Effects of Non-Opioid Analgesics on the Cell Membrane of Skin and Gastrointestinal Cancers
Abstract
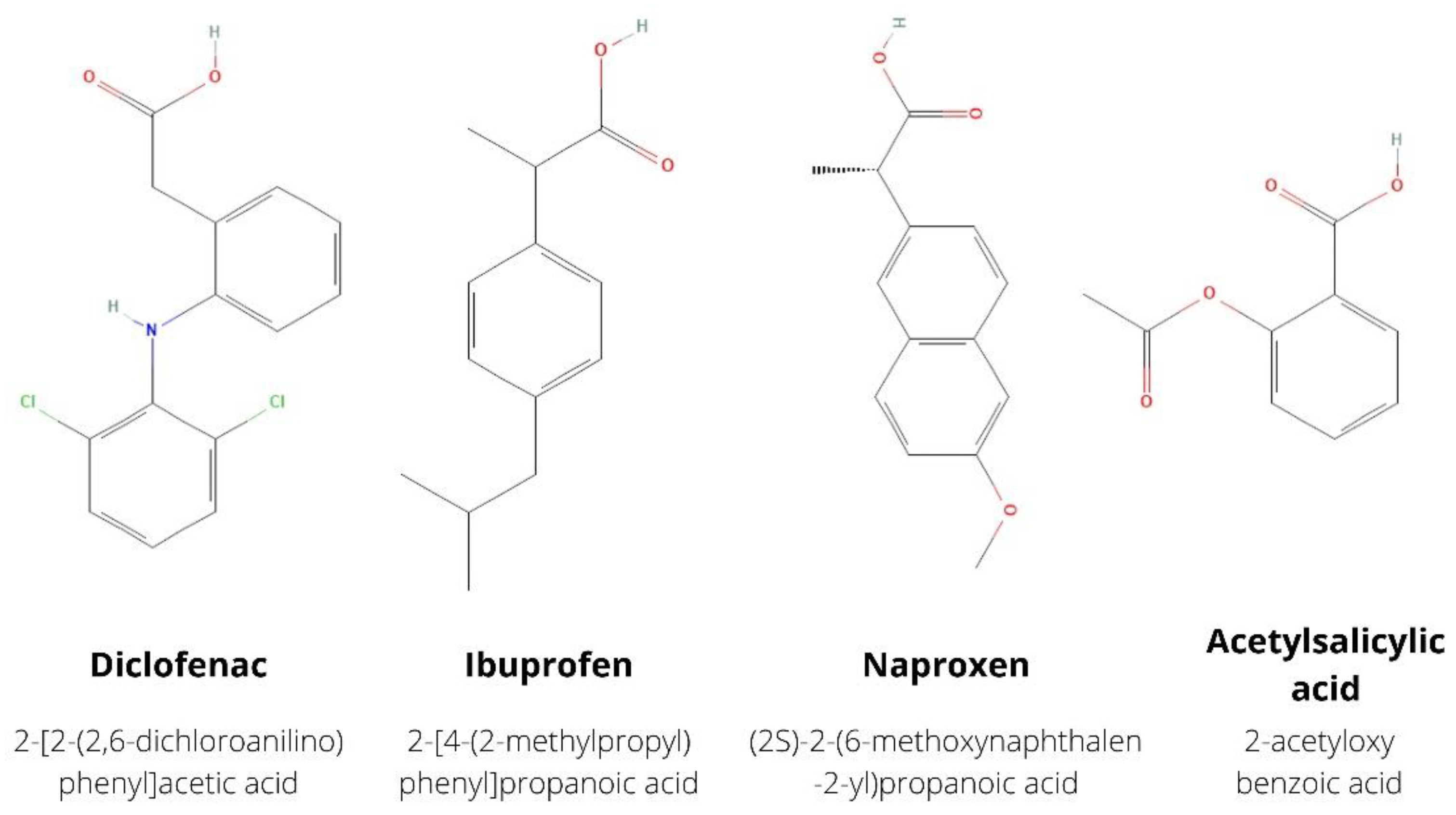
:1. Introduction
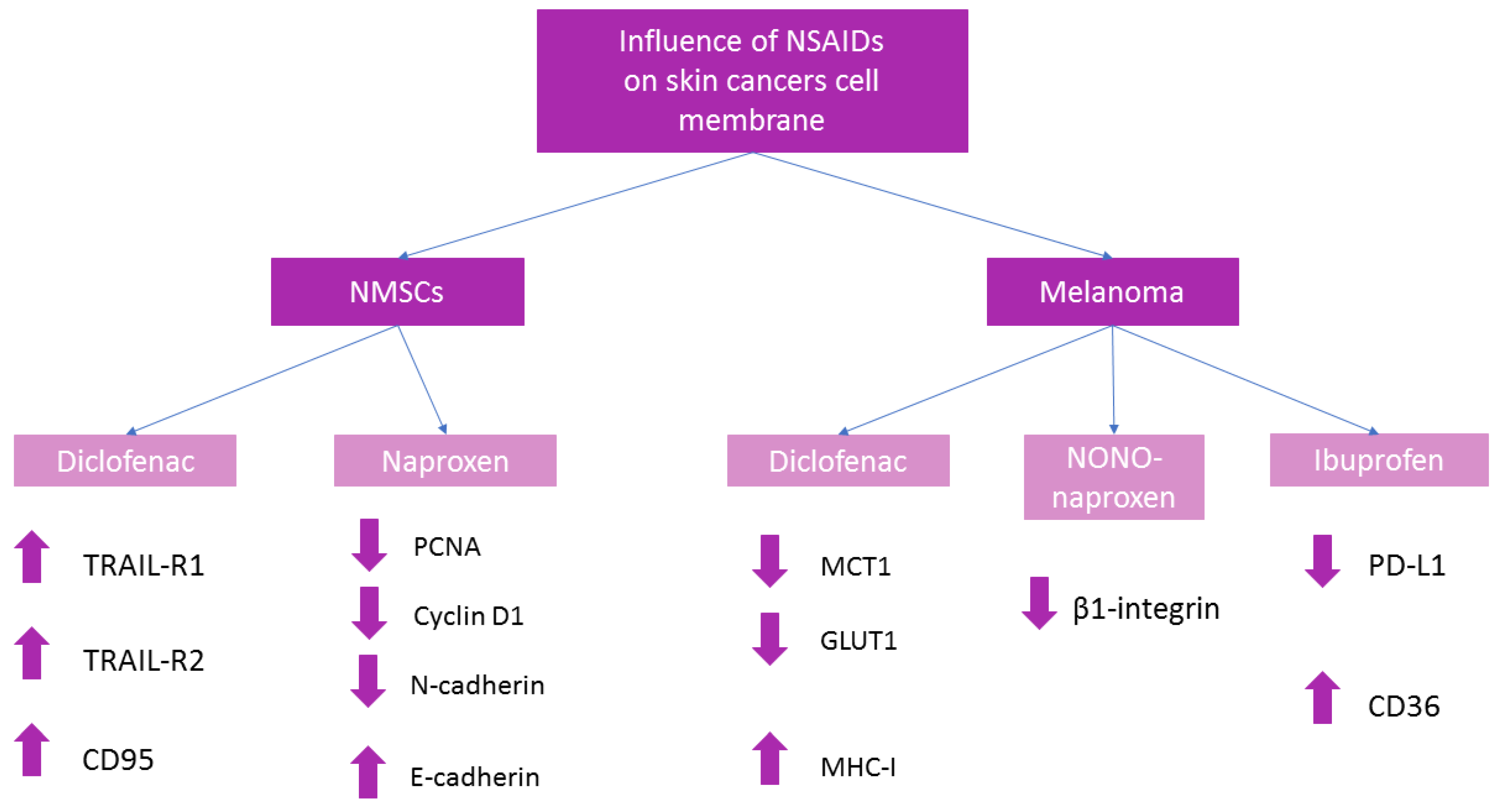
2. Non-Opioid Analgesics in Skin Cancers
2.1. Non-Melanoma Skin Cancers (NMSCs)
2.1.1. Diclofenac
2.1.2. Naproxen
2.2. Melanoma
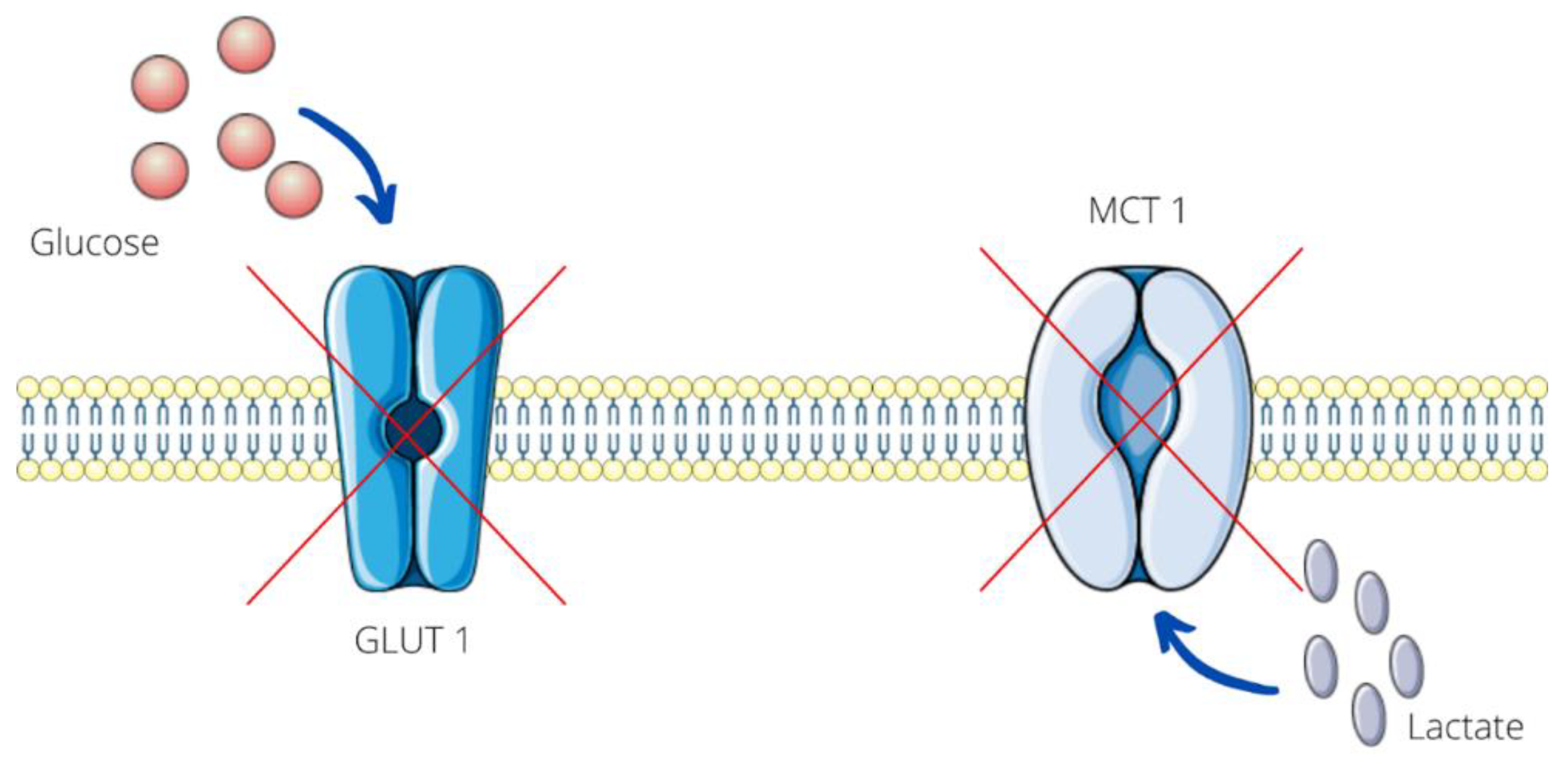
2.2.1. Diclofenac
2.2.2. Naproxen
2.2.3. Ibuprofen
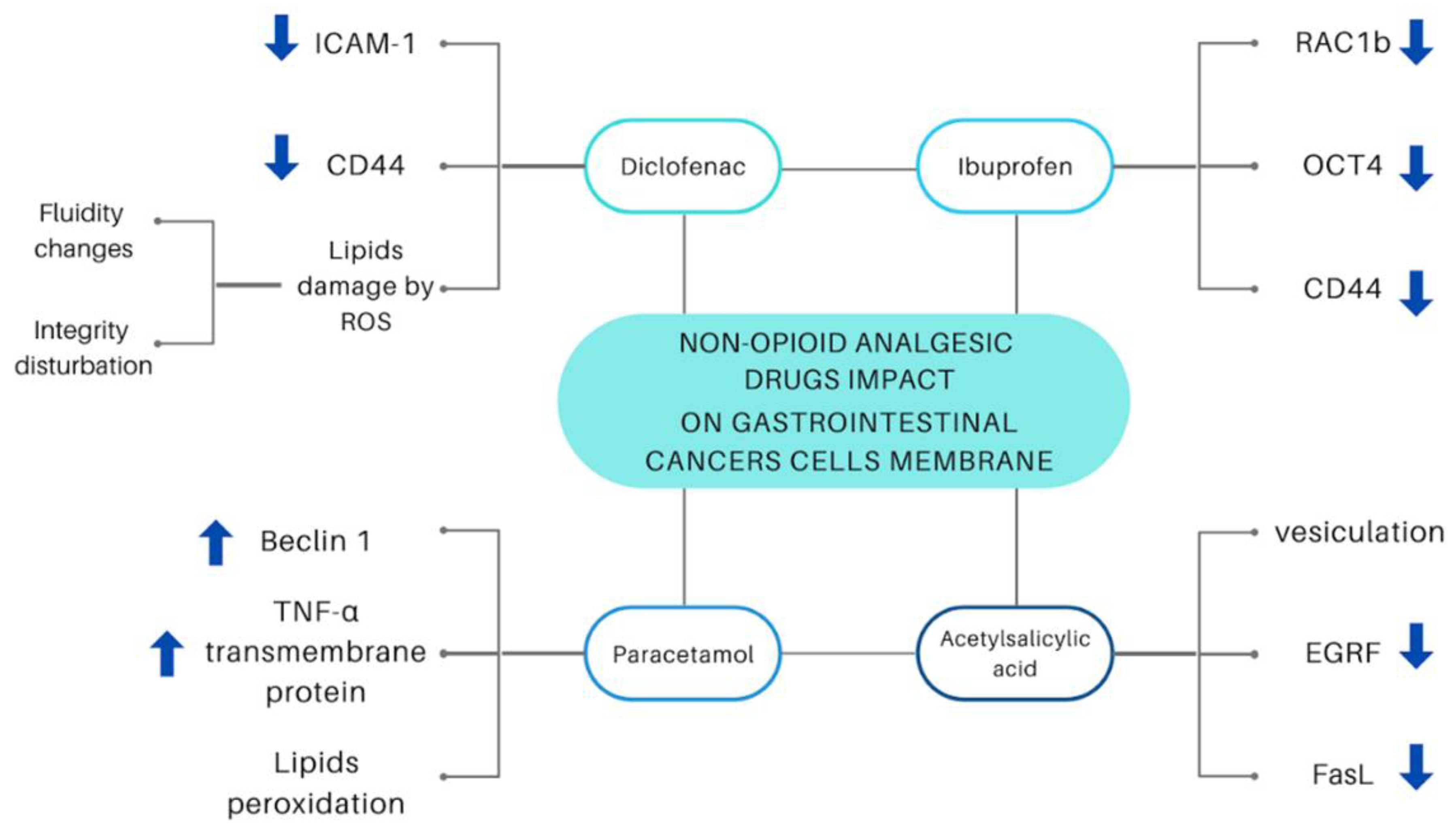
3. Non-Opioid Analgesics in Gastrointestinal Cancers
3.1. Diclofenac
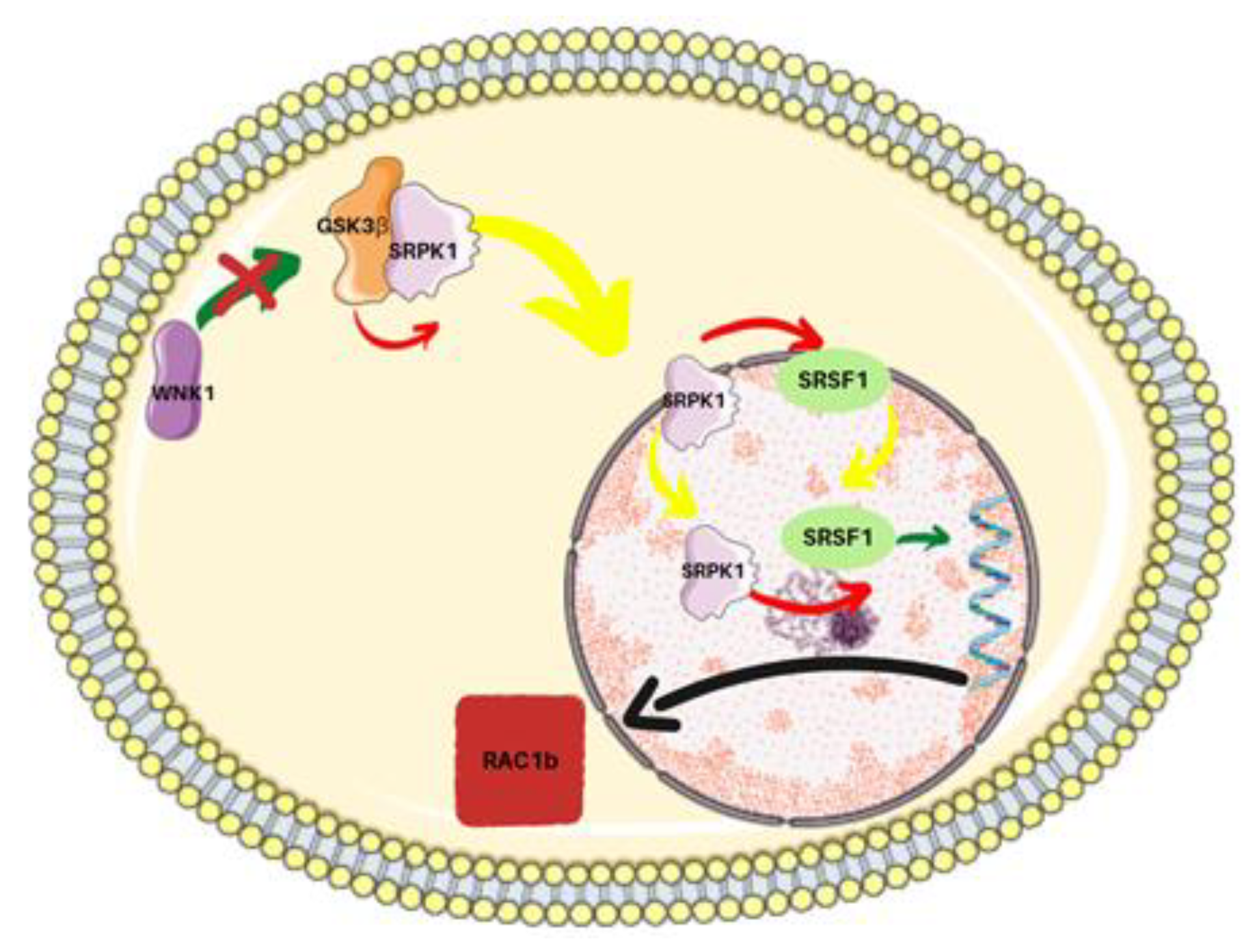
3.2. Ibuprofen
3.3. Acetylsalicylic Acid
3.4. Acetaminophen (Paracetamol)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seth, B. Non-opioid analgesics. Anaesth. Intensive Care Med. 2019, 20, 456–459. [Google Scholar] [CrossRef]
- Anekar, A.A.; Cascella, M. WHO Analgesic Ladder. J. R. Coll. Physicians Edinb. 2021, 38, 284. [Google Scholar] [CrossRef]
- Hadley, G.R.; Jones, M.R.; Kaye, A.D. Analgesic Ladder Approach. In Pain; Springer: Cham, Germany, 2019; pp. 949–951. [Google Scholar] [CrossRef]
- Yap, P.; Goh, K.-L. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Induced Dyspepsia. Curr. Pharm. Des. 2015, 21, 5073–5081. [Google Scholar] [CrossRef]
- GreGorczyk, I.; Maślanka, T. Effect of selected non-steroidal anti-inflammatory drugs on activation-induced CD25 expression on murine CD4+ and CD8+ T cells: An in vitro study. Cent. Eur. J. Immunol. 2019, 44, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002, 3, 166–174. [Google Scholar] [CrossRef]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The role of cyclooxygenase-2 in colorectal cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2021, 48, 3–19. [Google Scholar] [CrossRef]
- Minocha, R.; Damian, D.L.; Halliday, G.M. Melanoma and nonmelanoma skin cancer chemoprevention: A role for nicotinamide? Photodermatol. Photoimmunol. Photomed. 2018, 34, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Jeter, J.M.; Bowles, T.L.; Curiel-Lewandrowski, C.; Swetter, S.M.; Filipp, F.V.; Abdel-Malek, Z.A.; Geskin, L.J.; Brewer, J.D.; Arbiser, J.L.; Gershenwald, J.E.; et al. Chemoprevention agents for melanoma: A path forward into phase 3 clinical trials. Cancer 2019, 125, 18–44. [Google Scholar] [CrossRef] [Green Version]
