Human Sarcopenic Myoblasts Can Be Rescued by Pharmacological Reactivation of HIF-1α
Abstract
:1. Introduction
2. Results
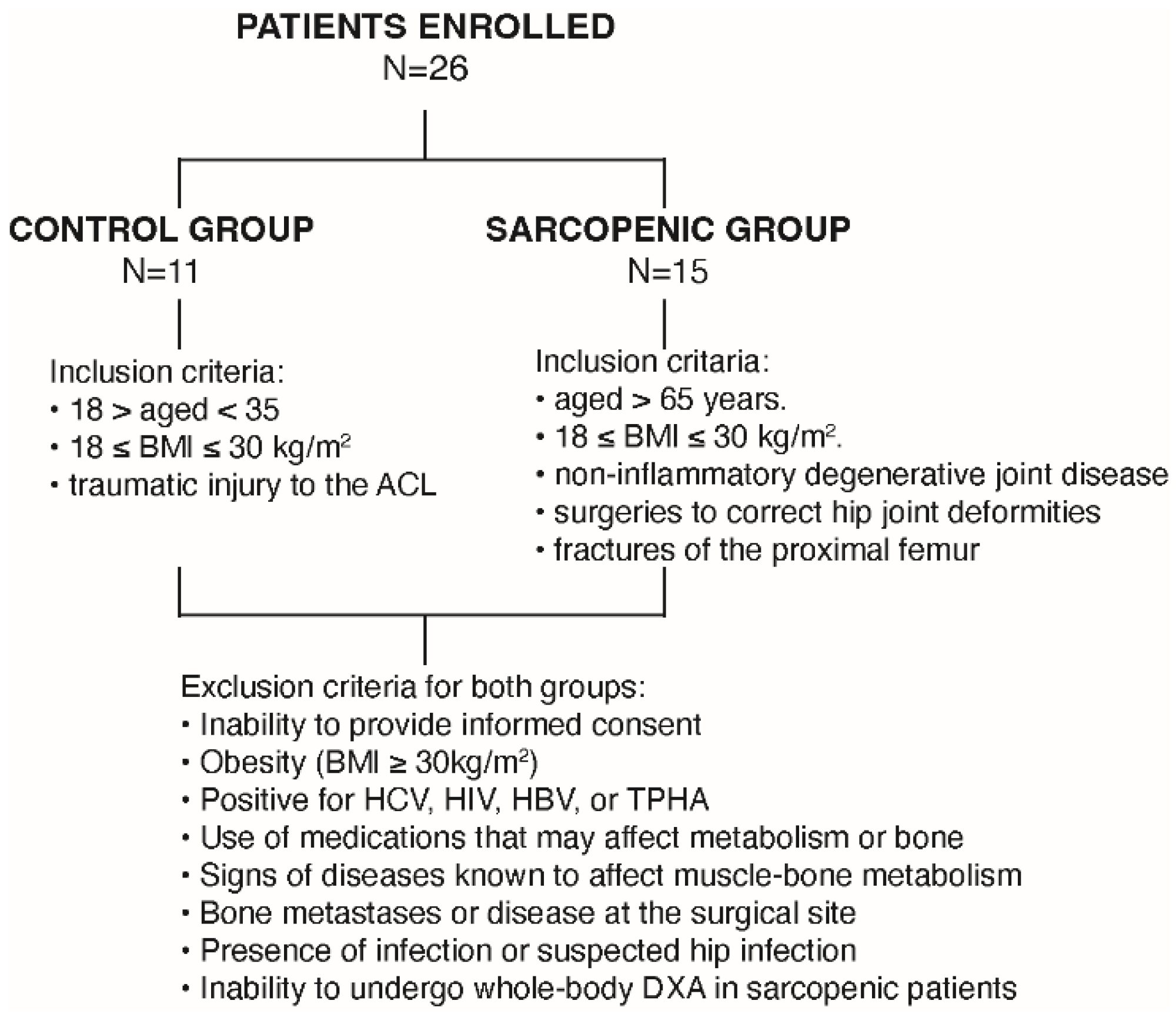
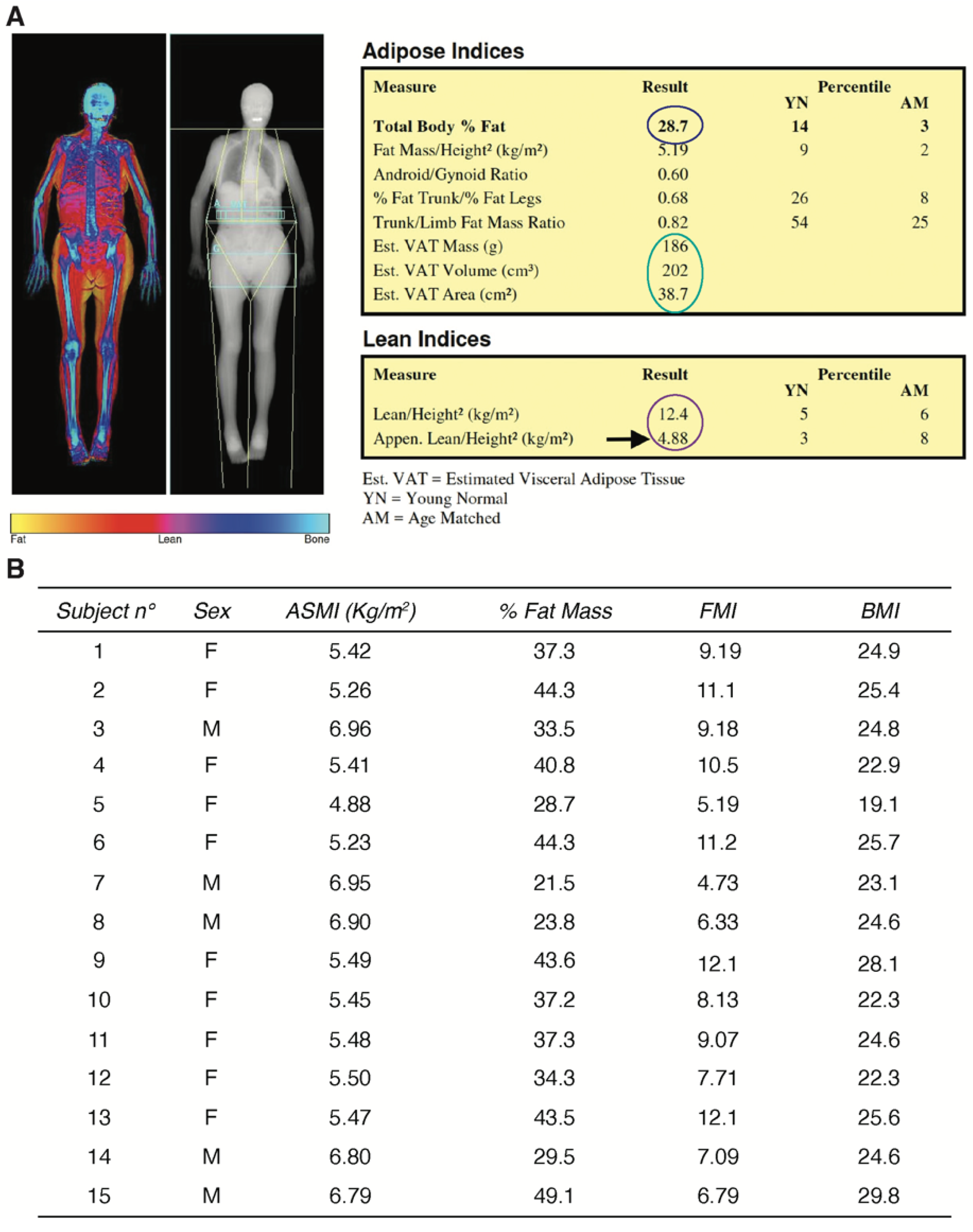
2.1. Study Population and Clinical Characterization of the Human Sarcopenic Phenotype
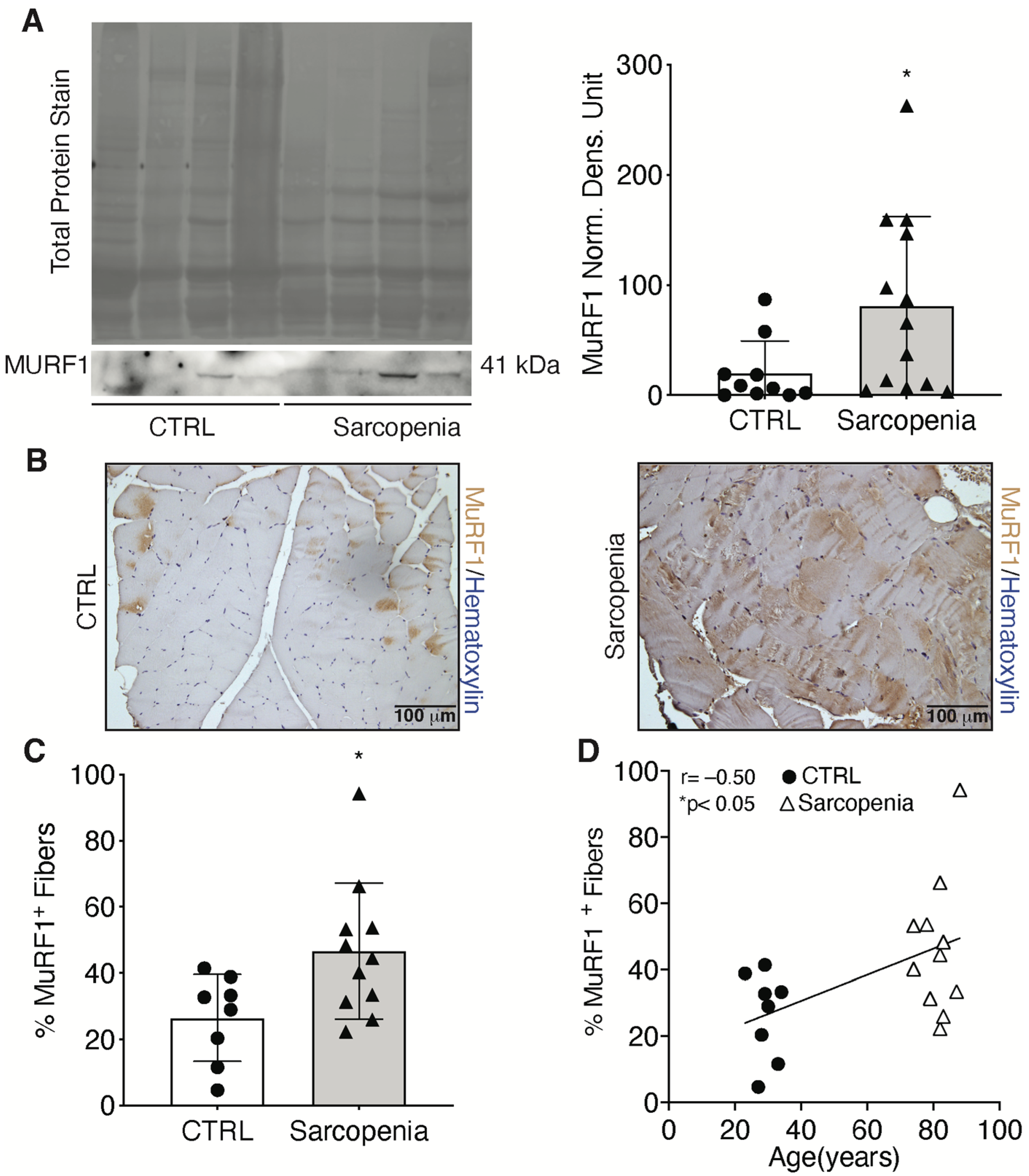
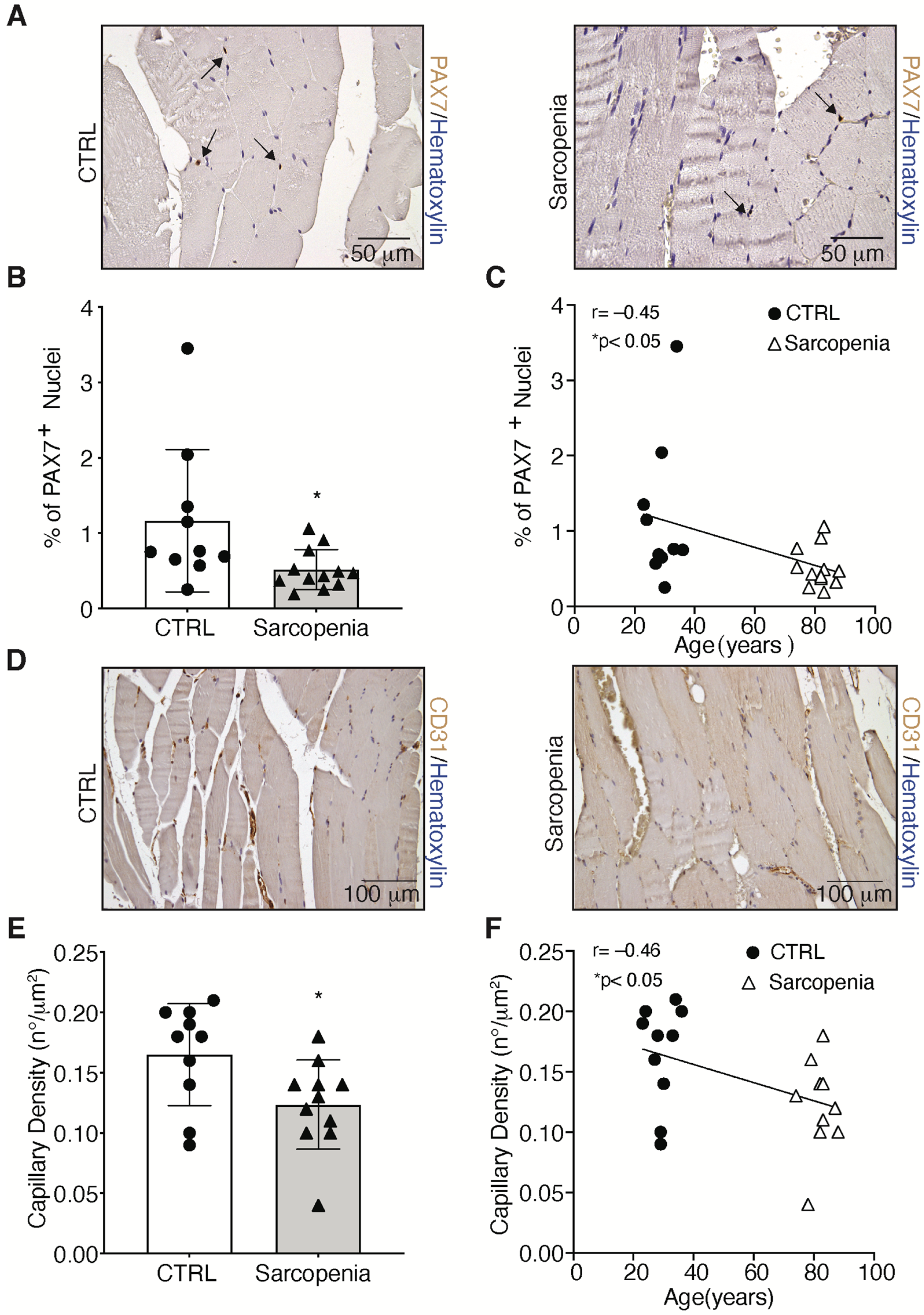
2.2. Sarcopenia Induces the Activation of MURF1 and Reduction of PAX7 in Human Biopsies
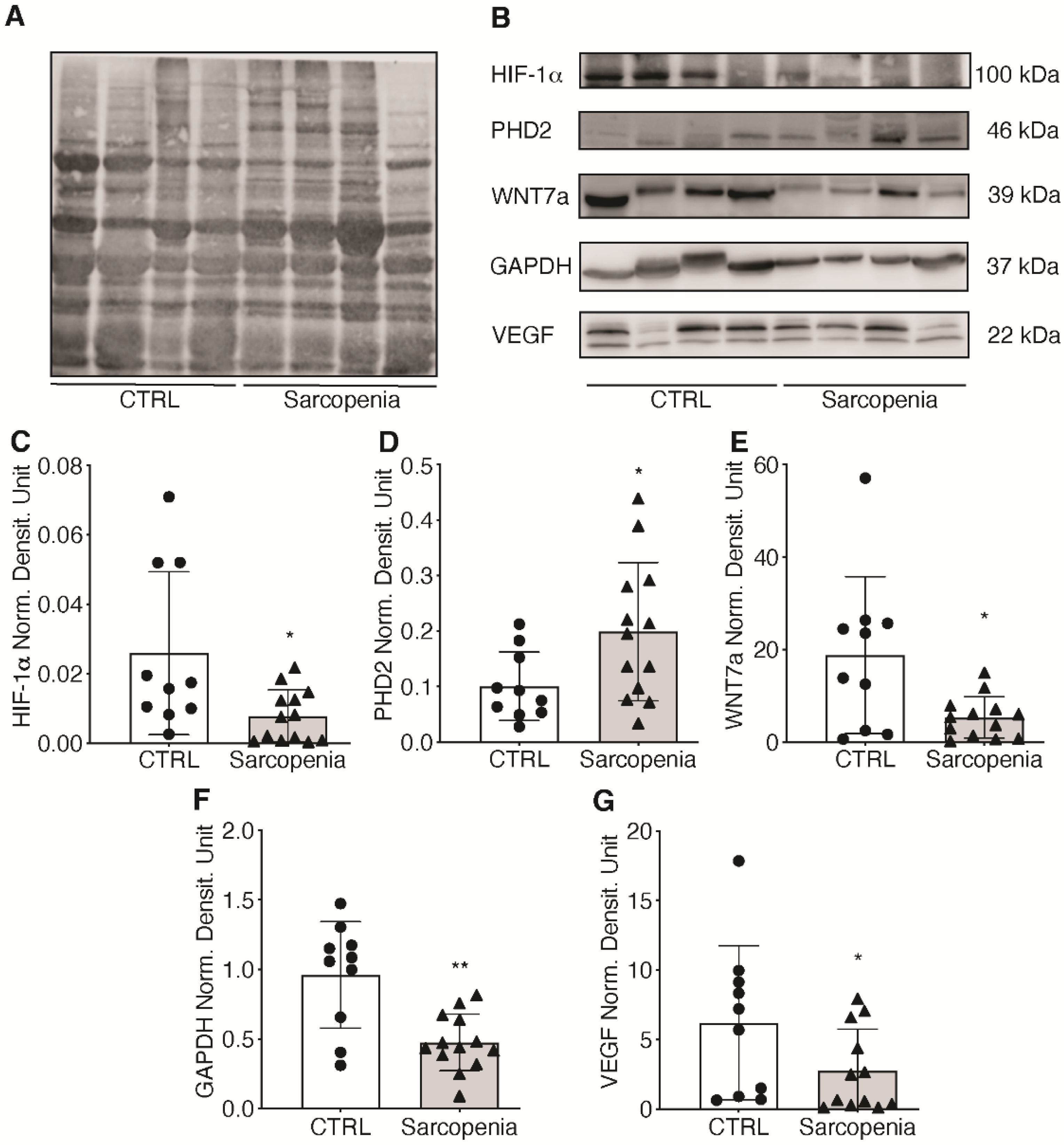
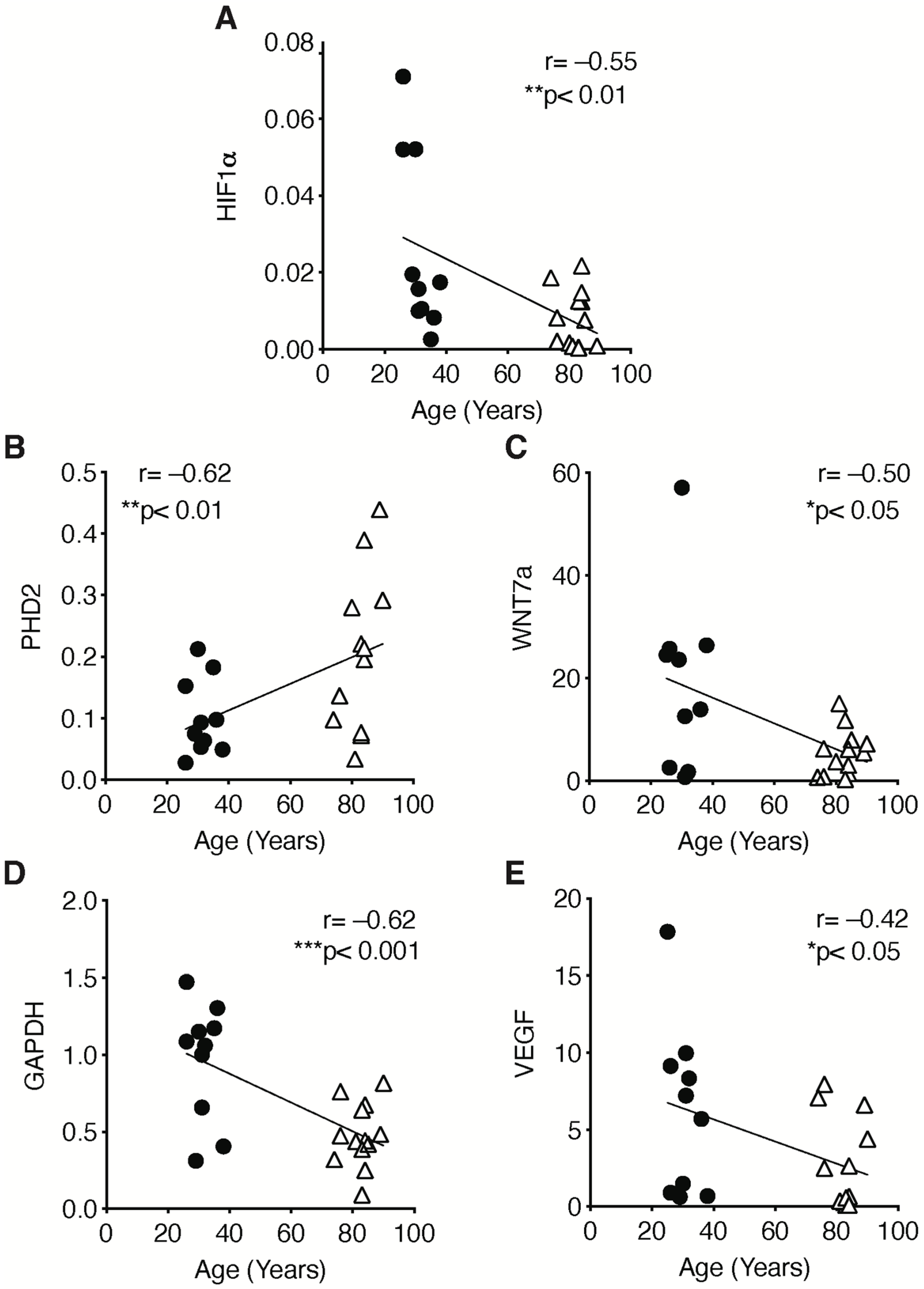
2.3. The Hypoxic Signaling Pathway Is Strongly Impaired in Human Sarcopenic Patients
2.4. The Pharmacological Activation of HIF-1α Counteracts the Sarcopenic Phenotype
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Anthropometric Examination
4.3. Blood Sample Analyses
4.4. Whole-Body DXA
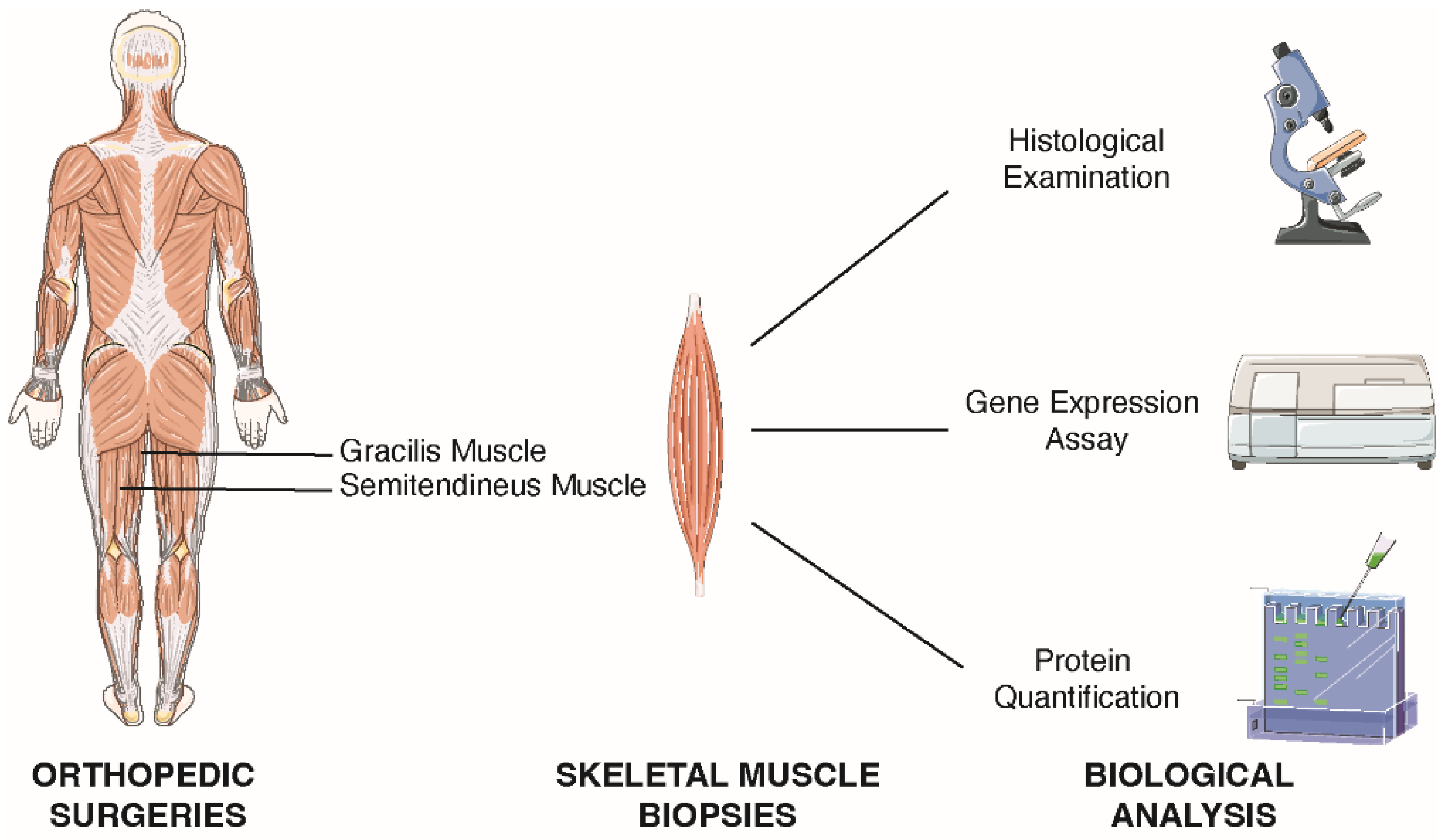
4.5. Skeletal Muscle Tissue Collection
4.6. Skeletal Muscle Cells Isolation
4.7. TRIzol RNA and Protein Extraction
4.8. Gene Expression by Real-Time Quantitative PCR (qPCR)
4.9. Western Blot
4.10. Immunohistochemistry and Quantification
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons; ST/ESA/SER.A/451; United Nations: New York, NY, USA, 2020. [Google Scholar]
