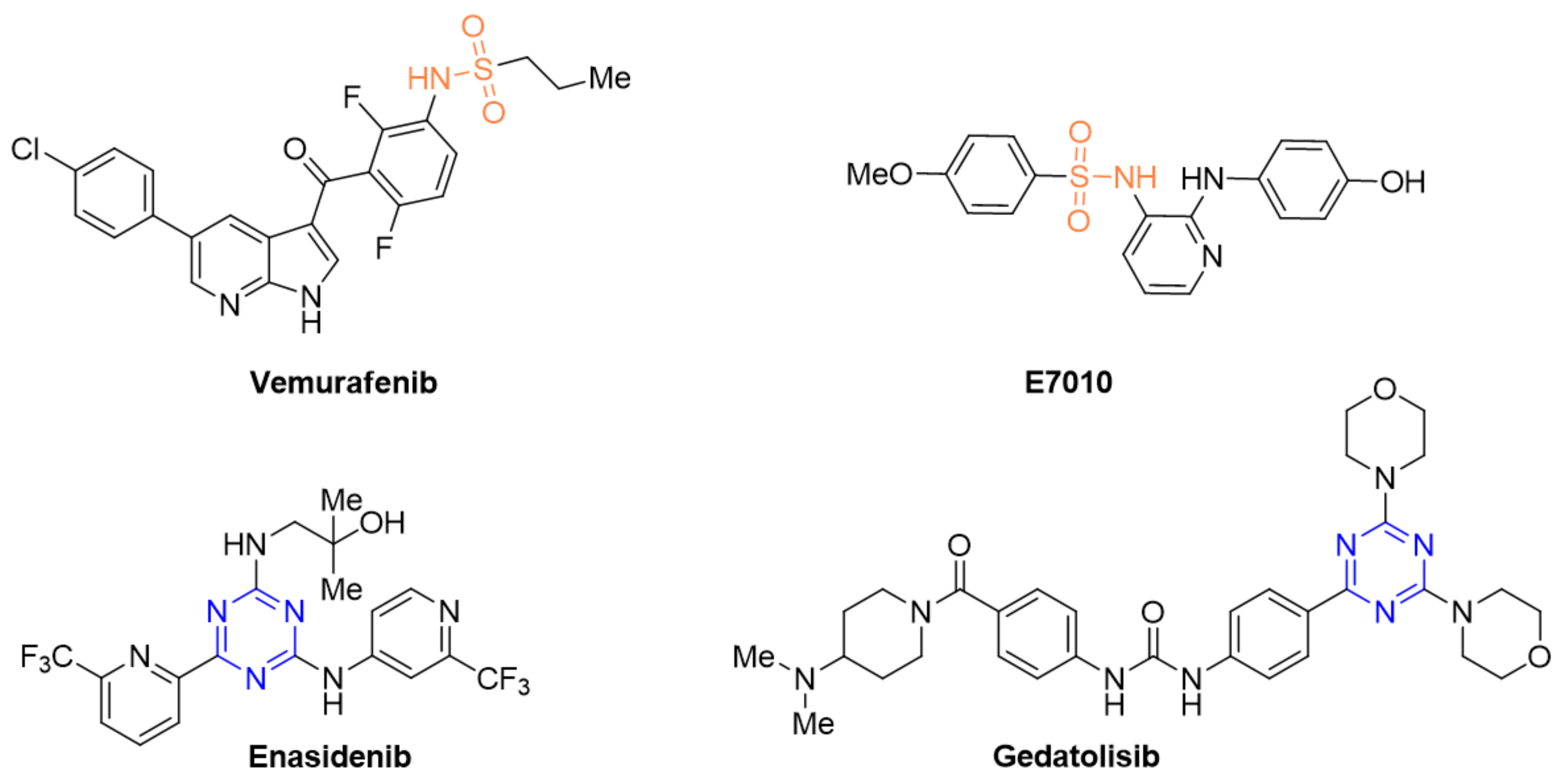
Figure 1.
Structures of exemplary anticancer drugs and compounds containing a sulfonamide or 1,3,5-triazine moiety which are in clinical trials, among others, for breast or colorectal cancer.
Figure 1.
