniculata Roth, as members of the tertiary gene pool of wheat [31], harbors many excellent traits for wheat breeding. So far, T.aestivum-Ae. geniculata progeny with excellent traits has been reported in large numbers, involving two genomes of the Ug and Mg genome. Firebe et al. [32] first carried out the complete set of T. aestivum-Ae. geniculata chromosome addition lines in 1999. Since then, the studies of the progeny of T. aestivum-Ae. geniculata became detailed and specific. Chromosome 4Mg from wheat-Ae. geniculata 4Mg (4B) substitution line can induce the formation of supernumerary florets. In addition to improving crop quality, Ae. geniculata has been widely studied in carrying disease resistance genes. Stoilova et al. [33] reported that chromosome 6U from wheat- Ae. geniculata addition line carrying powdery mildew resistance. The wheat-Ae. geniculata introgression T5DL 5DS-5MgS (0.95), with stripe rust resistance gene Yr40 and leaf rust resistance gene Lr57, is a source of resistance in Kansas and India [34]. For all that, although many pieces of researches on disease resistance have been reported, there is no report on the study of FHB in offspring from distant hybridization between the common wheat and Ae. geniculata. In this study, we develop and identify two wheat- Ae. geniculata 7Mg (7A) and 7Mg (7B) substitution lines carrying disease resistance to powdery mildew and FHB. Compared with previous studies, we believed that chromosomes 7Mg from Ae. geniculata not only have disease resistance to powdery mildew but also possess good resistance to Fusarium head blight. At the same time, the Ae. geniculata substitution line W623 and the disomic substitution line W637, both of which are highly resistant to stripe rust at the adult stage, since no identification of yellow rust at the adult stage was carried out on their parents, the sources of resistance to stripe rust was not clear. We do not know whether the stripe rust accompanied with the 7Mg substitution lines was related to the Mg genome from wheat-Ae. geniculata. However, it is undeniable that two substitution lines have high-level resistance to the three main diseases of wheat at the same time, which are rare bridge materials in chromosome engineering breeding.
Fusarium graminearum is a caused agent of Fusarium head blight (Fhb) [35] and produces the mycotoxin deoxynivalenol (DON) in grain, which is harmful to human and animal health [36]. It is well known that there are very few sources of parents of Fhb resistance in wheat breeding, mainly including Sumai 3 and its derivatives, Wangshuibai and Wuhan 1 [8,26]. Development and identification of Fhb resistance genes of wheat relatives and introduction of resistance genes to common wheat by chromosome engineering breeding are important methods to solve the shortage of resistance materials. Zhao et al. [37] reported that a wheat-Leymus mollis (NsNsXmXm, 2n = 4x = 28) double substitution line with resistance to yellow rust and Fhb. Wheat-Leymus racemosus alien introgression lines with Fhb resistance were described in 2008 [38]. Two wheat-Elymus repens (StStStStHH, 2n = 6x = 42) lines have determined that the chromosome 3St of E. repens harbors gene(s) highly resistant to Fhb [39]. And Fhb7 is a QTL introduced from Thinopyrum elongatum (EE, 2n = 14) was reported in 2020 [13]. However, few studies have been conducted on the pedigrees of the wheat-Ae. geniculata. Herein, we confirmed the resistance of Ae. geniculata, the wheat-Ae. geniculata 7Mg addition line, the wheat- Ae. geniculata 7Mg (7A) substitution line, and wheat-Ae. geniculata 7Mg (7B) disomic substitution line to Fhb. In consequence, we determined the resistance of wild relatives of Aegilops geniculata Roth, and enriched the sources of resistance to Fhb, and also identified the resistance of the 7Mg chromosome of Ae. geniculata.
Distant hybridization is important to develop new excellent germplasm and broaden the genetic base of cultivar wheat. In early studies, the substitution lines, translocation lines, addition lines, and introgression lines in research and breeding are generally produced by distant hybridization between the hexaploid wheat and wild relatives by crop breeders [32,40]. Compared with the substitution and addition lines are more easily to be used in breeding because they carry fewer alien chromosome fragments. To produce wheat-Ae. geniculata substitution lines of homologous group-7 in wheat, we used the wheat deletion lines of group-7 and the addition 7Mg wheat-Ae. geniculata to cross. In the initial stage of this study, we used the wheat-Ae. geniculata 7Mg disomic addition line W166 and the Abbondanza nullisomic lines of (7A and 7B) for hybridization, and purposefully substituted a pair of common wheat 7A and 7B chromosomes in the addition line to produce the substitution lines. In this process, we used cytological and disease resistance to powdery mildew analysis to select the substitution lines of the hybridization between the deletion lines and W166. To explore whether there were other excellent traits in W623 and W637, we inoculated them with the race PH-1, and we finally determined that these two substitution lines have resistance to Fhb.
