Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses
Abstract
:1. Introduction
2. Results
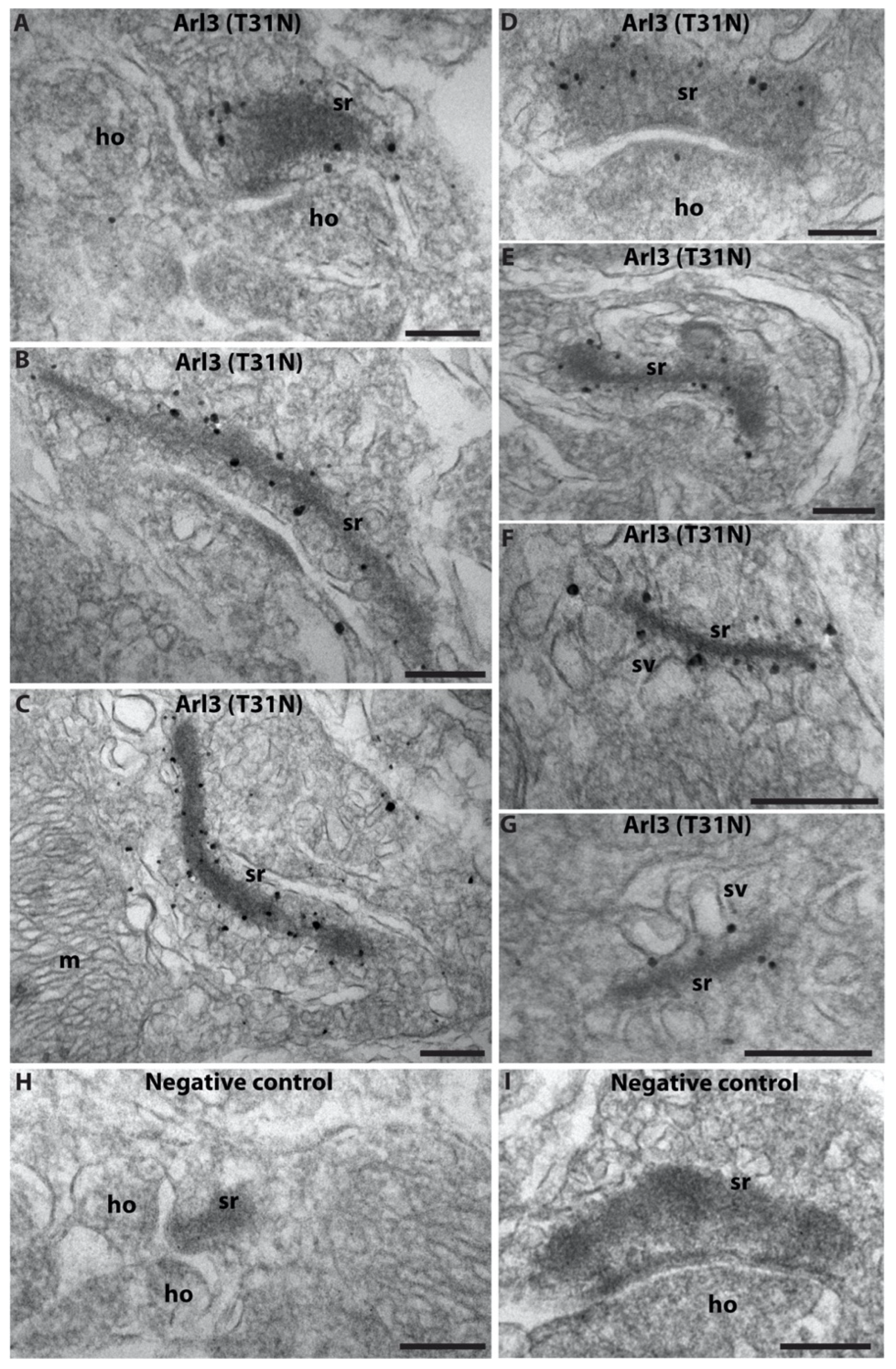
2.1. Arl3 Is Enriched at the Photoreceptor Synaptic Ribbon
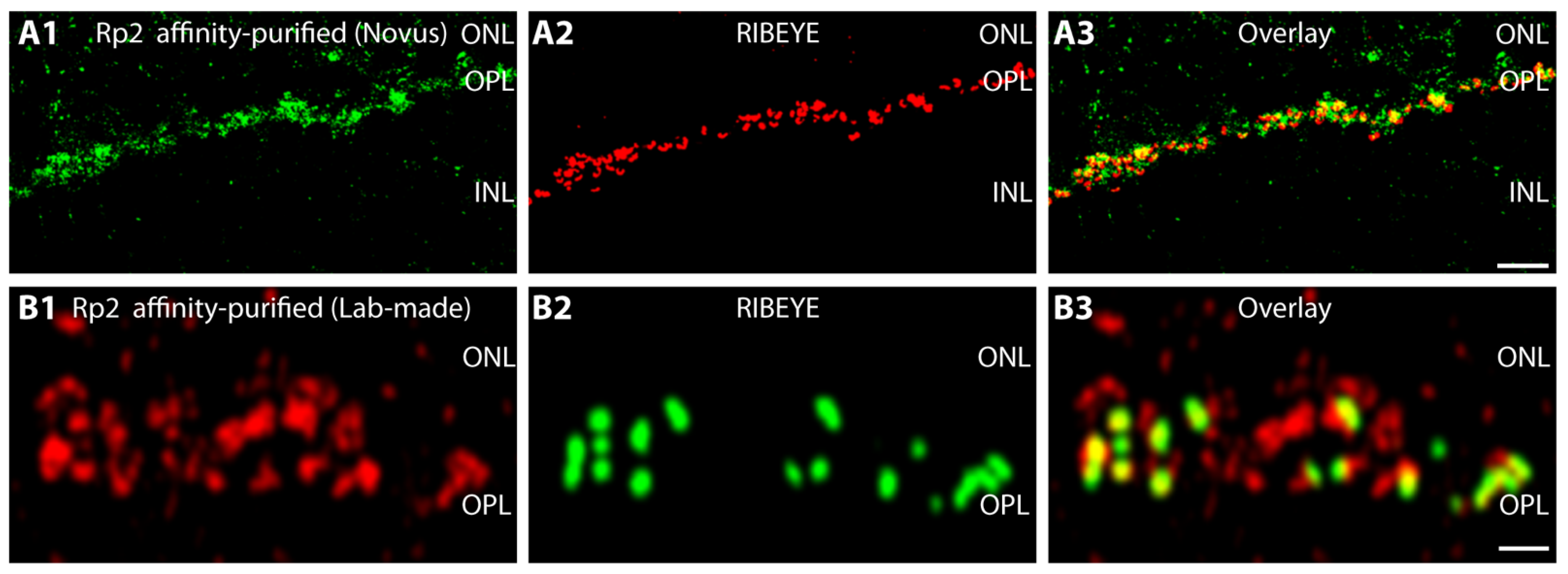
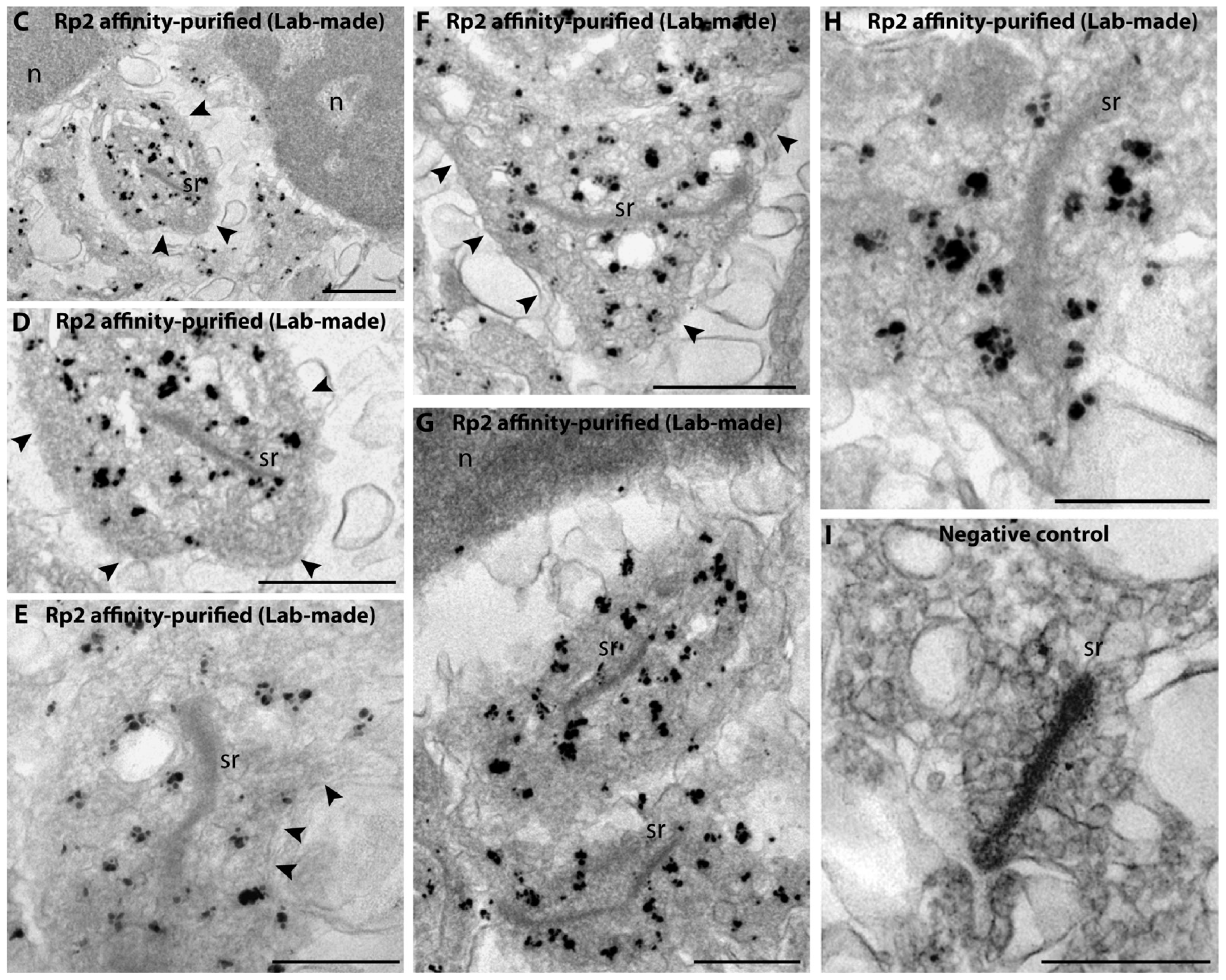
2.2. Rp2 Is Enriched on Synaptic Vesicles in the Presynaptic Photoreceptor Terminal
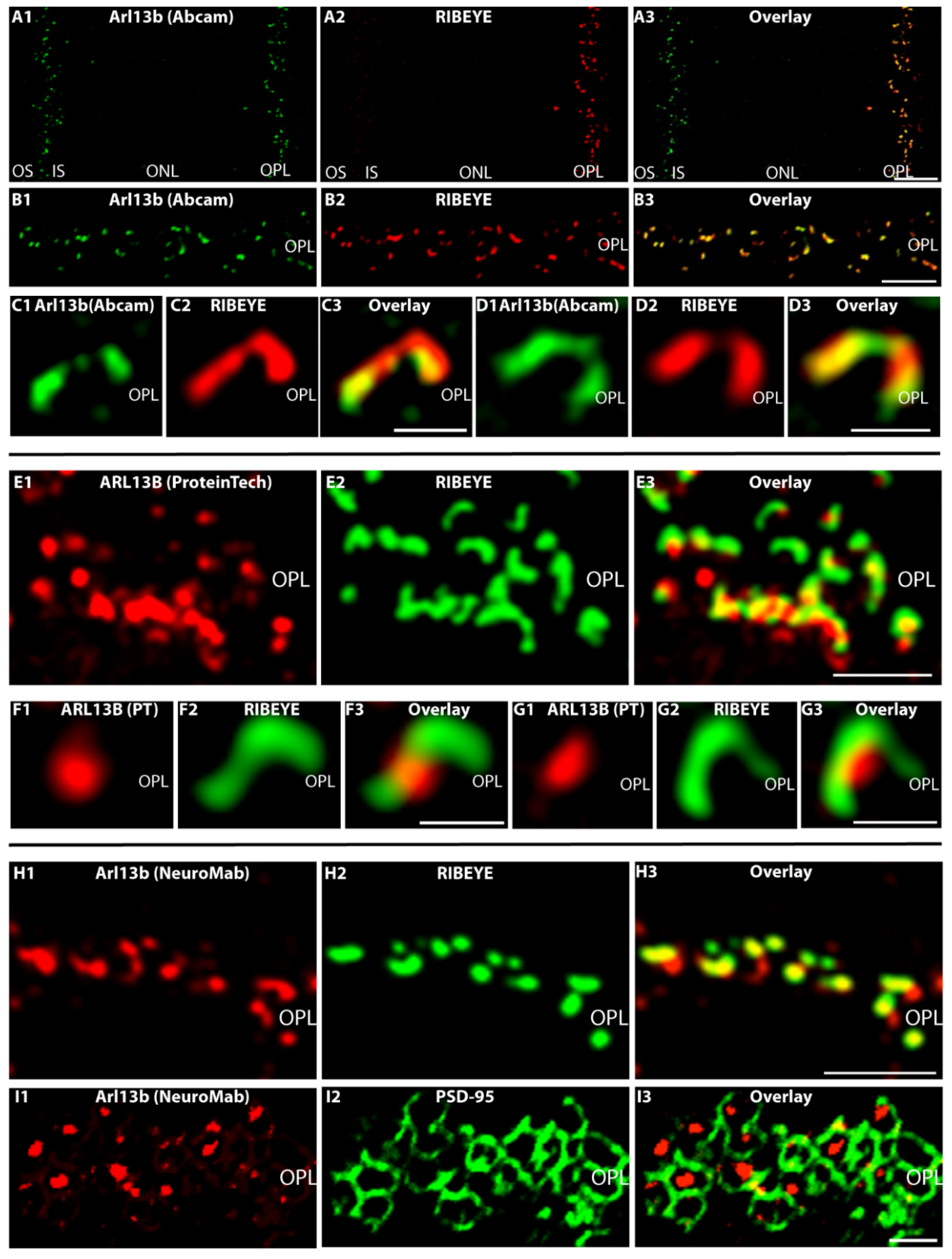
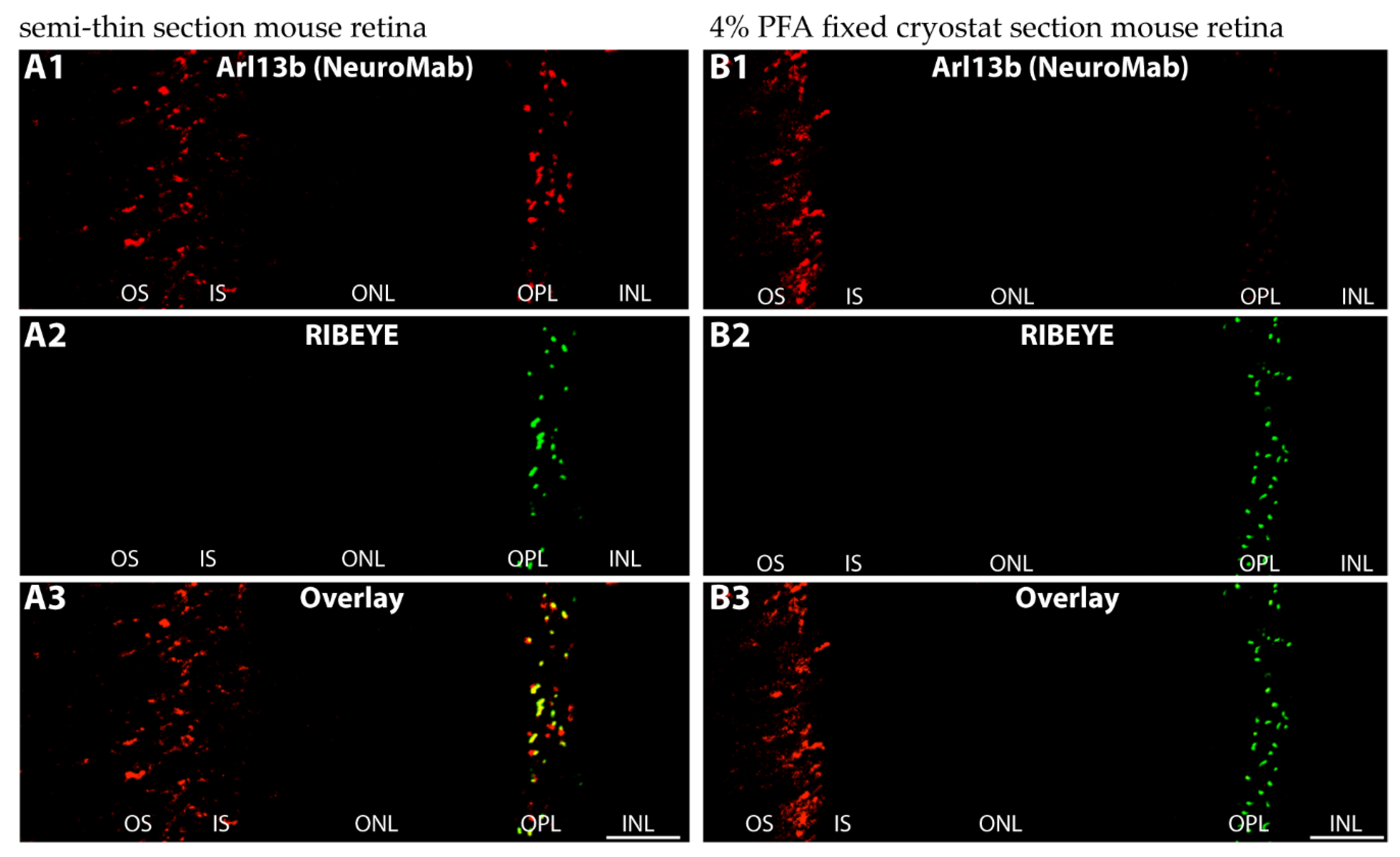
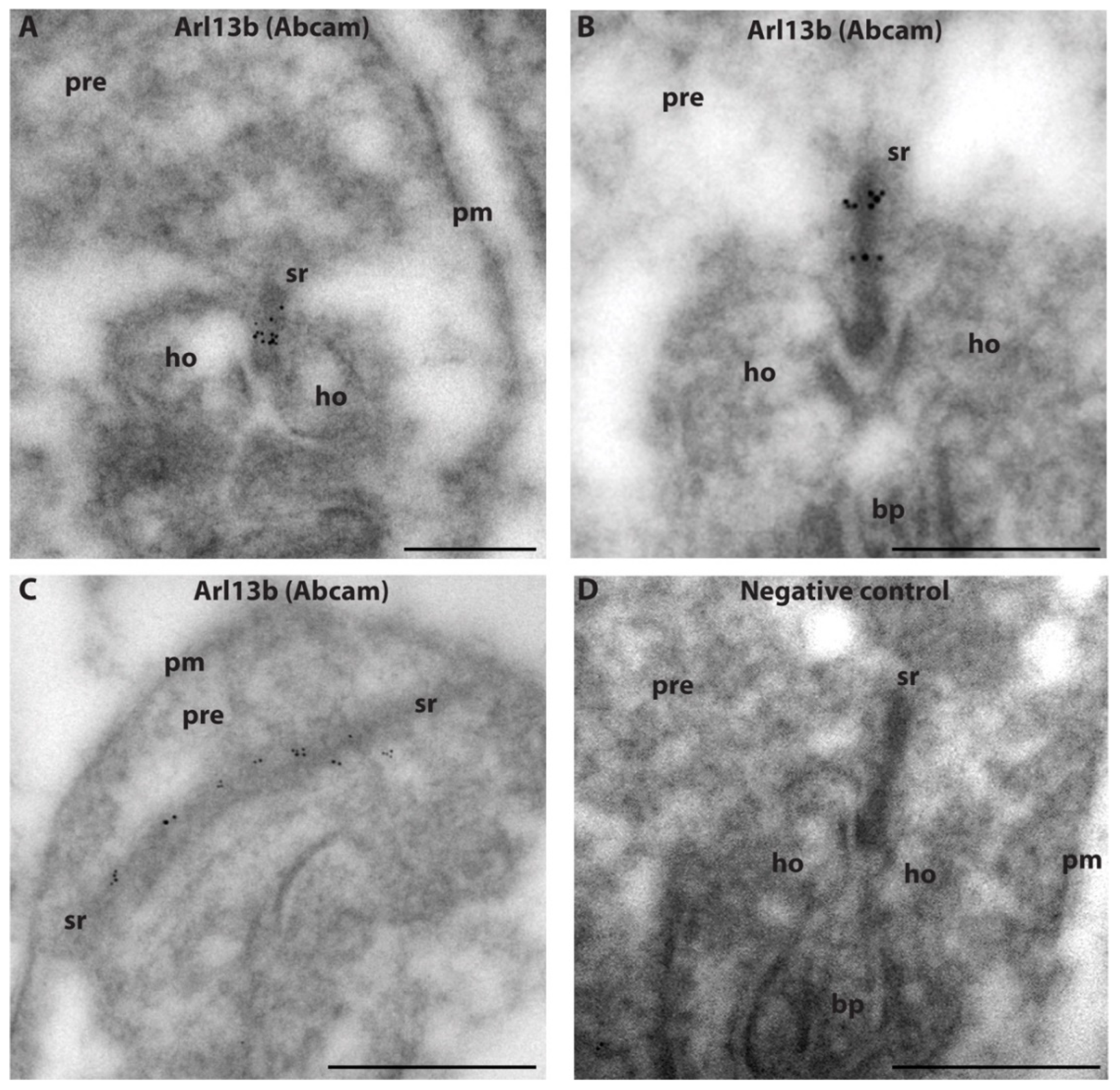
2.3. Arl13b Is Enriched on the Synaptic Ribbon in Rod Photoreceptor Terminals
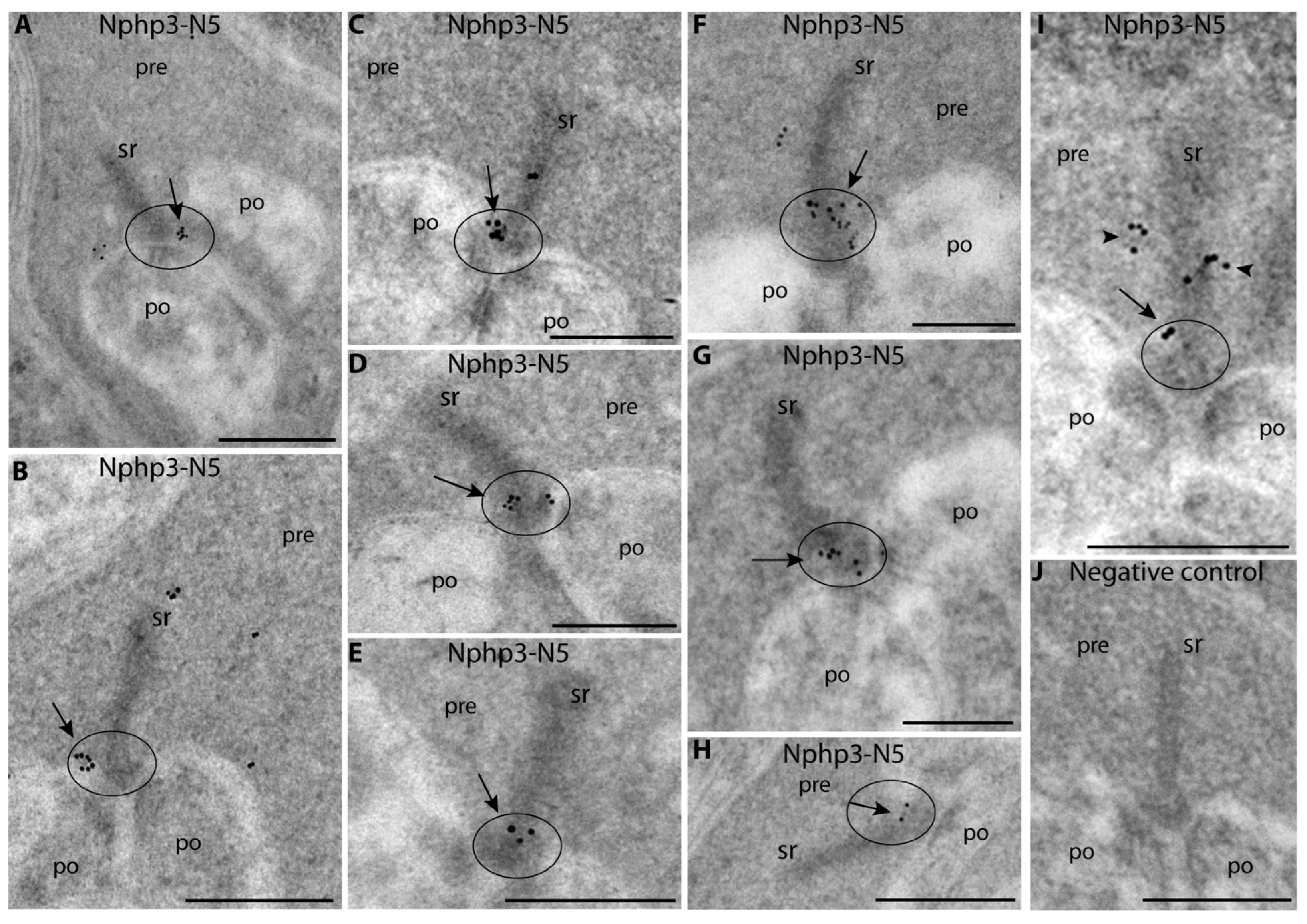
2.4. Nphp3 (Nephrocystin-3), a Disease-Relevant Client Protein of Unc119, Is Highly Enriched at the Synaptic Ribbon, Particularly at the Active Zone