- Maniewska, J.; Jeżewska, D. Non-Steroidal Anti-Inflammatory Drugs in Colorectal Cancer Chemoprevention. Cancers 2021, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Adegun, A.A.; Viriri, S. Deep learning-based system for automatic melanoma detection. IEEE Access 2020, 8, 7160–7172. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of skin cancer: Update 2019. In Advances in Experimental Medicine and Biology; Springer: Cham, Germany, 2020; Volume 1268, pp. 123–139. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cahlin, C.; Gelin, J.; Delbro, D.; Lönnroth, C.; Doi, C.; Lundholm, K. Effect of cyclooxygenase and nitric oxide synthase inhibitors on tumor growth in mouse tumor models with and without cancer cachexia related to prostanoids. Cancer Res. 2000, 60, 1742–1749. [Google Scholar] [PubMed]
- Panza, E.; De Cicco, P.; Ercolano, G.; Armogida, C.; Scognamiglio, G.; Anniciello, A.M.; Botti, G.; Cirino, G.; Ianaro, A. Differential expression of cyclooxygenase-2 in metastatic melanoma affects progression free survival. Oncotarget 2016, 7, 57077–57085. [Google Scholar] [CrossRef] [Green Version]
- Tudor, D.V.; Bâldea, I.; Lupu, M.; Kacso, T.; Kutasi, E.; Hopârtean, A.; Stretea, R.; Filip, A.G. COX-2 as a potential biomarker and therapeutic target in melanoma. Cancer Biol. Med. 2020, 17, 20–31. [Google Scholar] [CrossRef]
- Voiculescu, V.M.; Lisievici, C.V.; Lupu, M.; Vajaitu, C.; Draghici, C.C.; Popa, A.V.; Solomon, I.; Sebe, T.I.; Constantin, M.M.; Caruntu, C. Mediators of inflammation in topical therapy of skin cancers. Mediat. Inflamm. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: Potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5%. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical therapies for skin cancer and actinic keratosis. Eur. J. Pharm. Sci. 2015, 77, 279–289. [Google Scholar] [CrossRef]
- Dessinioti, C.; Antoniou, C.; Stratigos, A.J. New targeted approaches for the treatment and prevention of nonmelanoma skin cancer. Expert Rev. Dermatol. 2011, 6, 625–634. [Google Scholar] [CrossRef]