4. Materials and Methods
4.1. Plant Materials and Development of W623, W637
To generate the substitution lines, the crossing strategy was carried out as illustrated in Figure 1. Chinese Spring (CS) and Ae. geniculata were used as an early parent in this study. Lines W623 and W637 were selected from a progeny of a cross between Abbondanza nullisomic line 7A (genome composition: 12A + 14B + 14D, 2n = 40) and wheat-Ae. geniculata 7Mg addition line W166 (NA0973-5-4-1-2-9-1, AABBDD, 2n = 44) [14], and the Abbondanza nullisomic line 7B (genome composition:14A + 12B + 14D, 2n = 40) and the line W166, respectively. The wheat cultivars Shanyou 225, Huixianhong and Aikang 58 were used as the susceptible control of powdery mildew, stripe rust and FHB. The above-mentioned materials were planted at Yangling, Shaanxi Province, China (108°4′ E, 34°16′ N) during the 2018–2021 growing season.
The wheat-Ae. geniculata 7Mg disomic addition line W166, which has the characteristics of more kernels per spikelet, and higher thousand-kernel weight, as well as disease resistance to powdery mildew, was originally developed and identified from the crosses CS/Ae. geniculata//CS BC1F6 progenies [14]. To produce the wheat-Ae. geniculata 7Mg substitution lines, we crossed W166 with the Abbondanza nullisomic line 7A and Abbondanza nullisomic line 7B, and F1 was backcrossed with Abbondanza nullisomic line 7A and Abbondanza nullisomic line 7B, respectively. The crossing strategy was carried out as illustrated in Figure 1.
Then, identified pollen mother cell chromosomal configurations of F1 derived lines from between the addition line W166 and Abbondanza nullisomic line 7A and Abbondanza nullisomic line 7B, and screened out that plants for harvest and seed retention of the PMCs are 2n = 42 = 20II + I + I (Figure 7A) and resistant to powdery mildew. The seeds selected from F1 plants were bulked and advanced to the next generation. The next year, the pollen mother cell chromosomal configurations of BC1F1 interspecific hybrids were identified and investigated into the number and the configurations of the PMCs are 2n = 41 = 20II + I (Figure 7B) and also resistant to powdery mildew for continuous self-crossing until producing stable substitution lines. Two stable disomic substitution lines W623 and W637, with high resistance to powdery mildew over 2 years of observation, were isolated from 65 W166/2* Abbondanza nullisomic line 7A or Abbondanza nullisomic line 7B BC1F4 progeny (Figure 7).
4.2. Cytological Identification
When the flag leaf of wheat was spread from late Marth to early April, the young spikes were respectively collected and fixed in Carnoy’s solution to perform meiosis analysis, which was treated as described previously [41]. Fresh root tips were sampled from germinating seeds treated with nitrous oxide (N2O) for 2 h and then fixed in 90% glacial acid and stored in 70% v/v ethanol to perform mitosis analysis and chromosome slides preparation [40,42]. The pollen mother cells (PMCs) and the root tip cells (RTCs) were analyzed and photographed with an Olympus BX-43 microscope (Japan).
4.3. GISH and Sequential FISH-GISH
Genomic DNAs were isolated from Ae. geniculata and CS from fresh leaves by a modified CTAB method [43], then Ae. geniculata DNA was labeled fluorescein-12-dUTP (green) by the nick translation method [44,45] and used as a probe. Sheared genomic DNA of CS was used as blocking DNA. GISH analysis was performed according to Han et al. [44] and Gong et al. [39]. Probe labeling and FISH-GISH were performed as described in Tang et al. [46].
