Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage
Abstract
:1. Introduction
2. Results
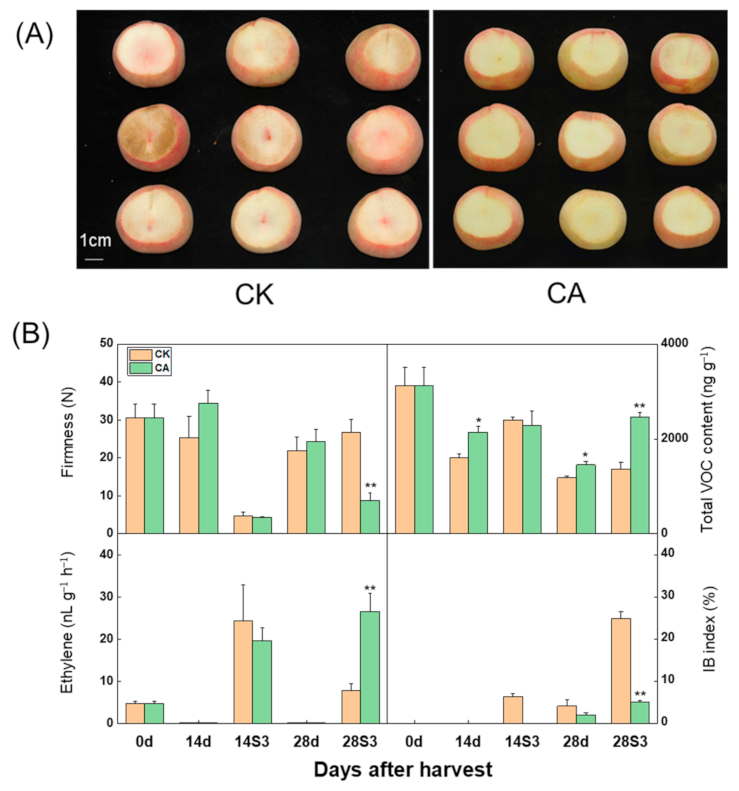
2.1. Influence of CA Treatment on Firmness, Ethylene Emission, Total VOC Production and Flesh Browning of Peach Fruit after Cold Storage
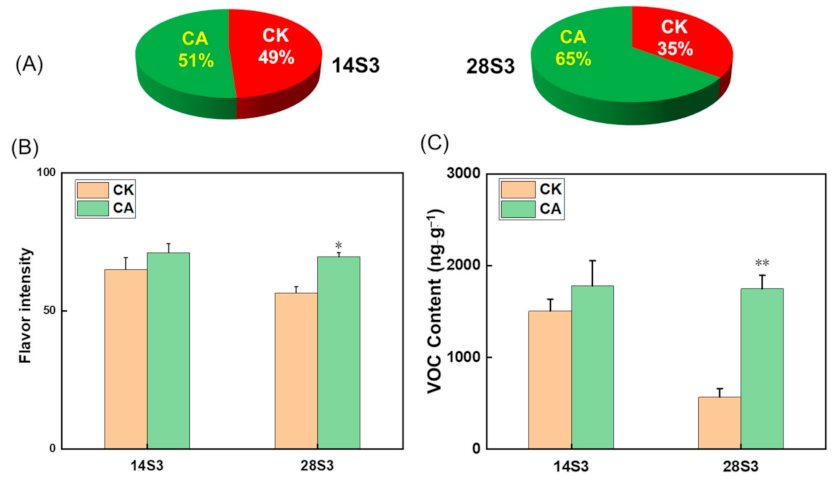
2.2. Sensory Quality Evaluation of Peach Fruit during Shelf-Life after Cold Storage
2.3. Production of Peach Fruit VOC under CA Treatment during Shelf-Life after Cold Storage
2.4. Clarifying Peach Fruit VOC Roles in Influencing Sensory Quality
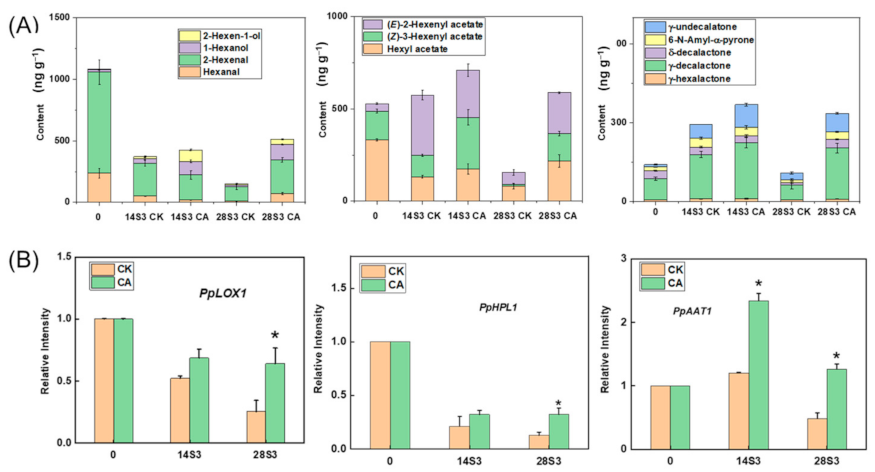
2.5. Effect of CA Treatment on Content of Fatty Acids and Derived VOCs
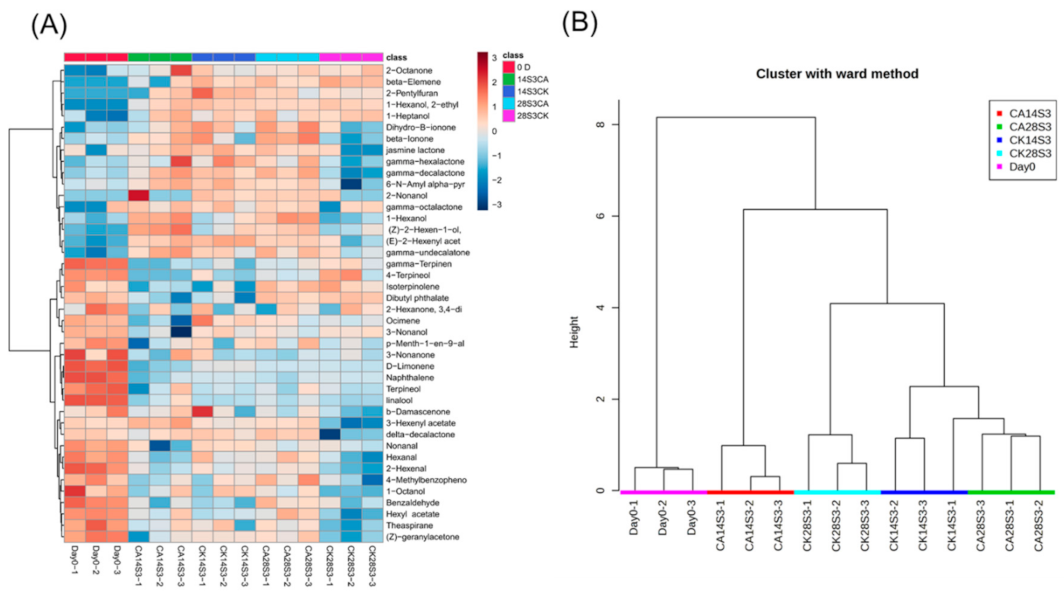
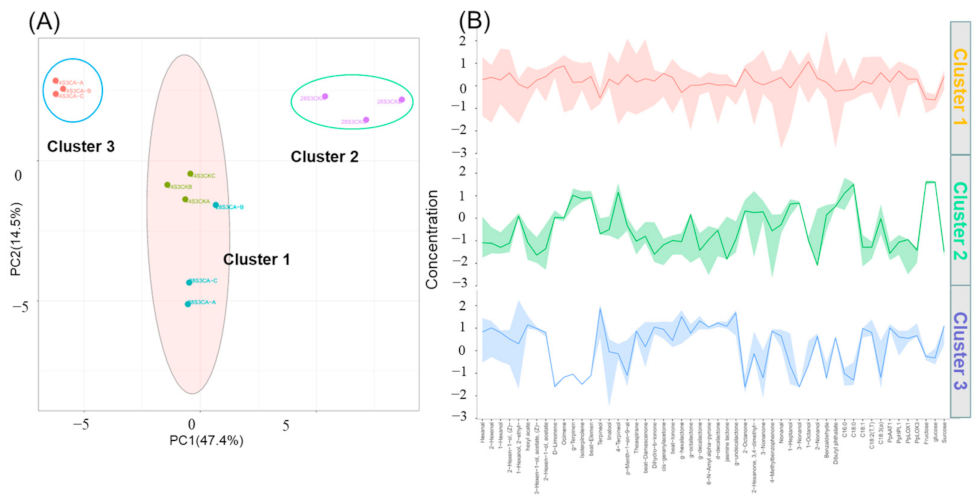
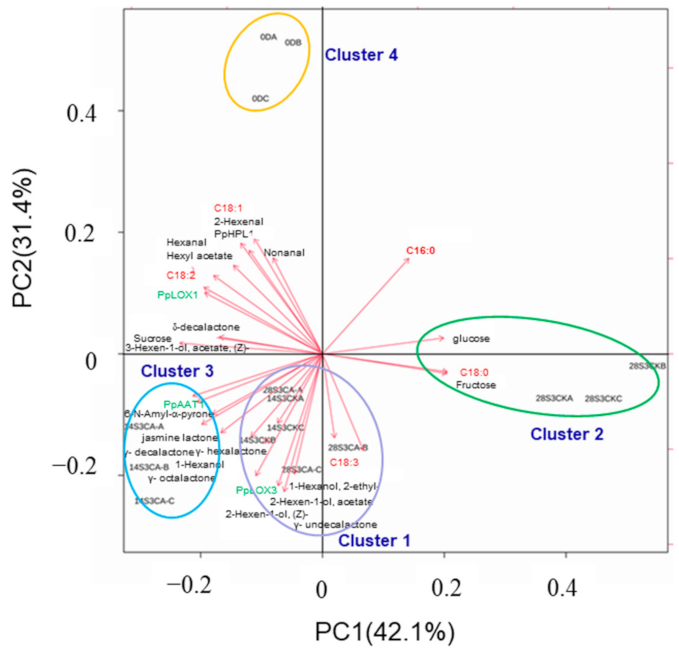
2.6. Analysis of Full-Data with K-Means and PCA Model during Shelf-Life after Cold Storage
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Analysis of the Firmness, Ethylene, and Flesh Browning
4.3. Determination of Aroma Volatile by HS-GC-MS
4.4. Sensory Analysis
4.5. RNA Extraction and Real-Time Quantitative PCR Analysis
4.6. Fatty acid Extraction and Determination
4.7. Soluble Sugar and Organic Acid Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, K.; Yang, X.; Li, Y.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wu, J.; Wang, L. New high-quality peach (Prunus persica L. Batsch) genome assembly to analyze the molecular evolutionary mechanism of volatile compounds in peach fruits. Plant J. 2021, 108, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Xi, W.-P.; Zhang, B.; Liang, L.; Shen, J.-Y.; Wei, W.-W.; Xu, C.-J.; Allan, A.C.; Ferguson, I.B.; Chen, K.-S. Postharvest temperature influences volatile lactone production via regulation of acyl-CoA oxidases in peach fruit. Plant Cell Environ. 2011, 35, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.; Chen, K.; Fei, Z.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef] [Green Version]