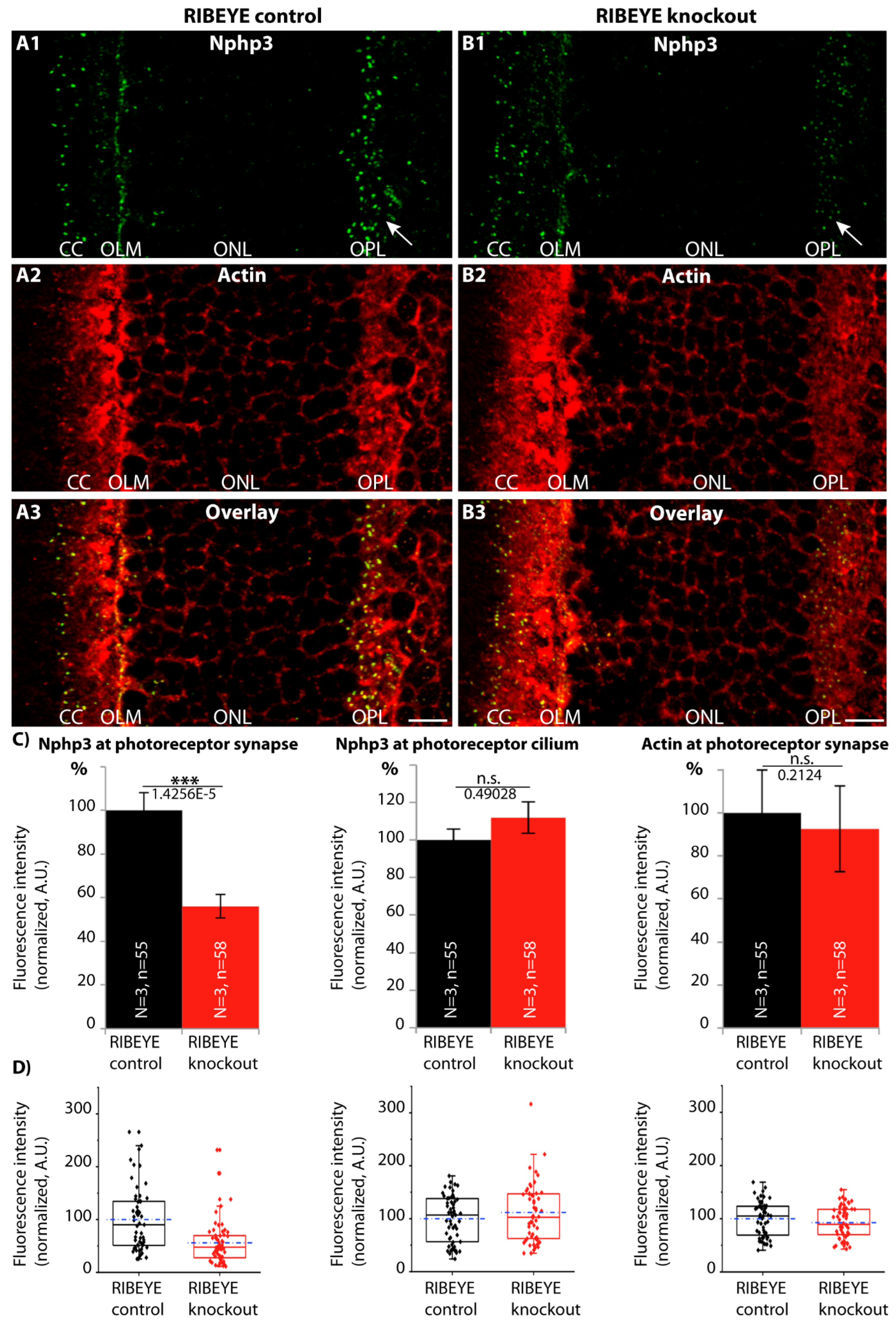
2.5. The Synaptic Ribbon Enhances Synaptic Enrichment of Nphp3
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Primary Antibodies
4.2.1. Arl3
- -
- Anti-Arl3(WT): Lab-made rabbit polyclonal antiserum against full-length rat Arl3. The antiserum was raised against a pGEX fusion protein construct encoding aa1-182 of rat Arl3. The full-length insert was cloned via NcoI/SalI in frame into the respective sites of pGEX-KG. The antiserum was used in a 1:100 dilution for IF and in a 1:500 dilution for WB. The affinity-purified antibody was used in a 1:50 dilution for IF and in a 1:100 dilution for WB.
- -
- Anti-Arl3(T31N): Lab-made rabbit polyclonal antiserum against a point-mutated Arl3(T31N)-MBP fusion protein. The Arl3(T31N) antibody