Th17 Activation and Th17/Treg Imbalance in Prolonged Anterior Intraocular Inflammation after Ocular Alkali Burn
Abstract
:1. Introduction
2. Results
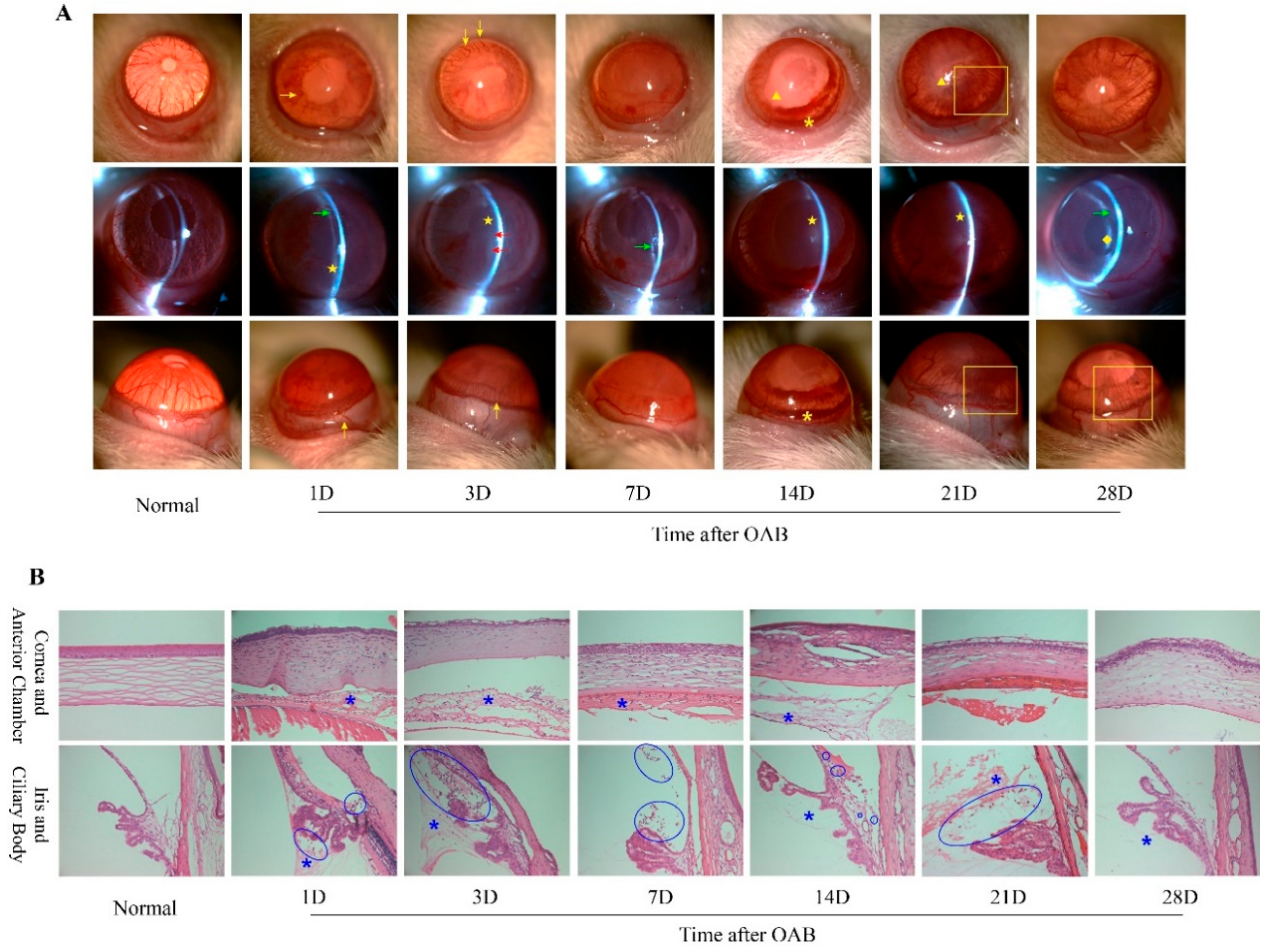
2.1. Prolonged Anterior Intraocular Inflammation Observed in Rat OAB Model
2.2. Elevated Intraocular IL-17A Level after the Acute Phase of OAB
2.3. Th17 Lymphocytes Detected in the Anterior Segment of OAB-Subjected Eyes
2.4. Increased Frequency of Th17 Population and Elevated Th17/Treg Ratio Rat OAB Model
3. Discussion
4. Methods and Materials
4.1. Animals and OAB Model
4.2. Slit-Lamp Examination
4.3. Hematoxylin and Eosin Staining and Immunofluorescence
4.4. Quantitative Real-Time PCR
4.5. Cell Processing and Flow Cytometry
4.6. Determination of Cytokine Levels in Intraocular Fluid
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cade, F.; Grosskreutz, C.L.; Tauber, A.; Dohlman, C.H. Glaucoma in eyes with severe chemical burn, before and after keratoprosthesis. Cornea 2011, 30, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Stefan, C.; Timaru, C.M.; Iliescu, D.A.; Schmitzer, S.; de Algerino, S.; Batras, M.; Hosseini-Ramhormozi, J. Glaucoma after chemical burns and radiation. Rom. J. Ophthalmol. 2016, 60, 209–215. [Google Scholar] [PubMed]
- Gu, J.; Zhai, J.; Zhou, S.; Chen, J. Boston Keratoprosthesis Outcomes in Severe Ocular Chemical Burns in Southern China: A Retrospective Study. Adv. Ther. 2016, 33, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Saeed, H.N.; Paschalis, E.I.; Chodosh, J. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: A systematic review. Ocul. Surf. 2018, 16, 272–281. [Google Scholar] [CrossRef]
- Dohlman, C.H.; Cade, F.; Regatieri, C.V.; Zhou, C.; Lei, F.; Crnej, A.; Harissi-Dagher, M.; Robert, M.C.; Papaliodis, G.N.; Chen, D.; et al. Chemical Burns of the Eye: The Role of Retinal Injury and New Therapeutic Possibilities. Cornea 2018, 37, 248–251. [Google Scholar] [CrossRef]
- Paschalis, E.I.; Zhou, C.; Lei, F.; Scott, N.; Kapoulea, V.; Robert, M.C.; Vavvas, D.; Dana, R.; Chodosh, J.; Dohlman, C.H. Mechanisms of Retinal Damage after Ocular Alkali Burns. Am. J. Pathol. 2017, 187, 1327–1342. [Google Scholar] [CrossRef] [Green Version]