- Akbudak, B.; Eris, A. Physical and chemical changes in peaches and nectarines during the modified atmosphere storage. Food Control 2004, 15, 307–313. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, W.-P.; Wei, W.-W.; Shen, J.-Y.; Ferguson, I.; Chen, K.-S. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Shinya, P.; Contador, L.; Frett, T.; Infante, R. Effect of prolonged cold storage on the sensory quality of peach and nectarine. Postharvest Biol. Technol. 2014, 95, 7–12. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Jankowski, P.; Radzanowska, J. Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruit (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol. Technol. 2014, 88, 21–33. [Google Scholar] [CrossRef]
- Yang, C.; Duan, W.; Xie, K.; Ren, C.; Zhu, C.; Chen, K.; Zhang, B. Effect of salicylic acid treatment on sensory quality, flavor-related chemicals and gene expression in peach fruit after cold storage. Postharvest Biol. Technol. 2019, 161, 111089. [Google Scholar] [CrossRef]
- Fernie, A.R.; Alseekh, S. Metabolomic selection–based machine learning improves fruit taste prediction. Proc. Natl. Acad. Sci. USA 2022, 119, e2201078119. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, C.M. Consumer and retailer satisfaction with the quality and size of california peaches and nectarines. J. Food Qual. 1995, 18, 241–256. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.-R.; Zhang, B.; Grierson, D.; Xu, C.-J.; Chen, K.-S. Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Graell, J.; López, M.L.; Echeverría, G.; Lara, I. Volatile ester-synthesising capacity in ‘Tardibelle’ peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chem. 2010, 123, 698–704. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, B.; Zhou, Q.; Tan, D.; Ji, S. 1-Methylcyclopropene alleviates chilling injury by regulating energy metabolism and fatty acid content in ‘Nanguo’ pears. Postharvest Biol. Technol. 2015, 109, 130–136. [Google Scholar] [CrossRef]
- Thewes, F.R.; Anese, R.O.; Thewes, F.R.; Ludwig, V.; Klein, B.; Wagner, R.; Nora, F.R.; Rombaldi, C.V.; Brackmann, A. Dynamic controlled atmosphere (DCA) and 1-MCP: Impact on volatile esters synthesis and overall quality of ‘Galaxy’ apples. Food Packag. Shelf Life 2020, 26, 100563. [Google Scholar] [CrossRef]
- Steffens, C.A.; Santana, G.R.O.; Amarante, C.V.T.D.; Antonovviski, J.L.; Miqueloto, T.; Anami, J.M.; Fenili, C.L. Treatment with nitric oxide in controlled atmosphere storage to preserve the quality of ‘Laetitia’ plums. LWT 2022, 158, 113033. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. Exogenous nitric oxide fumigation promoted the emission of volatile organic compounds in peach fruit during shelf life after long-term cold storage. Food Res. Int. 2020, 133, 109135. [Google Scholar] [CrossRef]