also detects wild-type Arl3 protein (see Supplementary Figure S1). The T31N point mutant of Arl3 mimics the conformation of GDP-bound Arl3 [37,38]. We used the Arl3(T31N) point mutant for immunization because we wanted to elicit an enhanced antibody response against Arl3 by exposing additional conformational epitopes of Arl3 to the immune system. It is known that Arl3 is a difficult protein for the generation of antibodies [68]. With the described strategy of using a point-mutated protein variant, we wanted to increase the likelihood of generating a good polyclonal antibody response against Arl3. Indeed, with this strategy we obtained an antibody that readily detected the Arl3 wild-type protein in Western blot analyses (Supplementary Figure S1). Therefore, we used this antibody as a second independent antibody for the localization of Arl3. For our present study, it is not relevant whether the antibody would also detect the T31N point mutation because we only analyzed wild-type retinas.
4.2.2. Anti-Rp2 Antibodies
- -
- Anti-Rp2 (lab-made rabbit polyclonal antiserum against full-length bovine RP2): The corresponding cDNA insert was amplified using forward primer AAAAGAATTCATGGGCTGCTTCTTCTCC, reverse primer AAAAGGATCCT CATATTCCCATCTGTATATC and Image clone 8433421 (BC153222; bovine RP2) as the template. The cDNA construct was cloned into the EcoRI/BamHI sites of pMalC2New [66]. The antibody was used for IF in a 1:100 dilution, for WB in a 1:500 dilution and for pre-embedding immunogold EM in a 1:100 dilution.