- Shen, Y.; Liu, D.; Li, S.; He, Y.; Tan, F.; Sun, X.; Li, D.; Xia, X.; Hao, Q. Effects of Exercise on Patients Important Outcomes in Older People With Sarcopenia: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Front. Med. 2022, 9, 811746. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; et al. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. BioMed Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Lena, A.; Anker, M.S.; Springer, J. Muscle Wasting and Sarcopenia in Heart Failure-The Current State of Science. Int. J. Mol. Sci. 2020, 21, 6549. [Google Scholar] [CrossRef]
- Han, P.; Yu, H.; Ma, Y.; Kang, L.; Fu, L.; Jia, L.; Chen, X.; Yu, X.; Hou, L.; Wang, L.; et al. The increased risk of sarcopenia in patients with cardiovascular risk factors in Suburb-Dwelling older Chinese using the AWGS definition. Sci. Rep. 2017, 7, 9592. [Google Scholar] [CrossRef]
- Frontera, W.R.; Suh, D.; Krivickas, L.S.; Hughes, V.A.; Goldstein, R.; Roubenoff, R. Skeletal muscle fiber quality in older men and women. Am. J. Physiol. Cell Physiol. 2000, 279, C611–C618. [Google Scholar] [CrossRef] [PubMed]
- Rotini, A.; Martinez-Sarra, E.; Duelen, R.; Costamagna, D.; Di Filippo, E.S.; Giacomazzi, G.; Grosemans, H.; Fulle, S.; Sampaolesi, M. Aging affects the in vivo regenerative potential of human mesoangioblasts. Aging Cell 2018, 17, e12714. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, A.; Contreras-Hernandez, I.; Castro-Sepulveda, M.; Campos, C.A.; Figueroa, R.; Tevy, M.F.; Eisner, V.; Casas, M.; Jaimovich, E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging 2018, 10, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Cataldi, A.; Di Giulio, C. “Oxygen supply” as modulator of aging processes: Hypoxia and hyperoxia models for aging studies. Curr. Aging Sci. 2009, 2, 95–102. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Sarcopenia and age-related endocrine function. Int. J. Endocrinol. 2012, 2012, 127362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenkel-Denkberg, G.; Gershon, D.; Levy, A.P. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999, 462, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Katschinski, D.M. Is there a molecular connection between hypoxia and aging? Exp. Gerontol. 2006, 41, 482–484. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Flann, K.L.; Cardinal, T.R.; Rathbone, C.R.; Liu, X.; Allen, R.E. Satellite cells isolated from aged or dystrophic muscle exhibit a reduced capacity to promote angiogenesis in vitro. Biochem. Biophys. Res. Commun. 2013, 440, 399–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giulio, C.; Bianchi, G.; Cacchio, M.; Artese, L.; Rapino, C.; Macri, M.A.; Di Ilio, C. Oxygen and life span: Chronic hypoxia as a model for studying HIF-1alpha, VEGF and NOS during aging. Respir. Physiol. Neurobiol. 2005, 147, 31–38. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Parizkova, J.; Eiselt, E.; Sprynarova, S.; Wachtlova, M. Body composition, aerobic capacity, and density of muscle capillaries in young and old men. J. Appl. Physiol. 1971, 31, 323–325. [Google Scholar] [CrossRef]
- DeLisser, H.M.; Christofidou-Solomidou, M.; Strieter, R.M.; Burdick, M.D.; Robinson, C.S.; Wexler, R.S.; Kerr, J.S.; Garlanda, C.; Merwin, J.R.; Madri, J.A.; et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 1997, 151, 671–677. [Google Scholar]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berra, E.; Benizri, E.; Ginouves, A.; Volmat, V.; Roux, D.; Pouyssegur, J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003, 22, 4082–4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirillo, F.; Resmini, G.; Angelino, E.; Ferrara, M.; Tarantino, A.; Piccoli, M.; Rota, P.; Ghiroldi, G.; Monasky, M.M.; Ciconte, G.; et al. HIF-1α Directly Controls WNT7A Expression During Myogenesis. Front. Cell Dev. Biol. 2020, 8, 593508. [Google Scholar] [CrossRef]