Structures of exemplary anticancer drugs and compounds containing a sulfonamide or 1,3,5-triazine moiety which are in clinical trials, among others, for breast or colorectal cancer.
Figure 2.
The general structure of a designed molecular hybrid consisting of two pharmacophores: a 2-mercaptobenzenesulfonamide fragment (A) and a 4-amino-6-R2-1,3,5-triazin-2-yl ring (B), modified by the R1 substituent (C) on the N-1 imidazolidine ring.
Figure 2.
The general structure of a designed molecular hybrid consisting of two pharmacophores: a 2-mercaptobenzenesulfonamide fragment (A) and a 4-amino-6-R2-1,3,5-triazin-2-yl ring (B), modified by the R1 substituent (C) on the N-1 imidazolidine ring.
Figure 3.
A pathway of the designing of 2-[(4-amino-6-R2-1,3,5-triazin-2-yl)methylthio]-N-(imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamide derivatives based on QSAR models.
Figure 3.
A pathway of the designing of 2-[(4-amino-6-R2-1,3,5-triazin-2-yl)methylthio]-N-(imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamide derivatives based on QSAR models.
Scheme 1.
Synthesis of ethyl 2-{2-[N-(1-R1-imidazolidin-2-ylidene)sulfamoyl]-5-chloro-4-methylphenylthio}acetate 11–18 and ethyl 2-{2-[N-{1-[4-(trifluoromethyl)benzyl]tetrahydropyrimidin-2(1H)-ylidene}sulfamoyl]-5-chloro-4-methylphenylthio}acetate 19. Reagents and conditions: (a) appropriate diamine (0.04 mol), anhydrous MeOH, reflux 70–240 h; (b) N-(4-trifluoromethylbenzyl)propane-1,3-diamine (0.04 mol), anhydrous MeOH, reflux 123 h; (c) ethyl 2-bromoacetate (0.024 mol), TEA (0.02 mol), DCM.
Scheme 1.
Synthesis of ethyl 2-{2-[N-(1-R1-imidazolidin-2-ylidene)sulfamoyl]-5-chloro-4-methylphenylthio}acetate 11–18 and ethyl 2-{2-[N-{1-[4-(trifluoromethyl)benzyl]tetrahydropyrimidin-2(1H)-ylidene}sulfamoyl]-5-chloro-4-methylphenylthio}acetate 19. Reagents and conditions: (a) appropriate diamine (0.04 mol), anhydrous MeOH, reflux 70–240 h; (b) N-(4-trifluoromethylbenzyl)propane-1,3-diamine (0.04 mol), anhydrous MeOH, reflux 123 h; (c) ethyl 2-bromoacetate (0.024 mol), TEA (0.02 mol), DCM.
Scheme 2.
Synthesis of 2-[(4-amino-6-R
2-1,3,5-triazin-2-yl)methylthio]-
N-(1-R
1-imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamides (
20–
157). Compounds synthesized previously [
31].
Scheme 2.
Synthesis of 2-[(4-amino-6-R
2-1,3,5-triazin-2-yl)methylthio]-
N-(1-R
1-imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamides (
20–
157). Compounds synthesized previously [
31].
Scheme 3.
Synthesis of 2-[(4-amino-6-R2-1,3,5-triazin-2-yl)methylthio]-N-{1-[4-(trifluoromethyl)benzyl]tetrahydropyrimidin-2(1H)-ylidene}-4-chloro-5-methylbenzenesulfonamides (158–162).
Scheme 3.
Synthesis of 2-[(4-amino-6-R2-1,3,5-triazin-2-yl)methylthio]-N-{1-[4-(trifluoromethyl)benzyl]tetrahydropyrimidin-2(1H)-ylidene}-4-chloro-5-methylbenzenesulfonamides (158–162).
Figure 4.
Molecular structure of compound 27, showing the atom-labelling scheme. Solvent molecules omitted. Displacement ellipsoids are shown at 50% probability.
Figure 4.
Molecular structure of compound 27, showing the atom-labelling scheme. Solvent molecules omitted. Displacement ellipsoids are shown at 50% probability.
Figure 5.