- -
- Anti-Rp2 (Novus, Wiesbaden, Germany, NBP1-56852, affinity-purified rabbit polyclonal antibody): Raised against the amino acid sequence LEFNGDGAVEVCQLIVNEIFNGTKMFVSESK ETASGDVDSFYNFADIQMG, this is an internal peptide region in the middle of human RP2. This antibody was used for IF in a 1:50 dilution and for WB in a 1:1000 dilution.
4.2.3. Anti-Arl13b Antibodies
- -
- An affinity-purified rabbit anti-Arl13b antibody (Proteintech; Planegg-Martinsried, Germany, 17711-1-AP) raised against the Arl13b-GST fusion protein (Ag12015, Proteintech), encoding full-length human ARL13B (encoding aa 1–321 of BC094725/NP659433 (full-length human ARL13B protein); amino acid sequence 86% identical to bovine ARL13B sequence XP_003585676.1; 75% identical amino acids to mouse Arl13b XP_006522568.1). The antibody was previously characterized (e.g., [39,70,71]) and verified for specificity on the mouse Arl13b knockout tissue by IF and WB [39]. This antibody was used for IF in a 1:50 dilution and for WB in a 1:1000 dilution. For preabsorption control experiments, the antibody was preabsorbed with the respective Arl13b-GST fusion protein that was used for immunization (Arl13b-GST fusion protein obtained from Proteintech (#Ag12015)). Further preabsorption control experiments were conducted with GST alone to check for possible non-specific preabsorption (see below).
- -
- An immunogen affinity-purified rabbit polyclonal anti-Arl13b from Abcam (Cambridge, UK, ab83879) raised against a synthetic peptide aa 251–300 of human ARL13B (NP_659433; VEPLNIDDCAPESPTPPPPPPPVGWGTPKVTRLPKLEPLGETHHN DK). This antibody was used for IF in a 1:500 dilution, for post-embedding immunogold in a 1:100 dilution and for WB in a 1:500 dilution.
- -
- A monoclonal Arl13b antibody (NIH NeuroMab/UC Davis, anti-Arl13b, Clone N295B/66) distributed by Antibodies-online, Aachen, Germany. This monoclonal antibody was raised against a fusion protein encoding amino acids 208–427 of the carboxyterminal half of mouse ARL13B (Q640N2, sequence identity in this stretch in comparison to bovine sequence 72%: XP_003585676). The specificity of this antibody was verified on knockout tissue by NeuroMab (documented on NeuroMab Clone N295B/66 datasheet). In WB analyses, this antibody selectively detects an ≈60 kDa band that is absent in the Arl13b knockout (datasheet NeuroMab).
4.2.4. Nphp3 (Nephrocystein-3)
- -
- Anti-Nphp3-N1: Rabbit polyclonal antibody raised against a peptide region covering aa 1–131 of human NPHP3 (AAP83423.1; Proteintech, Planegg-Martinsried, Germany; #22026-1-AP). This epitope corresponds to the first 7 aminoterminal amino acids (aa), followed by the peptide region encoded by the aminoterminal alternatively spliced exon (aa 8-131) of Nphp3 highlighted in Figure 7A (S). This antibody was previously characterized in WB and IF in human and mouse tissues [72,73]. This antibody was used for IF in a 1:200 dilution, for WB in a 1:500 dilution and for “pepspots“ analyses in a 1:1000 dilution.
- -
- Anti-Nphp3-N2 (lab-made rabbit polyclonal antibody): The antibody was raised against a peptide region comprising aa 1–180 of mouse Nphp3 (AAI15725.1, BC115724). From this cDNA clone, the N-terminal exon indicated in Figure 7A is spliced out. The corresponding cDNA was amplified by PCR using forward primer (AAAATCTAGAATGGGCACAGCCTCGTCG, XbaI), reverse primer (AAAAGTCGACAAGGTAGCACCTG ACAGG, SalI) and the mouse cDNA Image clone 40086844 (AAI15725.1, BC115724) as the template. The PCR product was cloned into pMalC2New [66], and the fusion protein was purified using standard techniques. This aminoterminal stretch is highly conserved (91% identical aa in mouse Nphp3 (AAI15725.1, BC115724) and bovine NPHP3 (XP_019817929.1)). This antibody was used for IF in a 1:100 dilution, for WB in a 1:500 dilution and for HEK293T cell WB transfection analyses in a 1:1000 dilution.
- -
- Anti-Nphp3-N4 (commercial Biorbyt [#orb221817] via Biozol; affinity-purified rabbit polyclonal antibody): The sequence against which the antibody was raised was not published. This antibody did not detect heterologously expressed Nphp3 (AAI15725.1/BC115724) with a spliced-out N-terminal exon (1204 aa in length) in WB (data not shown). Therefore, we assumed that this antibody is directed against a peptide sequence within the alternatively spliced exon and confirmed this assumption by overlapping peptide arrays (Supplementary Figure S4). The antibody was used for IF in a 1:500 dilution and for pepspot immunoblotting in a 1:2000 dilution.
- -
- Anti-Nphp3-N5: Mouse monoclonal Nphp3-N5 antibody (clone 5A3; IgA immunoglobulin) raised against the peptide sequence ENEIQDLLRAKRELESKLQRLQAQG and corresponding to aa 181–205 of bovine NPHP3 (XP_019817929.1) that is identical in mouse, rat, pig and human NPHP3. This amino acid sequence is located downstream of the alternatively spliced N-terminal exon (see Figure 7A) and is present both in the long and the short Nphp3 isoform. Mouse immunization, generation of hybridoma cells and ELISA selection of hybridoma cells, and antibody subtyping was performed by Absea, Beijing, China. This antibody was used for IF in a 1:200 dilution (corresponding to an immunoglobulin concentration of ≈0.5 µg/mL) and for WB in a 1:500 dilution (corresponding to an immunoglobulin concentration of ≈0.2 µg/mL) (Table 1 and Table 2).