4.4. Molecular Markers Analysis and Nulli-Tetrasomic Analysis
DNA was extracted from fresh leaves of W166 [14], W623, W627, Abbondanza nullisomic line 7A, Abbondanza nullisomic line 7B, and CS nulli-tetrasomic lines N7AT7B, N7BT7D, and N7DT7A, using the modified CTAB method, then used as the molecular marker analysis and nulli-tetrasomic analysis. The polymerase chain reaction (PCR)-based landmark unique gene (PLUG) [47] primer pairs were used to determine the alien chromatin of W623 and W627. The expressed sequence tagged sequence site (EST-STS) markers were used to identify the wheat chromatin in both lines. All PCR markers distributed in seven homoeologous groups of wheat were obtained from the Wheat Haplotype Polymorphisms website (https://wheat.pw.usda.gov/ accessed on 12 May 2020), and the details are shown in Table 1. The PCR amplification was conducted as previously described by Wu et al. [48].
4.5. Maintenance and Preparation of Inoculum
The F. graminearum inoculum used in all experiments was “PH-1 (NRRL 31084)”, a major pathogen of cultivated cereals and has completed genome sequencing [49], was kindly provided by Cong Jiang (Northwest A&F University, Yangling, China). Conidia were cultivated in mung bean (MB) liquid medium, and then conidia were harvested from 5-day-old at 25 °C MB and re-suspended to 2.5 × 105 spores per ml of PH-1between the lemma and palea in sterile distilled water (SDW) by blood counting chamber. The cultural conditions and fungal transformation were followed by Jiang et al. [50]. When the fluffy stigma and ovary appear in flowering heads of plants were inoculated with 10 μL of conidium suspensions at the spikelet. The plant inoculation experiments were performed using single-floret inoculation [26]. Inoculated plants were sprayed with and covered for 48 h with a plastic bag to maintain high humidity. Inoculated wheat heads were estimated for diseased spikelets at 18 or 21 dpi to estimate the disease index (number of diseased spikelets per head) [51], in which percentage of diseased spikelets (PDS) was used to measure Fhb resistance. Here PDS of plant material was calculated by dividing the number of spikelets with visible disease symptoms 21 days after the inoculation by the total number of spikelets in these spikes. The mean of the disease index was estimated with data from three independent replicates with at least 10 randomly chosen wheat heads examined in each replicate.
To detect the distribution of Ae. geniculata and two substitution lines in five major-effect QTL in common wheat, Fhb1 [9,10], Fhb4 [11], Fhb5 [12] and Fhb7 [13], several primers were used and optimized to determine the resistance gene for Fhb. Sumai 3 and Wangshuibai were the source of Fhb resistance in the population, which segregated for three known Fhb resistance QTL on Fhb1, Fhb4 and Fhb5. The plants were genotyped using microsatellite markers on chromosome 3BS, 4BL, and 5AS to facilitate selection of Ae. geniculata and their derived offspring for QTL intervals on 3BS, 4BL, and 5AS carrying the Fhb resistance gene (Table 2). TaHRC-STS [52] was known to deletion mutation in Fhb1 and validated in different types of populations, for the TaHRC-STS marker. The fragment of GST (Fhb7) (Gene ID: Tel7E01T1020600.1 https://ngdc.cncb.ac.cn/, accessed on 13 January 2021) on 7EL chromosome was amplified. The fragments amplified from Th. Elongatum (syn. Agropyrom Elongatum or Lophopyrum elongatum) was clone onto the pMD 19-T Vector (TaKaRa Biotech Co., Beijing, China) for sequencing. Thermal cycling included: 94 °C-3 min, 35 cycles of 94 °C-30 s, 56 °C-1 min, 72 °C-7 min. The sequence comparison analysis was carried out using the software DNAMAN.

Table 2.
Primers used to amplify four Fhb genes.
Table 2.
Primers used to amplify four Fhb genes.