Crystal packing and hydrogen bonding in 27. Molecules of the main component are linked by the ring-type hydrogen bond motif NH…N (using N4 and N8 atoms) located at the inversion center (drawn as the orange ball). The two dimethylsulfoxide molecules are interacting with the sulfonamide through NH…O, CH…O or NH…S hydrogen bonds.
Figure 5.
Crystal packing and hydrogen bonding in 27. Molecules of the main component are linked by the ring-type hydrogen bond motif NH…N (using N4 and N8 atoms) located at the inversion center (drawn as the orange ball). The two dimethylsulfoxide molecules are interacting with the sulfonamide through NH…O, CH…O or NH…S hydrogen bonds.
Figure 6.
Scatter plots for N-(imidazolidin-2-ylidene)benzenesulfonamides 20–44 (model 1), N-(1-benzylimidazolidin-2-ylidene)benzenesulfonamides 45–73 (model 2) and N-[1-(4-fluorobenzyl)imidazolidin-2-ylidene]benzenesulfonamides 82–101 (model 3), as well as N-[1-(3-trifluoromethylbenzyl)imidazolidin-2-ylidene]benzenesulfonamides 112–117, N-[1-(4-trifluoromethylbenzyl)imidazolidin-2-ylidene]benzenesulfonamides 118–143 and N-{1-[3,5-bis(trifluoromethyl)benzyl]imidazolidin-2-ylidene}benzenesulfonamides 144–157 (model 4) with experimental IC50 and predicted IC50 values of the training set (blue) and test set (red) for the HCT-116 cell line.
Figure 6.
Scatter plots for N-(imidazolidin-2-ylidene)benzenesulfonamides 20–44 (model 1), N-(1-benzylimidazolidin-2-ylidene)benzenesulfonamides 45–73 (model 2) and N-[1-(4-fluorobenzyl)imidazolidin-2-ylidene]benzenesulfonamides 82–101 (model 3), as well as N-[1-(3-trifluoromethylbenzyl)imidazolidin-2-ylidene]benzenesulfonamides 112–117, N-[1-(4-trifluoromethylbenzyl)imidazolidin-2-ylidene]benzenesulfonamides 118–143 and N-{1-[3,5-bis(trifluoromethyl)benzyl]imidazolidin-2-ylidene}benzenesulfonamides 144–157 (model 4) with experimental IC50 and predicted IC50 values of the training set (blue) and test set (red) for the HCT-116 cell line.
Figure 7.
Effects on MDM2 and p53 protein levels upon treatment. Western blot analysis showing MDM2 and p53 protein levels in MCF-7 cells, untreated and treated with the indicated compounds in concentrations 4 µM (A) and 10 µM (B) for 24 h. Reference: β-tubulin.
Figure 7.
Effects on MDM2 and p53 protein levels upon treatment. Western blot analysis showing MDM2 and p53 protein levels in MCF-7 cells, untreated and treated with the indicated compounds in concentrations 4 µM (A) and 10 µM (B) for 24 h. Reference: β-tubulin.
Figure 8.
Cell cycle arrest in MCF-7 cells induced by compounds 48 and 140. Cells were treated with the indicated concentrations of the compounds, and the cell cycle distribution was analyzed with flow cytometry. (A) Histograms showing the distribution of cell populations in the sub-G1, G0/G1, S and G2/M phases of the cell cycle. (B) Graphs demonstrating the percentages of cells in the depicted phases of the cell cycle. Values represent the mean ± SD of three independent experiments. Data were analyzed by one-way ANOVA with Tukey’s post hoc test [p < 0.05 (*), p < 0.01 (**), p < 0.001 (***)].
Figure 8.
Cell cycle arrest in MCF-7 cells induced by compounds 48 and 140. Cells were treated with the indicated concentrations of the compounds, and the cell cycle distribution was analyzed with flow cytometry. (A) Histograms showing the distribution of cell populations in the sub-G1, G0/G1, S and G2/M phases of the cell cycle. (B) Graphs demonstrating the percentages of cells in the depicted phases of the cell cycle. Values represent the mean ± SD of three independent experiments. Data were analyzed by one-way ANOVA with Tukey’s post hoc test [p < 0.05 (*), p < 0.01 (**), p < 0.001 (***)].
Figure 9.