4.3. Nphp3 Eukarzyotic Expression Plasmid
4.4. Various Reagents
5. Methods
5.1. Affinity Purification of Antibodies
5.2. Immunofluorescence Microscopy of Immunolabeled Semi-Thin Sections/Cryosections by Confocal and Super-Resolution Structured Illumination Microscopy (SR-SIM)
5.3. Preparation of Cryostat Section from PFA-Fixed Mouse Retinas
5.4. Triple Immunolabeling Experiments
5.5. PreAbsorption of Antibodies
5.5.1. Arl3
5.5.2. Arl13b (Abcam)
5.5.3. Nphp3 (Clone 5A3)
5.5.4. Rp2 (Lab-Made Rabbit Polyclonal Antiserum)
5.6. Immunostaining of PNA-stained Cone Synapses on Cryostat Sections of the Bovine Retina
5.7. Quantitative Analyses of Immunofluorescence Intensities and Statistical Evaluation
5.8. Pre-Embedding Immunogold Labeling (Rp2/Arl3(T31N))
5.9. Post-Embedding Immunogold Labeling (RIBEYE/Nphp3/Arl13b)
5.10. Quantification of Post-Embedding Immunogold Labeling Data
5.11. Multipeptide Arrays: Dot Blot Assays
5.12. Miscellaneous Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, V.L.; Leroux, M.R. Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 2017, 47, 83–91. [Google Scholar] [CrossRef] [PubMed]
- May-Simera, H.; Nagel-Wolfrum, K.; Wolfrum, U. Cilia—The sensory antennae in the eye. Prog. Ret. Eye Res. 2017, 60, 144–180. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Lyubarski, A.; Strissel, K.J.; Savchenko, A.B.; Govardovski, V.I.; Pugh, E.N., Jr.; Arshavsky, V.Y. Massive light-driven translocation of transducin between the two major compartments of rod cells: A novel mechanism of light adaptation. Neuron 2002, 34, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Slepak, V.Z.; Hurley, J.B. Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: Interaction-restricted diffusion. IUBMB Life 2008, 60, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Artemyev, N.O. Light-dependent compartmentalization of transducin in rod photoreceptors. Mol. Neurobiol. 2008, 37, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Constantine, R.; Zhang, H.; Gerstner, C.D.; Frederick, J.M.; Baehr, W. Uncoordinated (UNC) 119: Coordinating the trafficking of myristoylated proteins. Vis. Res. 2012, 75, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Ismail, S. A GDI/GDF-like system for sorting and shuttling ciliary proteins. Small GTPases 2017, 8, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Constantine, R.; Vorobiev, S.; Chen, Y.; Seetharaman, J.; Huang, Y.J.; Xiao, Z.; Montelione, G.T.; Gerstner, C.D.; Davis, M.W.; et al. UNC119 is required for G protein traffickig in sensory neurons. Nat. Neurosci. 2011, 14, 874–880. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.J.; Baye, L.M.; Olivier-Mason, A.; Mukhopadhyay, S.; Sang, L.; Kwong, M.; Wang, W.; Pretorius, P.R.; Sheffield, V.C.; Sengupta, P.; et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Develop. 2011, 25, 2347–2360. [Google Scholar] [CrossRef] [Green Version]
- Ismail, S.A.; Chen, Y.X.; Rusinova, A.; Chandra, A.; Bierbaum, M.; Gremer, L.; Triola, G.; Waldmann, H.; Bastiaens, P.I.; Wittinghofer, A. Arl2-GTP and Arl-GTP regulate a GDI-like transport system for farnesylated cargo. Nat. Chem. Biol. 2011, 7, 942–949. [Google Scholar] [CrossRef]
- Ismail, S.A.; Chen, Y.X.; Miertzschke, M.; Vetter, I.R.; Koerner, C.; Wittinghofer, A. Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J. 2012, 31, 4085–4094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baehr, W. Membrane protein transport in photoreceptors: The function of PDEd: The Proctor lecture. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8653–8666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, S.; Fansa, E.K.; Möbitz, S.; Ismail, S.A.; Winter, R.; Wittinghofer, A.; Weise, K. Effect of the N-terminal helix and nucleotide loading on the membrane and effector binding of Arl2/3. Biophys. J. 2015, 109, 1619–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fansa, E.K.; Wittinghofer, A. Sorting of lipidated cargo by the arl2/arl3 system. Small GTPases 2016, 7, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konitsiotis, A.D.; Roßmannek, L.; Stanoev, A.; Schmick, M.; Bastiaens, P.I.H. Spatial cycles mediated by UNC119 solubilisation maintain Src family kinases plasma membrane localization. Nat. Commun. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaya, T.; Tsutsumi, R.; Varner, L.R.; Maeda, Y.; Yoshida, S.; Furukuwa, T. Cul3-Klhl18 ubiquitin ligase modulates rod transducin translocation during light-dark adaptation. EMBO J. 2019, 38, e101409. [Google Scholar] [CrossRef]
- Jean, F.; Pilgrim, D. Coordinating the uncoordinated: UNC119 trafficking in cilia. Europ. J. Cell Biol. 2017, 96, 643–652. [Google Scholar] [CrossRef]
- Jaiswal, M.; Fansa, E.K.; Kösling, S.K.; Mejuch, T.; Waldmann, H.; Wittinghofer, A. Novel biochemical and structural insights into the interaction of myristoylated cargo with unc119 protein and their release by Arl2/3. J. Biol. Chem. 2016, 291, 20766–20778. [Google Scholar] [CrossRef] [Green Version]
- Higashide, T.; McLaren, M.J.; Inana, G. Localization of HRG4, a photoreceptor protein homologous to Unc-119, in ribbon synapses. Investig. Ophthalmol. Vis. Sci. 1998, 39, 690–698. [Google Scholar]
- Alpadi, K.; Magupalli, V.G.; Käppel, S.; Köblitz, L.; Schwarz, K.; Seigel, G.M.; Sung, C.H.; Schmitz, F. RIBEYE recruits Munc119, a mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses. J. Biol. Chem. 2008, 283, 26461–26467. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Majumder, A.; Belcastro, M.; Sokolov, M.; Artemyev, N.O. Expression and subcellular distribution of UNC119A, a protein partner of transducin a-subunit in rod photoreceptors. Cell. Signal. 2013, 25, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidelberger, R.; Thoreson, W.B.; Witkovsky, P. Synaptic transmission at retinal ribbon synapses. Prog. Retin. Eye Res. 2005, 24, 682–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, G.; Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010, 11, 812–822. [Google Scholar] [CrossRef]
- Schmitz, F. The making of synaptic ribbons. Neuroscientist 2009, 15, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Zenisek, D.; Steyer, J.A.; Almers, W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature 2000, 406, 854–949. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Matthews, G. Visualizing synaptic vesicle turnover and pool refilling driven by calcium nanodomains at presynaptic active zones of ribbon synapses. Proc. Natl. Acad. Sci. USA 2014, 111, 8655–8660. [Google Scholar] [CrossRef] [Green Version]
- Vaithianathan, T.; Henry, D.; Akmentin, W.; Matthews, G. Nanoscale dynamics of synaptic vesicle trafficking and fusion at the presynaptic active zone. Elife 2016, 5, e13245. [Google Scholar] [CrossRef]
- Moser, T.; Grabner, C.; Schmitz, F. Sensory processing at ribbon synapses in the retina and the cochlea. Physiol. Rev. 2020, 100, 103–144. [Google Scholar] [CrossRef]
- Schmitz, F.; Königstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insights into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Maxeiner, S.; Luo, F.; Tan, A.; Schmitz, F.; Südhof, T.C. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016, 35, 1098–1114. [Google Scholar] [CrossRef] [Green Version]
- Stephen, L.A.; Ismail, S. Shuttling and sorting lipid-modified cargo into the cilia. Biochem. Soc. Trans. 2016, 44, 1273–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltel, S.; Kravchanko, A.; Ismail, A.; Wittinghofer, A. Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. FEBS Lett. 2008, 582, 2501–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltel, S.; Gasper, R.; Eisenacher, E.; Wittinghofer, A. The retinitis pigmentosa 2 proteins gene product is a GTPase activating protein for Arf-like 3. Nat. Struct. Mol. Biol. 2008, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, K.; Lokaj, M.; Koerner, C.; Falk, N.; Gießl, A.; Wittinghofer, A. A G-protein activation cascade from Arl13b to Arl3 and implications for ciliary targeting of ciliated proteins. eLife 2015, 4, e11859. [Google Scholar] [CrossRef]