- Fu, A.; Zheng, Y.; Lv, Y.; Watkins, C.B.; Bai, C.; Ma, L.; Yuan, S.; Zheng, S.; Jia, L.; Gao, L.; et al. Multi-omics analysis reveals specific modifications associated with reduced chilling injury in bell pepper fruit by methyl jamonate. Postharvest Biol. Technol. 2021, 185, 111799. [Google Scholar] [CrossRef]
- Meng, X.; Han, J.; Wang, Q.; Tian, S. Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem. 2009, 114, 1028–1035. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; López, M.; Echeverría, G. Relationships between the instrumental and sensory characteristics of four peach and nectarine cultivars stored under air and CA atmospheres. Postharvest Biol. Technol. 2012, 75, 58–67. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Opara, U.L. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Wright, A.H.; Delong, J.M.; Arul, J.; Prange, R.K. The trend toward lower oxygen levels during apple (Malus × domestica Borkh) storage. J. Hortic. Sci. Biotechnol. 2015, 90, 1–13. [Google Scholar] [CrossRef]
- Wood, R.M.; Thewes, F.R.; Reynaud, M.; Kittemann, D.; Sautter, C.K.; Wünsche, J.N.; Neuwald, D.A. Apple fruit recovery from anoxia under controlled atmosphere storage. Food Chem. 2021, 371, 131152. [Google Scholar] [CrossRef]
- Tudela, J.A.; Marín, A.; Garrido, Y.; Cantwell, M.; Medina-Martínez, M.S.; Gil, M.I. Off-odour development in modified atmosphere packaged baby spinach is an unresolved problem. Postharvest Biol. Technol. 2013, 75, 75–85. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anwar, R.; Anjum, M.A.; Nawaz, A.; Shafique, M.; Naz, S. Combined application of ascorbic and oxalic acids delays postharvest browning of litchi fruits under controlled atmosphere conditions. Food Chem. 2021, 350, 129277. [Google Scholar] [CrossRef]