- Stingl, G.; Brüggen, M.C.; Vázquez-Strauss, M. Innate and adaptive components of the cutaneous immune barrier: The central role of dendritic cells. In Clinical and Basic Immunodermatology: Second Edition; Springer: Cham, Germany, 2017; pp. 1–10. ISBN 9783319297859. [Google Scholar] [CrossRef]
- Fecker, L.F.; Stockfleth, E.; Braun, F.K.; Rodust, P.M.; Schwarz, C.; Köhler, A.; Leverkus, M.; Eberle, J. Enhanced death ligand-induced apoptosis in cutaneous SCC Cells by treatment with diclofenac/hyaluronic acid correlates with downregulation of c-FLIP. J. Investig. Dermatol. 2010, 130, 2098–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampucci, S.; Carpi, S.; Digiacomo, M.; Polini, B.; Fogli, S.; Burgalassi, S.; MacChia, M.; Nieri, P.; Manera, C.; Monti, D. Diclofenac-derived hybrids for treatment of actinic keratosis and squamous cell carcinoma. Molecules 2019, 24, 1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, E.M.; Tober, K.L.; Riggenbach, J.A.; Schick, J.S.; Lamping, K.N.; Kusewitt, D.F.; Young, G.S.; Oberyszyn, T.M. Preventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh-1 mice in a model of UVB-induced cutaneous squamous cell carcinoma. Carcinogenesis 2013, 34, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Brinkhuizen, T.; Frencken, K.J.A.; Nelemans, P.J.; Hoff, M.L.S.; Kelleners-Smeets, N.W.J.; Zur Hausen, A.; Van Der Horst, M.P.J.; Rennspiess, D.; Winnepenninckx, V.J.L.; Van Steensel, M.A.M.; et al. The effect of topical diclofenac 3% and calcitriol 3 μg/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): A phase II, randomized controlled trial. J. Am. Acad. Dermatol. 2016, 75, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Kantari, C.; Walczak, H. Dual Philosophy in Death Receptor Signalling. Open Cell Signal. J. 2011, 3, 27–34. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, D.D.; Mello, B.P.; Pereira, W.O.; Amarante-Mendes, G.P. PRAME/EZH2-Mediated Regulation of TRAIL: A New Target for Cancer Therapy. Curr. Mol. Med. 2013, 13, 296–304. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Waseem, M.; Rana, M.; Xu, H.; Kopelovich, L.; Elmets, C.A.; Athar, M. Naproxen Inhibits UVB-induced Basal Cell and Squamous Cell Carcinoma Development in Ptch1+/−/SKH-1 Hairless Mice. Photochem. Photobiol. 2017, 93, 1016–1024. [Google Scholar] [CrossRef]