| Marker | QTL | Primer Sequence (5′-3′) | Chr. | Tm (°C) | Reference |
|---|---|---|---|---|---|
| TaHRC-STS | Fhb1 | F: ATTCCTACTAGCCGCCTGGT R: ACTGGGGCAAGCAAACATTG | 3BS | 65 | Su et al. [52] |
| Xgwm 149 | Fhb4 | F: CATTGTTTTCTGCCTCTAGCC R: CTAGCATCGAACCTGAACAAG | 4BS | 56 | Xue et al. [11] |
| Xgwm 304 | Fhb5 | F: AGGAAACAGAAATATCGCGG R: AGGACTGTGGGGAATGAATG | 5AL | 56 | Xue et al. [12] |
| F7-1 | Fhb7 | F: AGACTGGCCCTCAACTTCAA R: CGACAATCATGTCCGCATAC | 7EL | 56 | At this article |
| F7-7 | Fhb7 | F: GATGCAGTCCCTCCGAAACA R: ACCGACAATCATGTCCGCAT | 7EL | 55 | At this article |
4.6. Disease Resistance of Powdery Mildew and Stripe Rust
The powdery mildew resistance of plants was evaluated at seedling and adult stages. The wheat variety Shanyou 225 was employed for inoculation of Blumeria graminis f. sp. tritici (Bgt) pathotype E09 in a plant incubator and field. Powdery mildew reactions of two lines and their parents to Bgt race E09 at the seeding stage were assessed at green house. At 14 days after the initial inoculated, when the pustules were fully developed on Shanyou 225, the percentage of the powdery mildew spores covered the total area of the leaves at the same position on each plant were recorded by the plant responses. Powdery mildew infection type (IT) from 0 to 4 was identified as described by Jorgensen et al. [24]. The plants were evaluated and IT were recorded according with IT 0–2 were considered resistant, while those with IT 3–4 susceptible. The reactions to powdery mildew at the adult stage were tested on two substitution lines, addition line W166, their parents and susceptible cultivar Shanyou 225 during the 2018–2021 growing season at Yangling, Shaanxi Province, China. After wheat heading, when the susceptible control Shanyou 225 showed disease presentation. Powdery mildew disease reaction was were evaluated and IT recorded according to a 0–9 scale, which an IT of 0–4 was considered as resistant and an IT of 5–9 indicated susceptibility [53].
To assess the reactions of two lines to stripe rust at the adult stage, the plants were inoculated with a mixture of Pst CYR32, CYR33, and CYR34, as well as the wheat cultivar Huixianhong was used as the susceptible control, which are prevalent in China as used for artificial inoculation in field in early spring at Northwest A&F University, Yangling, China. When inoculating, spray the mixed strain containing diatomaceous earth on the susceptible material Huixianhong moistened with water in advance, and cover with a plastic bag to moisturize until the plastic bag is removed the next morning. After wheat heading, when the susceptible control Huixianhong showed disease presentation, the plant inoculation experiments and resistance evaluation were performed by Wu et al. [54]. In brief, wheat leaves responses to infection were scored using IT in a 0–4 scale, in which IT = 0, nearly immune; 1, highly resistant; 2, moderately resistant; 3, moderately susceptible and 4, susceptible.
5. Conclusions
In this study, we developed and charactered two stable wheat-Ae. geniculata 7Mg (7A) and 7Mg (7B) substitution lines W623 and W637 by cytogenetic analysis, sequential FISH-GISH, molecular markers, and disease resistance to powdery mildew, stripe rust and Fhb. Therefore, two substitution lines have the potential to serve as primary material for wheat genetic improvements. Further work is needed to transfer the excellent genes in the chromosomes of foreign genes to common wheat by hybridization or radiation, to identify the genomic composition of these lines by FISH and to evaluate their agronomic performance. In conclusion, the purpose is to transfer the disease resistance gene to common wheat for auxiliary breeding and shorten the breeding period of wheat. Thereby extending the genetic source of wheat breeding, enriching genetic diversity and improving the yield and quality of wheat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23137056/s1.