- Bodbodak, S.; Moshfeghifar, M. 2-Advances in controlled atmosphere storage of fruits and vegetables. Eco-Friendly Technology for Postharvest Produce Quality; MW Siddiqui, Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 39–76. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Collings, E.R.; Terry, L.A. Investigating the role of abscisic acid and its catabolites on senescence processes in green asparagus under controlled atmosphere (CA) storage regimes. Postharvest Biol. Technol. 2022, 188, 111892. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.-Y.; Guo, Y.-Y.; Wang, Z.-Q. Respiratory pathway metabolism and energy metabolism associated with senescence in postharvest Broccoli (Brassica oleracea L. var. italica) florets in response to O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Murray, R.; Lucangeli, C.; Polenta, G.; Budde, C. Combined pre-storage heat treatment and controlled atmosphere storage reduced internal breakdown of ‘Flavorcrest’ peach. Postharvest Biol. Technol. 2007, 44, 116–121. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, L.; Zhao, G.; Shen, C.; Yan, H.; Guan, J.; Yang, K. The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in ‘Yali’ pears during cold storage. LWT 2014, 60, 1243–1248. [Google Scholar] [CrossRef]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Zanella, A.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodriguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C.; et al. The Chemical Interactions Underlying Tomato Flavor Preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, M.F.R., Jr.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Yuanyuan, Z.; Wenjing, L.; Binbin, Z.; Yuyan, Z.; Zhixiang, C.; Hongfeng, S.; Ruijuan, M.; Mingliang, Y. Analysis of volatile compounds and their potential regulators in four high-quality peach (Prunus persica L.) cultivars with unique aromas. LWT-Food Sci. Technol. 2022, 160, 113195. [Google Scholar] [CrossRef]
- Duan, W.; Yang, C.; Cao, X.; Zhang, C.; Liu, H.; Chen, K.; Li, X.; Zhang, B. Transcriptome and DNA methylome analysis reveal new insights into methyl jasmonate-alleviated chilling injury of peach fruit after cold storage. Postharvest Biol. Technol. 2022, 189, 111915. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2018, 272, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Brizzolara, S.; Hertog, M.; Tosetti, R.; Nicolai, B.; Tonutti, P. Metabolic Responses to Low Temperature of Three Peach Fruit Cultivars Differently Sensitive to Cold Storage. Front. Plant Sci. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Shen, J.-Y.; Wei, W.-W.; Xi, W.-P.; Xu, C.-J.; Ferguson, I.; Chen, K. Expression of Genes Associated with Aroma Formation Derived from the Fatty Acid Pathway during Peach Fruit Ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yu, M.; Zhang, B.; Xu, J.; Ma, R. Differences in PpAAT1 Activity in High- and Low-Aroma Peach Varieties Affect γ-Decalactone Production. Plant Physiol. 2020, 182, 2065–2080. [Google Scholar] [CrossRef]
- Peng, B.; Xu, J.; Cai, Z.; Zhang, B.; Yu, M.; Ma, R. Different Roles of the Five Alcohol Acyltransferases in Peach Fruit Aroma Development. J. Am. Soc. Hortic. Sci. 2020, 145, 1–8. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of Storage Temperature, Storage Duration, and Subsequent Ripening on the Physicochemical Characteristics, Volatile Compounds, and Phytochemicals of Western Red Nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Ortiz, A.; Echeverría, G.; Graell, J.; Lara, I. Overall quality of ‘Rich Lady’ peach fruit after air- or CA storage. The importance of volatile emission. LWT 2009, 42, 1520–1529. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2020, 338, 128005. [Google Scholar] [CrossRef]
- Domínguez, T.; Hernández, M.L.; Pennycooke, J.C.; Jiménez, P.; Martínez-Rivas, J.M.; Sanz, C.; Stockinger, E.J.; Sánchez-Serrano, J.J.; Sanmartín, M. Increasing ω-3 Desaturase Expression in Tomato Results in Altered Aroma Profile and Enhanced Resistance to Cold Stress. Plant Physiol. 2010, 153, 655–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Zhang, H.-W.; Lilian, S.; Amnon, L. Lurie Susan Ethylene involvement in the cold storage disorder of ‘Flavortop’ nectarine. Postharvest Biol. Technol. 2001, 23, 105–115. [Google Scholar]
- Both, V.; Thewes, F.R.; Brackmann, A.; Anese, R.D.O.; Ferreira, D.D.F.; Wagner, R. Effects of dynamic controlled atmosphere by respiratory quotient on some quality parameters and volatile profile of ‘Royal Gala’ apple after long-term storage. Food Chem. 2017, 215, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jeong, M.; Baek, M.; Choi, J.; Lee, H.; Jeong, C.; Tilahun, S. Transcriptome Analysis of Pre-Storage 1-MCP and High CO2-Treated ‘Madoka’ Peach Fruit Explains the Reduction in Chilling Injury and Improvement of Storage Period by Delaying Ripening. Int. J. Mol. Sci. 2021, 22, 4437. [Google Scholar] [CrossRef]