- Paschalis, E.I.; Lei, F.; Zhou, C.; Kapoulea, V.; Thanos, A.; Dana, R.; Vavvas, D.G.; Chodosh, J.; Dohlman, C.H. The Role of Microglia and Peripheral Monocytes in Retinal Damage after Corneal Chemical Injury. Am. J. Pathol. 2018, 188, 1580–1596. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yuan, M.; Duan, F.; Yang, Y.; Lou, B.; Lin, X. Inhibition of endoplasmic reticulum stress by 4-phenylbutyrate alleviates retinal inflammation and the apoptosis of retinal ganglion cells after ocular alkali burn in mice. Inflamm. Res. 2022, 71, 577–590. [Google Scholar] [CrossRef]
- Grant, W.M. Experimental investigation of paracentesis in the treatment of ocular ammonia burns. Arch. Ophthalmol. 1950, 44, 399–404. [Google Scholar] [CrossRef]
- Bizrah, M.; Yusuf, A.; Ahmad, S. An update on chemical eye burns. Eye 2019, 33, 1362–1377. [Google Scholar] [CrossRef]
- Choi, H.; Phillips, C.; Oh, J.Y.; Stock, E.M.; Kim, D.K.; Won, J.K.; Fulcher, S. Comprehensive Modeling of Corneal Alkali Injury in the Rat Eye. Curr. Eye Res. 2017, 42, 1348–1357. [Google Scholar] [CrossRef]
- Zhou, C.; Robert, M.C.; Kapoulea, V.; Lei, F.; Stagner, A.M.; Jakobiec, F.A.; Dohlman, C.H.; Paschalis, E.I. Sustained Subconjunctival Delivery of Infliximab Protects the Cornea and Retina Following Alkali Burn to the Eye. Investig. Opthalmol. Vis. Sci. 2017, 58, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.W.; Bian, D.M.; Hu, N.; Zhang, J.F. Effects of amniotic membrane transplantation on cytokines expression in chemically burned rat corneas. Int. J. Ophthalmol. 2011, 4, 33–36. [Google Scholar]
- Miyamoto, F.; Sotozono, C.; Ikeda, T.; Kinoshita, S. Retinal cytokine response in mouse alkali-burned eye. Ophthalmic Res. 1998, 30, 168–171. [Google Scholar] [CrossRef]
- Sotozono, C.; He, J.; Matsumoto, Y.; Kita, M.; Imanishi, J.; Kinoshita, S. Cytokine expression in the alkali-burned cornea. Curr. Eye Res. 1997, 16, 670–676. [Google Scholar] [CrossRef]
- Kao, W.W.; Zhu, G.; Benza, R.; Kao, C.W.; Ishizaki, M.; Wander, A.H. Appearance of immune cells and expression of MHC II DQ molecule by fibroblasts in alkali-burned corneas. Cornea 1996, 15, 397–408. [Google Scholar] [CrossRef]
- Kruit, P.J.; Broersma, L.; van der Gaag, R.; Kijlstra, A. Clinical and experimental studies concerning circulating antibodies to corneal epithelium antigens, Documenta ophthalmologica. Adv. Ophthalmol. 1986, 64, 43–51. [Google Scholar]