- Kundu, K.; Ghosh, S.; Sarkar, R.; Edri, A.; Brusilovsky, M.; Gershoni-Yahalom, O.; Yossef, R.; Shemesh, A.; Soria, J.C.; Lazar, V.; et al. Inhibition of the NKp44-PCNA immune checkpoint using a MAb to PCNA. Cancer Immunol. Res. 2019, 7, 1120–1134. [Google Scholar] [CrossRef]
- Sato, F.; Bhawal, U.K.; Tojyo, I.; Fujita, S.; Murata, S.I.; Umuragaki, Y. Differential expression of claudin-4, occludili, SOX2 and proliferating cell nuclear antigen between basaloid squamous cell carcinoma and squamous cell carcinoma. Mol. Med. Rep. 2019, 20, 1977–1985. [Google Scholar] [CrossRef]
- Chen, K.; Jiao, X.; Ashton, A.; Di Rocco, A.; Pestell, T.G.; Sun, Y.; Zhao, J.; Casimiro, M.C.; Li, Z.; Lisanti, M.P.; et al. The membrane-associated form of cyclin D1 enhances cellular invasion. Oncogenesis 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.; Lang, S.A.; Renner, K.; Bosserhoff, A.; Gronwald, W.; Rehli, M.; Einhell, S.; Gedig, I.; Singer, K.; Seilbeck, A.; et al. New Aspects of an Old Drug—Diclofenac Targets MYC and Glucose Metabolism in Tumor Cells. PLoS ONE 2013, 8, e66987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, M.; Kitz, J.; Pilavakis, Y.; Hakroush, S.; Wolff, H.A.; Canis, M.; Rieken, S.; Schirmer, M.A. Monocarboxylate transporter-1 (MCT1) protein expression in head and neck cancer affects clinical outcome. Sci. Rep. 2021, 11, 4578. [Google Scholar] [CrossRef] [PubMed]
- Renner, K.; Bruss, C.; Schnell, A.; Koehl, G.; Becker, H.M.; Fante, M.; Menevse, A.N.; Kauer, N.; Blazquez, R.; Hacker, L.; et al. Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy. Cell Rep. 2019, 29, 135–150.e9. [Google Scholar] [CrossRef] [Green Version]
- Renner, K.; Singer, K.; Koehl, G.E.; Geissler, E.K.; Peter, K.; Siska, P.J.; Kreutz, M. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front. Immunol. 2017, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Al-Nimer, M.S.M.; Hameed, H.G.; Mahmood, M.M. Antiproliferative effects of aspirin and diclofenac against the growth of cancer and fibroblast cells: In vitro comparative study. Saudi Pharm. J. 2015, 23, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Seliger, B. Different regulation of MHC Class i antigen processing components in human tumors. J. Immunotoxicol. 