Author Contributions
Y.W. (Yajuan Wang) and W.J. designed the study. X.Y. analyzed the data and wrote the article. X.Y. and M.X. performed the research. X.Y. and Y.W. (Yajuan Wang) contributed the development to the material. Y.W. (Yongfu Wang), X.Y. and M.X. contributed to cytological analysis, molecular markers analysis. C.H., X.C., C.W. and C.C. contributed to disease resistance analysis. H.Z. and T.L. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project of science and technology of Shaanxi province of China (2021NY-081), and Crop Germ plasm Resources Protection (No. 2019NWB036-02-1). We are grateful for their financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bai, G.; Shaner, G. Scab of wheat-prospects control. Plant Dis. 1994, 78, 760–766. [Google Scholar]
- Walter, S.; Nicholson, P.; Doohan, F.M. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010, 185, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Boenisch, M.J.; Schaefer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Xu, D.A.; Cheng, S.H.; Gao, C.B.; Xia, X.C.; Hao, Y.F.; He, Z.H. Characterization of Fusarium Head Blight Resistance Gene Fhb1 and Its Putative Ancestor in Chinese Wheat Germplasm. Acta Agron. Sin. 2018, 44, 473–482. (In Chinese) [Google Scholar] [CrossRef]
- Wang, D.; Zhang, K.; Dong, L.; Dong, Z.; Li, Y.; Hussain, A.; Zhai, H. Molecular genetic and genomic analysis of wheat milling and end-use traits in China: Progress and perspectives. Crop J. 2018, 6, 68–81. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Huber, K.; Heckmann, J.; Steiner, B.; Nelson, J.C.; Buerstmayr, H. Mapping of QTL for Fusarium head blight resistance and morphological and developmental traits in three backcross populations derived from Triticum Dicoccum × Triticum durum. Theor. Appl. Genet. 2012, 125, 1751–1765. [Google Scholar] [CrossRef]
- Jia, H.Y.; Zhou, J.Y.; Xue, S.L.; Li, G.Q.; Yan, H.S.; Ran, C.F.; Zhang, Y.D.; Shi, J.X.; Jia, L.; Wang, X.; et al. A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai. Crop J. 2018, 6, 48–59. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nature Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nature Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Xue, S.; Li, G.; Jia, H.; Xu, F.; Lin, F.; Tang, M.; Wang, Y.; An, X.; Xu, H.; Zhang, L.; et al. Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xu, F.; Tang, M.; Zhou, Y.; Li, G.; An, X.; Lin, F.; Xu, H.; Jia, H.; Zhang, L.; et al. Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 123, 1055–1063. [Google Scholar] [CrossRef]
- Wang, H.W.; Sun, S.L.; Ge, W.Y.; Zhao, L.F.; Hou, B.Q.; Wang, K.; Lyu, Z.F.; Chen, L.Y.; Xu, S.S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, W.; Peng, N.; Wang, C.; Yang, X.; Liu, X.; Zhang, H.; Chen, C.; Ji, W. Molecular cytogenetic identification of a wheat-Aegilops geniculata Roth 7 M(g) disomic addition line with powdery mildew resistance. Mol. Breed. 2016, 36, 40. [Google Scholar] [CrossRef]
- Guo, L.; Yu, L.; Tong, J.; Zhao, Y.; Yang, Y.; Ma, Y.; Cui, L.; Hu, Y.; Wang, Z.; Gao, X. Addition of Aegilops geniculata 1Ug chromosome improves the dough rheological properties by changing the composition and micro-structure of gluten. Food Chem. 2021, 358, 129850. [Google Scholar] [CrossRef] [PubMed]
- Zeller, F.J.; Kong, L.; Hartl, L.; Mohler, V.; Hsam, S.L.K. Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.) 7. Gene Pm29 in line Pova. Euphytica 2002, 123, 187–194. [Google Scholar] [CrossRef]
- Liu, W.X.; Rouse, M.; Friebe, B.; Jin, Y.; Gill, B.; Pumphrey, M.O. Discovery and molecular mapping of a new gene conferring resistance to stem rust, Sr53, derived from Aegilops geniculata and characterization of spontaneous translocation stocks with reduced alien chromatin. Chromosome Res. 2011, 19, 669–682. [Google Scholar] [CrossRef]