- Li, Y.; Ling, K.; Hu, J. The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J. Cell. Biochem. 2012, 113, 2201–2207. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, N.; Hardcastle, A.J.; Cheetham, M.E. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vis. Res. 2012, 75, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Linari, M.; Hanzal-Bayer, M.; Becker, J. The delta subunit of rod-specific cyclic GMP phosphodiesterase, PDEd, interacts with the Arf-like protein Arl3 in a GTP-specific manner. FEBS Lett. 1999, 458, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Y.; Zhang, Y.; Torres, V.E.; Harris, P.C.; Ling, K.; Hu, J. GTP-binding of ARL-3 is activated by ARL-13 as a GEF and stabilized by UNC-119. Sci. Rep. 2016, 6, 24534. [Google Scholar] [CrossRef] [Green Version]
- Hanke-Gogokhia, C.; Wu, Z.; Sharif, A.; Yazigi, H.; Frederick, J.M.; Baehr, W. The guanine nucleotide Arl13b factor Arf-like protein 13b is essential for assembly of mouse photoreceptor transition zone and outer segment. J. Biol. Chem. 2017, 292, 21442–21456. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Ferland, R.J. Fixation methods can differentially affect ciliary protein immunolabeling. Cilia 2017, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Kim, J.H.; Yu, Y.S.; Ko, H.W.; Kim, J.H. Localization of primary cilia in mouse retina. Acta Histochem. 2013, 115, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, H.; Fliegauf, M.; Hoefele, J.; Kispert, A.; Otto, E.; Volz, A.; Wolf, M.T.; Sasmaz, G.; Trauer, U.; Reinhardt, R.; et al. Mutations in a novel gene, NPHP3, cause adolescent nephroniphthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat. Genet. 2003, 34, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ronquillo, C.C.; Bernstein, P.S.; Baehr, W. Senior-Løken-Syndrome: A syndromic form of retinal dystrophy associated with nephronophthisis. Vis. Res. 2012, 75, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayson, C.; Bartolini, F.; Chapple, J.P.; Willison, K.R.; Bhamidipati, A.; Lewis, S.A.; Luthert, P.J.; Hardcastle, A.J.; Cowan, N.J.; Cheetham, M.E. Localization in the human retina of the X-linked retinitis pigmentosa protein 2 RP2, its homologue cofactor C and the RP2 interacting protein arl3. Hum. Mol. Genet. 2002, 11, 3065–3074. [Google Scholar] [CrossRef]
- Holopainen, J.M.; Cheng, C.L.; Molday, L.L.; Johal, G.; Coleman, J.; Dyka, F.; Hii, T.; Ahn, J.; Molday, R.S. Interaction and localization of the retinitis pigmentosa protein RP2 and NSF in retinal photoreceptors. Biochemistry 2010, 49, 7439–7447. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.J.; Schwarz, N.; Nagel-Wolfrum, K.; Wolfrum, U.; Hardcastle, A.J.; Cheetham, M.E. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet. 2010, 19, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Hanke-Gogokhia, C.; Frederick, J.M.; Zhang, H.; Baehr, W. Binary function of ARL3-GTP revealed by gene knockouts. Adv. Exp. Med. Biol. 2018, 1074, 317–325. [Google Scholar]
- Nachury, M.V.; Mick, D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukuda, M. Slp2-a controls renal epithelial size through regulation of Rap-ezrin signaling independently of Rab27. J. Cell Sci. 2014, 127, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M. Rab27 and its effectors in secretaory granule exocytosis: A novel docking machinery omposed of Rab27 effector complex. Biochem. Soc. Trans. 2006, 34, 691–695. [Google Scholar] [CrossRef]
- Tsuboi, T. Molecular mechanism of attachment process of dense-core vesicles to the plasma membrane in neuroendocrine cells. Neurosci. Res. 2009, 63, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Grossman, G.H.; Pauer, G.J.T.; Narendra, U.; Peachey, N.S.; Hagstrom, S.A. Early synaptic defects in tulp1 -/- mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3074–3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, S.; Magupalli, V.G.; Dembla, M.; Katiyar, R.; Schwarz, R.; Köblitz, L.; Alpadi, K.; Krause, E.; Rettig, J.; Sung, C.H.; et al. The disease protein Tulp1 is essential for periactive zone endocytosis in photoreceptor ribbon synapses. J. Neurosci. 2016, 36, 2473–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebke, L.A.; Sinha, S.; Pauer, G.J.T.; Hagstrom, S.A. Photoreceptor compartment-specific Tulp1 interactomes. Int. J. Mol. Sci. 2021, 22, 8066. [Google Scholar] [CrossRef] [PubMed]
- Muresan, V.; Lyass, A.; Schnapp, B.J. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J. Neurosci. 1999, 19, 1027–1037. [Google Scholar] [CrossRef]
- tom Dieck, S.; Altrock, W.D.; Kessels, M.M.; Qualmann, B.; Regus, H.; Brauner, D.; Fejtova, A.; Bracko, O.; Gundelfinger, E.D.; Brandstätter, J.H. Molecular dissection of the photoreceptor synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the synaptic ribbon complex. J. Cell Biol. 2005, 168, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, M.A.; Gleeson, J.G. The primary cilium as a cellular signaling center: Lessons from disease. Curr. Opin. Genet. Dev. 2009, 19, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Verhey, K.J.; Dishinger, J.; Kee, H.L. Kinesin motors and primary cilia. Biochem. Soc. Trans. 2011, 39, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Malicki, J.; Besharse, J.C. Kinesin-2 family motors in the unusual photoreceptor cilium. Vis. Res. 2012, 75, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Won, J.; de Evsikova, C.M.; Smith, R.S.; Hicks, W.L.; Edwards, M.M.; Longo-Guess, C.; Li, T.; Naggert, J.K.; Nishina, P.M. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum. Mol. Genet. 2011, 20, 482–496. [Google Scholar] [CrossRef] [Green Version]
- Morgans, C.W.; Brandstätter, J.H.; Kellerman, J.; Betz, H.; Wässle, H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J. Neurosci. 1996, 16, 6713–6721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, J.Z.; Zhao, Y.; Sung, C.H. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 2007, 130, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, L.; Datta, P.; Liu, X.; Bogdanova, N.; Heidelberger, R.; Janz, R. Syntaxin3b is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina. Neuroscience 2010, 166, 832–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakakhel, M.; Tebbe, L.; Makia, M.S.; Conley, S.M.; Sherry, D.M.; Al-Ubaidi, M.R.; Naash, M.I. Syntaxin 3 is essential for photoreceptor outer segment protein trafficking and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 20615–20624. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Katiyar, R.; Schmitz, F. A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to the synaptic ribbon. J. Neurosci. 2013, 33, 10278–10300. [Google Scholar] [CrossRef]
- Dembla, M.; Wahl, S.; Katiyar, R.; Schmitz, F. ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms a NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis. J. Neurosci. 2014, 34, 5245–5260. [Google Scholar] [CrossRef] [Green Version]
- Dembla, M.; Kesharwani, A.; Natarajan, S.; Fecher-Trost, C.; Fairless, R.; Williams, S.K.; Flockerzi, V.; Diem, R.; Schwarz, K.; Schmitz, F. Early auto-immune targeting of photoreceptor ribbon synapses in mouse models of multiple sclerosis. EMBO Mol. Med. 2018, 10, e8926. [Google Scholar] [CrossRef]
- Hanke-Gogokhia, C.; Wu, Z.; Gerstner, C.D.; Frederick, J.M.; Zhang, H.; Baehr, W. Arf-like protein 3 (Arl3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. J. Biol. Chem. 2016, 291, 7142–7155. [Google Scholar] [CrossRef] [Green Version]