- Zhao, M.; Chen, J.; Yang, P. Immunologic experimental studies on the alkali burn of cornea in rats. Zhonghua Yan Ke Za Zhi 2000, 36, 40–42, 44. [Google Scholar]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Singh, R.P.; Hasan, S.; Sharma, S.; Nagra, S.; Yamaguchi, D.T.; Wong, D.T.; Hahn, B.H.; Hossain, A. Th17 cells in inflammation and autoimmunity. Autoimmun. Rev. 2014, 13, 1174–1181. [Google Scholar] [CrossRef]
- Luger, D.; Silver, P.B.; Tang, J.; Cua, D.; Chen, Z.; Iwakura, Y.; Bowman, E.P.; Sgambellone, N.M.; Chan, C.C.; Caspi, R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 2008, 205, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattri, R.; Cox, T.; Yasayko, S.A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [Green Version]
- Chabaud, M.; Durand, J.M.; Buchs, N.; Fossiez, F.; Page, G.; Frappart, L.; Miossec, P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999, 42, 963–970. [Google Scholar] [CrossRef]
- Bettelli, E.; Korn, T.; Oukka, M.; Kuchroo, V.K. Induction and effector functions of T(H)17 cells. Nature 2008, 453, 1051–1057. [Google Scholar] [CrossRef]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef]
- Yin, X.T.; Zobell, S.; Jarosz, J.G.; Stuart, P.M. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J. Immunol. 2015, 194, 4029–4038. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Su, G.; Kijlstra, A.; Yang, P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog. Retin. Eye Res. 2021, 80, 100866. [Google Scholar] [CrossRef]
- Rios-Rios, W.J.; Sosa-Luis, S.A.; Torres-Aguilar, H. T Cells Subsets in the Immunopathology and Treatment of Sjogren’s Syndrome. Biomolecules 2020, 10, 1539. [Google Scholar] [CrossRef]
- Sisto, M.; Lorusso, L.; Tamma, R.; Ingravallo, G.; Ribatti, D.; Lisi, S. Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjogren’s syndrome. Clin. Exp. Immunol. 2019, 198, 261–272. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, S.; Cao, Z.; Hu, Y.; Yang, H.; Wang, D. Interleukin-17 enhanced immunoinflammatory lesions in a mouse model of recurrent herpetic keratitis. Microbes Infect. 2013, 15, 126–139. [Google Scholar] [CrossRef]
- Chen, L.; Yang, P.; Zhou, H.; He, H.; Ren, X.; Chi, W.; Wang, L.; Kijlstra, A. Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3475–3482. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Liu, B.; Wei, H.; Wu, S.; Guo, L.; Xu, F.; Liu, T.; Bi, H.; Guo, D. Activation of the Notch signaling pathway disturbs the CD4+/CD8+, Th17/Treg balance in rats with experimental autoimmune uveitis. Inflamm. Res. 2019, 68, 761–774. [Google Scholar] [CrossRef]