- Liu, H.; Cao, X.; Liu, X.; Xin, R.; Wang, J.; Gao, J.; Wu, B.; Gao, L.; Xu, C.; Zhang, B.; et al. UV-B irradiation differentially regulates terpene synthases and terpene content of peach. Plant, Cell Environ. 2017, 40, 2261–2275. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Gao, J.; Huang, Y.; Zhang, B.; Chen, K. Two ω-3 FADs Are Associated with Peach Fruit Volatile Formation. Int. J. Mol. Sci. 2016, 17, 464. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]




| Compound (mg × g−1 FW) | Treatment | 0 days | 14 days | 14 S3 | 28 days | 28 S3 |
|---|---|---|---|---|---|---|
| Fructose | CK | 14.88 | 13.43 a | 13.94 a | 21.18 a | 41.80 a |
| CA | 12.53 a | 16.39 a | 12.20 b | 13.78 b | ||
| glucose | CK | 19.98 | 19.45 a | 14.63 a | 27.88 a | 46.29 a |
| CA | 19.07 a | 17.57 a | 18.27 b | 14.05 b | ||
| Sucrose | CK | 151.1 | 155.91 a | 151.37 b | 127.45 b | 104.80 b |
| CA | 145.40 b | 165.61 a | 153.35 a | 138.20 a | ||
| Total sugar | CK | 185.95 | 188.79 a | 179.93 b | 176.51 a | 192.90 a |
| CA | 177.00 b | 199.57 a | 183.82 a | 166.02 b | ||
| Malic acid | CK | 6.96 | 6.5 | 6.67 | 5.42 | 6.38 |
| CA | 5.92 | 7 | 5.33 | 6.92 | ||
| quinic acid | CK | 1.5 | 1.21 | 0.88 | 0.58 | 0.46 |
| CA | 1.08 | 0.96 | 1.04 | 0.71 | ||
| Citric acid | CK | 2.13 | 2.54 | 2 | 2.21 | 2.13 |
| CA | 2.5 | 2.08 | 2.38 | 1.67 | ||
| Total acid | CK | 10.58 | 10.25 | 9.54 | 8.21 | 8.96 |
| CA | 9.5 | 10.04 | 8.75 | 9.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; He, H.; Liu, C.; Wang, C.; Qiao, Y.; Zhang, B. Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage. Int. J. Mol. Sci. 2022, 23, 7141. https://doi.org/10.3390/ijms23137141
Liu H, He H, Liu C, Wang C, Qiao Y, Zhang B. Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage. International Journal of Molecular Sciences. 2022; 23(13):7141. https://doi.org/10.3390/ijms23137141
Chicago/Turabian StyleLiu, Hongru, Hui He, Chenxia Liu, Chunfang Wang, Yongjin Qiao, and Bo Zhang. 2022. "Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage" International Journal of Molecular Sciences 23, no. 13: 7141. https://doi.org/10.3390/ijms23137141