2008, 5, 361–367. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K.; Bhardwaj, T.R. Therapeutic role of nitric oxide as emerging molecule. Biomed. Pharmacother. 2017, 85, 182–201. [Google Scholar] [CrossRef]
- Hathi, D.; Chanswangphuwana, C.; Cho, N.; Fontana, F.; Maji, D.; Ritchey, J.; O’Neal, J.; Ghai, A.; Duncan, K.; Akers, W.J.; et al. Ablation of VLA4 in multiple myeloma cells redirects tumor spread and prolongs survival. Sci. Rep. 2022, 12, 30. [Google Scholar] [CrossRef]
- Cheng, H.; Mollica, M.Y.; Lee, S.H.; Wang, L.; Velázquez-Martínez, C.A.; Wu, S. Effects of nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NONO-NSAIDs) on melanoma cell adhesion. Toxicol. Appl. Pharmacol. 2012, 264, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Botti, G.; Fratangelo, F.; Cerrone, M.; Liguori, G.; Cantile, M.; Anniciello, A.M.; Scala, S.; D’Alterio, C.; Trimarco, C.; Ianaro, A.; et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J. Transl. Med. 2017, 15, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan, B.L.; Bailey, E.; Gill, D.M.; Agarwal, N. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front. Oncol. 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.; Krykbaeva, I.; Damsky, W.; Kluger, H.M.; Bosenberg, M. Evaluating the role of the COX2/PGE2 pathway in anti-melanoma immunity. J. Clin. Oncol. 2019, 37, e14114. [Google Scholar] [CrossRef]
- Chen, M.; Pych, E.; Corpron, C.; Harmon, C.M. Regulation of Cd36 Expression. In Human Melanoma Cells; Springer: Boston, MA, USA, 2002; pp. 337–342. [Google Scholar] [CrossRef]
- Martini, C.; DeNichilo, M.; King, D.P.; Cockshell, M.P.; Ebert, B.; Dale, B.; Ebert, L.M.; Woods, A.; Bonder, C.S. CD36 promotes vasculogenic mimicry in melanoma by mediating adhesion to the extracellular matrix. BMC Cancer 2021, 21, 765. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Qu, Y.Y.; Wang, J.; Wang, H.K.; Wan, F.N.; Zhang, H.L.; Ye, D.W.; Zhao, J.Y. Elevated CD36 expression correlates with increased visceral adipose tissue and predicts poor prognosis in ccRCC patients. J. Cancer 2019, 10, 4522–4531. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Carrié, L.; Dufau, C.; Nieto, L.; Ségui, B.; Levade, T.; Riond, J.; Andrieu-Abadie, N. Lipid metabolic Reprogramming: Role in Melanoma Progression and Therapeutic Perspectives. Cancers 2020, 12, 3147. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Yilmaz, Ç.; Köksoy, S.; Çeker, T.; Aslan, M. Diclofenac down-regulates COX-2 induced expression of CD44 and ICAM-1 in human HT29 colorectal cancer cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 2259–2272. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, H.R.; Kim, Y.J.; Lee, J.H.; Park, Y.S.; Cho, S.H.; Joo, J.K. Expression of standard CD44 in human colorectal carcinoma: Association with prognosis. Pathol. Int. 2009, 59, 241–246. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Xie, L.; Huang, A.; Xue, C.; Gu, Z.; Wang, K.; Zong, S. The prognostic and clinical value of CD44 in colorectal cancer: A meta-analysis. Front. Oncol. 2019, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kang, S.M.; Sawada, T.; Nishiguchi, Y.; Yashiro, M.; Ogawa, Y.; Ohira, M.; Ishikawa, T.; Chung, K.H.Y. Expression of intercellular adhesion molecule-1 and prognosis in colorectal cancer. Oncol. Rep. 2002, 9, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Özbolat, S.N.; Ayna, A. Chrysin Suppresses HT-29 Cell Death Induced by Diclofenac through Apoptosis and Oxidative Damage. Nutr. Cancer 2021, 73, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Ralph, S.J.; Pritchard, R.; Rodríguez-Enríquez, S.; Moreno-Sánchez, R.; Ralph, R.K. Hitting the Bull’s-Eye in Metastatic Cancers—NSAIDs Elevate ROS in Mitochondria, Inducing Malignant Cell Death. Pharmaceuticals 2015, 8, 62–106. [Google Scholar] [CrossRef] [Green Version]
- Elsayed Azab, A.; A Adwas, A.; Ibrahim Elsayed, A.S.; A Adwas, A.; Ibrahim Elsayed, A.S.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Gonçalves, V.; Henriques, A.F.A.; Matos, P.; Jordan, P. Ibuprofen disrupts a WNK1/GSK3β/SRPK1 protein complex required for expression of tumor-related splicing variant RAC1B in colorectal cells. Oncotarget 2020, 11, 4421–4437. [Google Scholar] [CrossRef]