- Yin, X.; Wei, H.; Wu, S.; Wang, Z.; Liu, B.; Guo, L.; Bi, H.; Guo, D. DAPT reverses the Th17/Treg imbalance in experimental autoimmune uveitis in vitro via inhibiting Notch signaling pathway. Int. Immunopharmacol. 2020, 79, 106107. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Li, H.; Su, W.; Xie, Y.; Pan, Y.; Chen, X.; Liang, D. Apremilast Regulates the Teff/Treg Balance to Ameliorate Uveitis via PI3K/AKT/FoxO1 Signaling Pathway. Front. Immunol. 2020, 11, 581673. [Google Scholar] [CrossRef]
- Pfister, R.R.; Friend, J.; Dohlman, C.H. The anterior segments of rabbits after alkali burns. Metabolic and histologic alterations. Arch. Ophthalmol. 1971, 86, 189–193. [Google Scholar] [CrossRef]
- Bansal, S.; Barathi, V.A.; Iwata, D.; Agrawal, R. Experimental autoimmune uveitis and other animal models of uveitis: An update. Indian J. Ophthalmol. 2015, 63, 211–218. [Google Scholar]



| Gene | Primers | |
|---|---|---|
| IL-1β | forward | GCAGCTTTCGACAGTGAGGAGA |
| reverse | CAATAGAGGAAACGCAGGTG | |
| IL-6 | forward | CAGCGATGATGCACTGTCAGA |
| reverse | GGAGAGCATTGGAAGTTGGG | |
| TNF-α | forward | GCCACCACGCTCTTCTGTCTA |
| reverse | CGCTTGGTGGTTTGCTACGA | |
| IL-10 | forward | TGCTCTTACTGGCTGGAGTG |
| reverse | CCTGGGGCATCACTTCTACC | |
| IL-17 | forward | ATCCAGCAAGAGATCCTGGT |
| reverse | CAATAGAGGAAACGCAGGTG | |
| GAPDH | forward | GGATGGAATTGTGAGGGAGA |
| reverse | GTGGACCTCATGGCCTACAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Qian, X.; Huang, Y.; Ma, X.; Duan, F.; Yang, Y.; Lou, B.; Lin, X. Th17 Activation and Th17/Treg Imbalance in Prolonged Anterior Intraocular Inflammation after Ocular Alkali Burn. Int. J. Mol. Sci. 2022, 23, 7075. https://doi.org/10.3390/ijms23137075
Yuan M, Qian X, Huang Y, Ma X, Duan F, Yang Y, Lou B, Lin X. Th17 Activation and Th17/Treg Imbalance in Prolonged Anterior Intraocular Inflammation after Ocular Alkali Burn. International Journal of Molecular Sciences. 2022; 23(13):7075. https://doi.org/10.3390/ijms23137075
Chicago/Turabian StyleYuan, Miner, Xiaobing Qian, Yanqiao Huang, Xinqi Ma, Fang Duan, Yao Yang, Bingsheng Lou, and Xiaofeng Lin. 2022. "Th17 Activation and Th17/Treg Imbalance in Prolonged Anterior Intraocular Inflammation after Ocular Alkali Burn" International Journal of Molecular Sciences 23, no. 13: 7075. https://doi.org/10.3390/ijms23137075