- Higashimura, Y.; Nakajima, Y.; Yamaji, R.; Harada, N.; Shibasaki, F.; Nakano, Y.; Inui, H. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch. Biochem. Biophys. 2011, 509, 1–8. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Bentzinger, C.F.; Rudnicki, M.A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat. Cell Biol. 2011, 14, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigelso, A.; Dybboe, R.; Hansen, C.N.; Dela, F.; Helge, J.W.; Guadalupe Grau, A. GAPDH and beta-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J. Appl. Physiol. 2015, 118, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; McGough, R.; Aswad, B.; Block, J.A.; Terek, R. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J. Orthop. Res. 2004, 22, 1175–1181. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Eckardt, K.U. HIF prolyl hydroxylase inhibitors for the treatment of renal Nat. Rev. Nephrol. 2016, 12, 157–168. [Google Scholar]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J. Cell. Biochem. 2016, 117, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, F.; Resmini, G.; Ghiroldi, A.; Piccoli, M.; Bergante, S.; Tettamanti, G.; Anastasia, L. Activation of the hypoxia-inducible factor 1alpha promotes myogenesis through the noncanonical Wnt pathway, leading to hypertrophic myotubes. FASEB J. 2017, 31, 2146–2156. [Google Scholar] [CrossRef] [Green Version]