- Melzer, C.; Hass, R.; Lehnert, H.; Ungefroren, H. RAC1B: A Rho GTPase with Versatile Functions in Malignant Transformation and Tumor Progression. Cells 2019, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Matos, P.; Jordan, P. Expression of Rac1b stimulates NF-κB-mediated cell survival and G1/S progression. Exp. Cell Res. 2005, 305, 292–299. [Google Scholar] [CrossRef]
- Matos, P.; Kotelevets, L.; Gonçalves, V.; Henriques, A.; Zerbib, P.; Moyer, M.P.; Chastre, E.; Jordan, P. Ibuprofen inhibits colitis-induced overexpression of tumor- related Rac1b. Neoplasia 2013, 15, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Akrami, H.; Aminzadeh, S.; Fallahi, H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumor Biol. 2015, 36, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mai, Z.; Zhao, M.; Wang, B.; Yu, S.; Wang, X.; Chen, T. Aspirin induces oncosis in tumor cells. Apoptosis 2019, 24, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Fujita, K.; Gong, J.; Nakahara, M.; Iwama, H.; Liu, S.; Yoneyama, H.; Morishita, A.; Nomura, T.; Tani, J.; et al. Aspirin inhibits hepatocellular carcinoma cell proliferation in vitro and in vivo via inducing cell cycle arrest and apoptosis. Oncol. Rep. 2020, 44, 457–468. [Google Scholar] [CrossRef]
- Olejniczak-Kęder, A.; Szaryńska, M.; Wrońska, A.; Siedlecka-Kroplewska, K.; Kmieć, Z. Effects of 5-FU and anti-EGFR antibody in combination with ASA on the spherical culture system of HCT116 and HT29 colorectal cancer cell lines. Int. J. Oncol. 2019, 55, 223–242. [Google Scholar] [CrossRef]
- Wang, M.; Su, P. The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: An update. Syst. Biol. Reprod. Med. 2018, 64, 93–102. [Google Scholar] [CrossRef]
- Ohashi, N.; Kohno, T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front. Pharmacol. 2020, 11, 1916. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Pan, B.; Chen, Y.; Zhang, T.; Tang, N. Interleukin 33 mediates hepatocyte autophagy and innate immune response in the early phase of acetaminophen-induced acute liver injury. Toxicology 2021, 456, 152788. [Google Scholar] [CrossRef]