- Chapple, J.P.; Hardcastle, A.J.; Grayson, C.; Willison, K.R.; Cheetham, M.E. Delineation of plasma membrane targeting domain of the X-linked retinitis pigmentosa protein RP2. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2015–2020. [Google Scholar]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Danés, A.; Larsimont, J.C.; Liagre, M.; Muñoz-Couselo, E.; Lapouge, G.; Brisebarre, A.; Dubois, C.; Suppa, M.; Sukuraman, V.; Del Marmol, V.; et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 2018, 562, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Phua, S.C.; DeRose, R.; Chiba, S.; Narita, K.; Kalugin, P.N.; Katada, T.; Kontani, K.; Takeda, S.; Inoue, T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat. Meth. 2013, 10, 1105–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, N.; Carr, A.J.; Lane, A.; Moeller, F.; Chen, L.L.; Aguilà, M.; Nommiste, B.; Muthiah, M.N.; Kanuga, N.; Wolfrum, U. Translational read-through of the RP2 Arg120stop mutation in patient iPSC-derived retinal pigment epithelium cells. Hum. Mol. Genet. 2015, 24, 972–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoch, S.; Mittelstaedt, T.; Kaeser, P.; Padgett, D.; Feldmann, N.; Chevalevre, V.; Castillo, P.E.; Hammer, R.E.; Wan, W.; Schmitz, F.; et al. Redundant functions of RIM1alpha and RIM2alpha in Ca2+-triggered neurotransmitter release. EMBO J. 2006, 25, 5852–5863. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.; Ayoubian, H.; Schmitz, F. Differential synaptic distribution of the scaffold proteins Cask and Caskin in the bovine retina. Mol. Cell. Neurosci. 2014, 62, 19–29. [Google Scholar] [CrossRef]
- Dembla, E.; Dembla, M.; Maxeiner, S.; Schmitz, F. Synaptic ribbons foster active zone stability and illumination-dependent active zone enrichment of RIM2 and Cav1.4 at photoreceptor synapses. Sci. Rep. 2020, 10, 5957. [Google Scholar] [CrossRef] [Green Version]
- Buckley, K.; Kelly, R.B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J. Cell Biol. 1985, 100, 1284–1294. [Google Scholar] [CrossRef] [Green Version]
- Irie, M.; Hata, Y.; Takeuchi, M.; Ichtchenko, K.; Toyoda, A.; Hirao, K.; Takai, Y.; Rosahl, T.W.; Südhof, T.C. Binding of neuroligins to PSD-95. Science 1997, 277, 1511–1515. [Google Scholar] [CrossRef]
- Olmstedt, J.B. Affinity-purification of antibodies from diazotized paper blots of heterogeneous protein samples. J. Biol. Chem. 1981, 256, 11955–11957. [Google Scholar] [CrossRef]
- Mukherjee, A.; Katiyar, R.; Dembla, E.; Dembla, M.; Kumar, P.; Belkacemi, A.; Jung, M.; Beck, A.; Flockerzi, V.; Schwarz, K.; et al. Disturbed presynaptic Ca2+ signaling in photoreceptors in the EAE mouse model of multiple sclerosis. iScience 2020, 23, 101830. [Google Scholar] [CrossRef]
- Kesharwani, A.; Schwarz, K.; Dembla, E.; Dembla, M.; Schmitz, F. Early changes in exocytosis and endocytosis in the EAE mouse model of multiple sclerosis correlate with decreased synaptic ribbon size and reduced ribbon-associated vesicle pools. Int. J. Mol. Sci. 2021, 221, 789. [Google Scholar]
- Eich, M.L.; Dembla, E.; Wahl, S.; Dembla, M.; Schwarz, K.; Schmitz, F. The calcineurin-binding, activity-dependent splice variant dynamin1xb is highly enriched in synapses in various regions of the central nervous system. Front. Mol. Neurosci. 2017, 210, 230. [Google Scholar] [CrossRef] [PubMed]
- Shankhwar, S.; Schwarz, K.; Katiyar, R.; Jung, M.; Maxeiner, S.; Südhof, T.C.; Schmitz, F. RIBEYE B-domain is essential for RIBEYE A-domain stability and assembly of synaptic ribbons. Front. Mol. Neurosci. 2022, 15, 838311. [Google Scholar] [CrossRef]
- Suiwal, S.; Kiefer, G.; Schmitz, F.; Schwarz, K. An easy, fast and “low-tech“-equipment-requiring alternative method to optimize immunolabelling conditions for pre-embedding immunogold electron microscopy and to correlate light and electron microscopical immunogold labelling results. J. Immunol. Meth. 2017, 44, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Harsman, A.; Kopp, A.; Wagner, R.; Zimmermann, R.; Jung, M. Calmodulin regulation of the calcium-leak channel Sec61 is unique to vertebrates. Channels 2011, 5, 293–298. [Google Scholar] [CrossRef] [Green Version]



| Primary Antibodies | References/Source | Dilution |
|---|---|---|
| Anti-RIBEYE mouse monoclonal antibody (2D9) against the C-terminus of RIBEYE(B)-domain/CtBP2 | [67] | 1:200 (IF) (≈2.5 µg/mL final concentration); 1:100 (WB) (≈5 µg/mL final concentration) |
| Anti-RIM1/2 rabbit polyclonal antibody | [74,75,76] | 1:200 (IF) |
| Anti-SV2 mouse monoclonal antibody | [77] | 1:50 (IF) |
| Anti-GFAP rabbit polyclonal IgG | Sigma (Taufkirchen, Germany), G9269 | 1:300 (WB) |
| Anti-actin (clone C4) mouse monoclonal antibody | Millipore (Darmstadt, Germany), MAB1501 | 1:100 (IF) 1:2000 (WB) |
| Anti-RIBEYE(B)/CtBP2 mouse monoclonal antibody | BD Transduction Laboratories (Heidelberg, Germany), 612044 | 1:1000 (IF) |
| DyLight 650 directly labeled anti-RIBEYE(B)/CtBP2 | [65] | 1:20 (IF) |
| Anti-PSD95 rabbit polyclonal (L667) | [78] | 1:750 (IF) |
| Anti-RIBEYE rabbit polyclonal antibody (U2656) against RIBEYE(B)-domain | [29] | 1:1000 (IF) 1:5000 (EM) |
| Antibody | Source | Dilution |
|---|---|---|
| Chicken anti-mouse Alexa488 | Invitrogen Molecular Probes (Karlsruhe, Germany), A-21200 | 1:1000 (IF) |
| Donkey anti-mouse Alexa488 | Invitrogen Molecular Probes (Karlsruhe, Germany), A-21202 | 1:1000 (IF) |
| Donkey anti-rabbit Alexa568 | Invitrogen, Molecular Probes (Karlsruhe, Germany), A-10042 | 1:1000 (IF) |
| Chicken anti-rabbit Alexa488 | Invitrogen, Molecular Probes (Karslruhe, Germany), A-21441 | 1:1000 (IF) |
| Donkey anti-mouse Alexa568 | Invitrogen, Molecular Probes (Karlsruhe, Germany), A-10037 | 1:1000 (IF) |
| Goat anti-mouse Alexa647 | Invitrogen, Molecular Probes (Karlsruhe, Germany), A-21236 | 1:1000 (IF) |
| Donkey anti-mouse Alexa647 | Invitrogen, Molecular Probes (Karslruhe, Germany), A-31571 | 1:1000 (IF) |
| Goat anti-rabbit peroxidase-conjugated (POX) IgG | Sigma (Taufkirchen, Germany), A-6154 | 1:3000 (WB) |
| Goat anti-mouse (IgG, IgM, IgA), conjugated to peroxidase | Sigma (Taufkirchen, Germany), SAB3701048 | 1:3000 (WB) |
| Goat anti-mouse POX IgG | Sigma (Taufkirchen, Germany), A-3673 | 1:3000 (WB) |
| Monovalent Fab fragments goat anti-rabbit (unconjugated) (H&L) | Rockland Immunochemicals, 811–1102 via Biomol GmbH | 1:100 (IF) |
| Rabbit anti-mouse IgA | Novus (Wiesbaden, Germany), NB7506 | 1:100 (IF) 1:1000(WB) 1:100 (EM) |
| Goat anti-rabbit antibody conjugated to ultrasmall (1.4 nm-diameter) gold particles | Nanoprobes, #2003 via Biozol (Eching, Germany) | 1:50 (EM) |
| Goat anti-rabbit secondary antibody conjugated to 5 nm gold particles | Sigma (Taufkirchen, Germany), G7277 | 1:100 (EM) |
| Goat anti-mouse secondary antibody conjugated to 5 nm gold particles | Sigma (Taufkirchen, Germany), G7527 | 1:100 (EM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suiwal, S.; Dembla, M.; Schwarz, K.; Katiyar, R.; Jung, M.; Carius, Y.; Maxeiner, S.; Lauterbach, M.A.; Lancaster, C.R.D.; Schmitz, F. Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses. Int. J. Mol. Sci. 2022, 23, 7135. https://doi.org/10.3390/ijms23137135
Suiwal S, Dembla M, Schwarz K, Katiyar R, Jung M, Carius Y, Maxeiner S, Lauterbach MA, Lancaster CRD, Schmitz F. Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses. International Journal of Molecular Sciences. 2022; 23(13):7135. https://doi.org/10.3390/ijms23137135
Chicago/Turabian StyleSuiwal, Shweta, Mayur Dembla, Karin Schwarz, Rashmi Katiyar, Martin Jung, Yvonne Carius, Stephan Maxeiner, Marcel A. Lauterbach, C. Roy D. Lancaster, and Frank Schmitz. 2022. "Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses" International Journal of Molecular Sciences 23, no. 13: 7135. https://doi.org/10.3390/ijms23137135
APA StyleSuiwal, S., Dembla, M., Schwarz, K., Katiyar, R., Jung, M., Carius, Y., Maxeiner, S., Lauterbach, M. A., Lancaster, C. R. D., & Schmitz, F. (2022). Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses. International Journal of Molecular Sciences, 23(13), 7135. https://doi.org/10.3390/ijms23137135