- Wang, Y.J.; Long, D.Y.; Wang, Y.Z.; Wang, C.Y.; Liu, X.L.; Zhang, H.; Tian, Z.R.; Chen, C.H.; Ji, W.Q. Characterization and Evaluation of Resistance to Powdery Mildew of Wheat-Aegilops geniculata Roth 7M(g) (7A) Alien Disomic Substitution Line W16998. Int. J. Mol. Sci. 2020, 21, 1861. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Wen, J.; Nie, X.J.; Xu, L.H.; Chen, N.B.; Li, Z.X.; Wang, Q.L.; Zheng, Z.Q.; Li, M.; et al. Frequent intra- and inter-species introgression shapes the landscape of genetic variation in bread wheat. Genome Biol. 2019, 20, 136. [Google Scholar] [CrossRef]
- Ciferri, R. The First Interspecific Wheat Hybrids. J. Hered. 1955, 46, 81–83. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, C.Y.; Wang, Y.Z.; Wang, Y.J.; Chen, C.H.; Ji, W.Q. Molecular cytogenetic identification of two wheat-Thinopyrum ponticum substitution lines conferring stripe rust resistance. Mol. Breed. 2019, 39, 143–153. [Google Scholar] [CrossRef]
- Zheng, Q.; Luo, Q.; Niu, Z.; Li, H.; Li, B.; Xu, S.S.; Li, Z. Variation in Chromosome Constitution of the Xiaoyan Series Partial Amphiploids and Its Relationship to Stripe Rust and Stem Rust Resistance. J. Genet. Genom. 2015, 42, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Jiao, C.; Hou, J.; Li, T.; Liu, H.; Wang, Y.; Zheng, J.; Liu, H.; Bi, Z.; Xu, F.; et al. Resequencing of 145 Landmark Cultivars Reveals Asymmetric Sub-genome Selection and Strong Founder Genotype Effects on Wheat Breeding in China. Mol. Plant 2020, 13, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H. Discovery, Characterization and Exploitation of Mlo Powdery Mildew resistance in Barley. Euphytica 1992, 63, 141–152. [Google Scholar] [CrossRef]
- Chen, X.M. Epidemiology and control of stripe rust Puccinia striiformis f. sp tritici on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- An, D.; Ma, P.; Zheng, Q.; Fu, S.; Li, L.; Han, F.; Han, G.; Wang, J.; Xu, Y.; Jin, Y.; et al. Development and molecular cytogenetic identification of a new wheat-rye 4R chromosome disomic addition line with resistances to powdery mildew, stripe rust and sharp eyespot. Theor. Appl. Genet. 2018, 132, 257–272. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Hou, Y.; Cai, J.; Shen, X.; Zhou, T.; Xu, H.; Ohm, H.W.; Wang, H.; Li, A.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef]
- Hao, Y.B.; Wang, T.; Wang, K.; Wang, X.J.; Fu, Y.P.; Huang, L.L.; Kang, Z.S. Transcriptome Analysis Provides Insights into the Mechanisms Underlying Wheat Plant Resistance to Stripe Rust at the Adult Plant Stage. PLoS ONE 2016, 11, e0150717. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Taketa, S.; Takeda, K. Production and characterization of a complete set of wheat-wild barley (Hordeum vulgare ssp spontaneum) chromosome addition lines. Breed. Sci. 2001, 51, 199–206. [Google Scholar] [CrossRef]
- Mahjoub, A.; El Gharbi, M.S.; Mguis, K.; El Gazzah, M.; Brahim, N.B. Evaluation of genetic diversity in Aegilops geniculata Roth accessions using morphological and RAPD markers. Pak. J. Biol. Sci. PJBS 2009, 12, 994–1003. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friebe, B.R.; Tuleen, N.A.; Gill, B.S. Development and identification of a complete set of Triticum aestivum Aegilops geniculata chromosome addition lines. Genome 1999, 42, 374–380. [Google Scholar] [CrossRef]
- Stoilova, T.; Spetsov, P. Chromosome 6U from Aegilops geniculata Roth carrying powdery mildew resistance in bread wheat. Breed. Sci. 2006, 56, 351–357. [Google Scholar] [CrossRef]
- Kuraparthy, V.; Sood, S.; See, D.R.; Gill, B.S. Development of a PCR Assay and Marker-Assisted Transfer of Leaf Rust and Stripe Rust Resistance Genes Lr57 and Yr40 into Hard Red Winter Wheats. Crop Sci. 2009, 49, 120–126. [Google Scholar] [CrossRef]
- Xu, H.J.; Ye, M.; Xia, A.L.; Jiang, H.; Huang, P.P.; Liu, H.Q.; Hou, R.; Wang, Q.H.; Li, D.G.; Xu, J.R.; et al. The Fng3 ING protein regulates H3 acetylation and H4 deacetylation by interacting with two distinct histone modifying complexes. New Phytol. 2022. [Google Scholar] [CrossRef]
- Sari, E.; Cabral, A.L.; Polley, B.; Tan, Y.; Hsueh, E.; Konkin, D.J.; Knox, R.E.; Ruan, Y.; Fobert, P.R. Weighted gene co-expression network analysis unveils gene networks associated with the Fusarium head blight resistance in tetraploid wheat. BMC Genom. 2019, 20, 925. [Google Scholar] [CrossRef]