- Li, B.X.; Li, C.Y.; Peng, R.Q.; Wu, X.J.; Wang, H.Y.; Wan, D.S.; Zhu, X.F.; Zhang, X.S. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy 2009, 5, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Ginting, C.N.; Lister, I.N.E.; Girsang, E.; Widowati, W.; Yusepany, D.T.; Azizah, A.M.; Kusuma, H.S.W. Hepatotoxicity prevention in Acetaminophen-induced HepG2 cells by red betel (Piper crocatum Ruiz and Pav) extract from Indonesia via antioxidant, anti-inflammatory, and anti-necrotic. Heliyon 2021, 7, e05620. [Google Scholar] [CrossRef]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Jannuzzi, A.T.; Kara, M.; Alpertunga, B. Celastrol ameliorates acetaminophen-induced oxidative stress and cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2018, 37, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.; Saczko, J.; Chwilkowska, A. [Oxidative stress in cells damage processes]. Pol. Merkur. Lek. 2009, 27, 44–47. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, N.; Sałek, A.; Sawińska, M.; Kuchar, E.; Wiela-Hojeńska, A.; Karłowicz-Bodalska, K. Effects of Non-Opioid Analgesics on the Cell Membrane of Skin and Gastrointestinal Cancers. Int. J. Mol. Sci. 2022, 23, 7096. https://doi.org/10.3390/ijms23137096
Janicka N, Sałek A, Sawińska M, Kuchar E, Wiela-Hojeńska A, Karłowicz-Bodalska K. Effects of Non-Opioid Analgesics on the Cell Membrane of Skin and Gastrointestinal Cancers. International Journal of Molecular Sciences. 2022; 23(13):7096. https://doi.org/10.3390/ijms23137096
Chicago/Turabian StyleJanicka, Natalia, Agnieszka Sałek, Magdalena Sawińska, Ernest Kuchar, Anna Wiela-Hojeńska, and Katarzyna Karłowicz-Bodalska. 2022. "Effects of Non-Opioid Analgesics on the Cell Membrane of Skin and Gastrointestinal Cancers" International Journal of Molecular Sciences 23, no. 13: 7096. https://doi.org/10.3390/ijms23137096
APA StyleJanicka, N., Sałek, A., Sawińska, M., Kuchar, E., Wiela-Hojeńska, A., & Karłowicz-Bodalska, K. (2022). Effects of Non-Opioid Analgesics on the Cell Membrane of Skin and Gastrointestinal Cancers. International Journal of Molecular Sciences, 23(13), 7096. https://doi.org/10.3390/ijms23137096