- Verdijk, L.B.; Koopman, R.; Schaart, G.; Meijer, K.; Savelberg, H.H.; van Loon, L.J. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E151–E157. [Google Scholar] [CrossRef] [Green Version]
- Brack, A.S.; Bildsoe, H.; Hughes, S.M. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell Sci. 2005, 118, 4813–4821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open Biol. 2020, 10, 200048. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, S.F.; Jaspers, R.T.; Jones, D.A.; Degens, H. Time-course of changes in the myonuclear domain during denervation in young-adult and old rat gastrocnemius muscle. Muscle Nerve 2011, 43, 212–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, R.; Zweyer, M.; Knauf, U.; Mundegar, R.R.; Wernig, A. The ontogeny of soleus muscles in mdx and wild type mice. Neuromuscul. Disord. 2005, 15, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Landers-Ramos, R.Q.; Prior, S.J. The Microvasculature and Skeletal Muscle Health in Aging. Exerc. Sport Sci. Rev. 2018, 46, 172–179. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Joanisse, S.; Snijders, T.; Ivankovic, V.; Baker, S.K.; Phillips, S.M.; Parise, G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J. Cachexia Sarcopenia Muscle 2016, 7, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Kaelin, W.G., Jr. The von Hippel-Lindau tumor suppressor protein: New insights into oxygen sensing and cancer. Curr. Opin. Genet. Dev. 2003, 13, 55–60. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [Green Version]
- Pircher, T.; Wackerhage, H.; Aszodi, A.; Kammerlander, C.; Bocker, W.; Saller, M.M. Hypoxic Signaling in Skeletal Muscle Maintenance and Regeneration: A Systematic Review. Front. Physiol. 2021, 12, 684899. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardani, R.; Baldassa, S.; Botta, A.; Rinaldi, F.; Novelli, G.; Mancinelli, E.; Meola, G. Ribonuclear inclusions and MBNL1 nuclear sequestration do not affect myoblast differentiation but alter gene splicing in myotonic dystrophy type 2. Neuromuscul. Disord. 2009, 19, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zaccagnini, G.; Palmisano, A.; Canu, T.; Maimone, B.; Lo Russo, F.M.; Ambrogi, F.; Gaetano, C.; De Cobelli, F.; Del Maschio, A.; Esposito, A.; et al. Magnetic Resonance Imaging Allows the Evaluation of Tissue Damage and Regeneration in a Mouse Model of Critical Limb Ischemia. PLoS ONE 2015, 10, e0142111. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, F.; Mangiavini, L.; La Rocca, P.; Piccoli, M.; Ghiroldi, A.; Rota, P.; Tarantino, A.; Canciani, B.; Coviello, S.; Messina, C.; et al. Human Sarcopenic Myoblasts Can Be Rescued by Pharmacological Reactivation of HIF-1α. Int. J. Mol. Sci. 2022, 23, 7114. https://doi.org/10.3390/ijms23137114
Cirillo F, Mangiavini L, La Rocca P, Piccoli M, Ghiroldi A, Rota P, Tarantino A, Canciani B, Coviello S, Messina C, et al. Human Sarcopenic Myoblasts Can Be Rescued by Pharmacological Reactivation of HIF-1α. International Journal of Molecular Sciences. 2022; 23(13):7114. https://doi.org/10.3390/ijms23137114
Chicago/Turabian StyleCirillo, Federica, Laura Mangiavini, Paolo La Rocca, Marco Piccoli, Andrea Ghiroldi, Paola Rota, Adriana Tarantino, Barbara Canciani, Simona Coviello, Carmelo Messina, and et al. 2022. "Human Sarcopenic Myoblasts Can Be Rescued by Pharmacological Reactivation of HIF-1α" International Journal of Molecular Sciences 23, no. 13: 7114. https://doi.org/10.3390/ijms23137114
APA StyleCirillo, F., Mangiavini, L., La Rocca, P., Piccoli, M., Ghiroldi, A., Rota, P., Tarantino, A., Canciani, B., Coviello, S., Messina, C., Ciconte, G., Pappone, C., Peretti, G. M., & Anastasia, L. (2022). Human Sarcopenic Myoblasts Can Be Rescued by Pharmacological Reactivation of HIF-1α. International Journal of Molecular Sciences, 23(13), 7114. https://doi.org/10.3390/ijms23137114