- Zhao, J.X.; Liu, Y.; Cheng, X.N.; Pang, Y.H.; Li, J.C.; Su, Z.Q.; Wu, J.; Yang, Q.H.; Bai, G.H.; Chen, X.H. Development and identification of a dwarf wheat-Leymus mollis double substitution line with resistance to yellow rust and Fusarium head blight. Crop J. 2019, 7, 516–526. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef]
- Gong, B.R.; Zhu, W.; Li, S.Y.; Wang, Y.Q.; Xu, L.L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.N.; Zhang, H.Q.; et al. Molecular cytogenetic characterization of wheat-Elymus repens chromosomal translocation lines with resistance to Fusarium head blight and stripe rust. BMC Plant Biol. 2019, 19, 590. [Google Scholar] [CrossRef]
- Fu, S.L.; Lv, Z.L.; Qi, B.; Guo, X.; Li, J.; Liu, B.; Han, F.P. Molecular Cytogenetic Characterization of Wheat-Thinopyrum elongatum Addition, Substitution and Translocation Lines with a Novel Source of Resistance to Wheat Fusarium Head Blight. J. Genet. Genom. 2012, 39, 103–110. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, P.Y.; Qi, X.L.; Bao, Y.G.; Wang, H.G.; Wang, R.R.C.; Li, X.F. Characterization of chromosome constitution in three wheat-Thinopyrum intermedium amphiploids revealed frequent rearrangement of alien and wheat chromosomes. BMC Plant Biol. 2021, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Han, H.M.; Li, Q.F.; Zhang, J.P.; Lu, Y.Q.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Identification and genetic analysis of multiple P chromosomes of Agropyron cristatum in the background of common wheat. J. Integr. Agric. 2018, 17, 1697–1705. [Google Scholar] [CrossRef]
- Doyle JJ, D.J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull 1987, 19, 11–15. [Google Scholar]
- Han, F.P.; Gao, Z.; Birchler, J.A. Reactivation of an Inactive Centromere Reveals Epigenetic and Structural Components for Centromere Specification in Maize. Plant Cell 2009, 21, 1929–1939. [Google Scholar] [CrossRef]
- Han, F.P.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef]
- Tang, Z.X.; Yang, Z.J.; Fu, S.L. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Ishikawa, G.; Nakamura, T.; Ashida, T.; Saito, M.; Nasuda, S.; Endo, T.R.; Wu, J.; Matsumoto, T. Localization of anchor loci representing five hundred annotated rice genes to wheat chromosomes using PLUG markers. Theor. Appl. Genet. 2009, 118, 499–514. [Google Scholar] [CrossRef]
- Wu, S.Y.; Pumphrey, M.; Bai, G.H. Molecular Mapping of Stem-Rust-Resistance Gene Sr40 in Wheat. Crop Sci. 2009, 49, 1681–1686. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Gueldener, U.; Xu, J.-R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.-J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef]
- Jiang, C.; Hei, R.; Yang, Y.; Zhang, S.; Wang, Q.; Wang, W.; Zhang, Q.; Yan, M.; Zhu, G.; Huang, P.; et al. An orphan protein of Fusarium graminearum modulates host immunity by mediating proteasomal degradation of TaSnRK1 alpha. Nat. Commun. 2020, 11, 4382. [Google Scholar] [CrossRef]
- Jonkers, W.; Dong, Y.H.; Broz, K.; Kistler, H.C. The Wor1-like Protein Fgp1 Regulates Pathogenicity, Toxin Synthesis and Reproduction in the Phytopathogenic Fungus Fusarium graminearum. PLoS Pathog. 2012, 8, e1002724. [Google Scholar] [CrossRef]
- Su, Z.Q.; Jin, S.J.; Zhang, D.D.; Bai, G.H. Development and validation of diagnostic markers for Fhb1 region, a major QTL for Fusarium head blight resistance in wheat. Theor. Appl. Genet. 2018, 131, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Duan, X. Improvement of scale 0–9 method for scoring adult plant resistance to powdery mildew of wheat. Beijing Agric. Sci. 1991, 1, 38–39. [Google Scholar]
- Wu, J.; Liu, S.; Wang, Q.; Zeng, Q.; Mu, J.; Huang, S.; Yu, S.; Han, D.; Kang, Z. Rapid identification of an adult plant stripe rust resistance gene in hexaploid wheat by high-throughput SNP array genotyping of pooled extremes. Theor. Appl. Genet. 2018, 131, 43–58. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).