Induction of apoptosis by compounds 48 and 140 in MCF-7 cells. Cells were treated with the indicated concentrations of compounds 48 and 140 for 72 h, stained with Annexin V-PE and 7-AAD and analyzed with flow cytometry. (A) Dot plots showing early apoptotic (bottom right quadrant), late apoptotic (upper right quadrant), viable (lower left quadrant) and necrotic cell populations (upper left quadrant). (B) Graphs indicating early and late apoptotic cells. Values represent the mean ± SD of three repetitions. Data were analyzed by one-way ANOVA with Tukey’s post hoc test [p < 0.05 (*), p < 0.01 (**), p < 0.001 (***)].
Figure 9.
Induction of apoptosis by compounds 48 and 140 in MCF-7 cells. Cells were treated with the indicated concentrations of compounds 48 and 140 for 72 h, stained with Annexin V-PE and 7-AAD and analyzed with flow cytometry. (A) Dot plots showing early apoptotic (bottom right quadrant), late apoptotic (upper right quadrant), viable (lower left quadrant) and necrotic cell populations (upper left quadrant). (B) Graphs indicating early and late apoptotic cells. Values represent the mean ± SD of three repetitions. Data were analyzed by one-way ANOVA with Tukey’s post hoc test [p < 0.05 (*), p < 0.01 (**), p < 0.001 (***)].
Figure 10.
Induction of apoptosis by compounds 48 and 140 in T47D cells. Cells were treated with the indicated concentrations of compounds 48 and 140 for 72 h, stained with Annexin V-PE and 7-AAD, and analyzed with flow cytometry. (A) Dot plots showing early apoptotic (bottom right quadrant), late apoptotic (upper right quadrant), viable (lower left quadrant) and necrotic cell populations (upper left quadrant). (B) Graphs indicating early and late apoptotic cells. Values represent the mean ± SD of three repetitions. Data were analyzed by one-way ANOVA with Tukey’s post hoc test [p < 0.01 (**), p < 0.001 (***)].
Figure 10.
Induction of apoptosis by compounds 48 and 140 in T47D cells. Cells were treated with the indicated concentrations of compounds 48 and 140 for 72 h, stained with Annexin V-PE and 7-AAD, and analyzed with flow cytometry. (A) Dot plots showing early apoptotic (bottom right quadrant), late apoptotic (upper right quadrant), viable (lower left quadrant) and necrotic cell populations (upper left quadrant). (B) Graphs indicating early and late apoptotic cells. Values represent the mean ± SD of three repetitions. Data were analyzed by one-way ANOVA with Tukey’s post hoc test [p < 0.01 (**), p < 0.001 (***)].
Table 1.
Cytotoxicity of N-(imidazolidin-2-ylidene)benzenesulfonamides 20–44 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
Table 1.
Cytotoxicity of N-(imidazolidin-2-ylidene)benzenesulfonamides 20–44 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
| Compd | Substituent | IC50 [µM] |
|---|
| HCT-116 | MCF-7 | HeLa | HaCaT |
|---|
| 20 | | 110.0 ± 2.0 | 220.0 ± 6.0 | 115.0 ± 7.0 | NT |
| 21 | 350.0 ± 7.0 | 190.0 ± 2.0 | 510.0 ± 5.0 | NT |
| 22 | 140.0 ± 3.0 | 130.0 ± 4.0 | 110.0 ± 4.0 | NT |
| 23 | 142.0 ± 4.0 | 130.0 ± 3.0 | 150.0 ± 2.0 | NT |
| 24 | 20.0 ± 0.4 | 27.0 ± 0.3 | 22.0 ± 0.2 | 41.0 ± 1.0 |
| 25 | 45.0 ± 2.0 | 46.0 ± 2.0 | 46.0 ± 2.0 | 54.0 ± 2.0 |
| 26 | 33.0 ± 2.0 | 33.0 ± 1.0 | 34.0 ± 1.0 | 45.0 ± 2.0 |
| 27 | 39.0 ± 2.0 | 44.0 ± 1.0 | 74.0 ± 2.0 | 75.0 ± 3.0 |
| 28 | 58.0 ± 0.6 | 45.0 ± 0.5 | 80.0 ± 1.0 | NT |
| 29 | 25.0 ± 0.5 | 24.0 ± 0.2 | 32.0 ± 0.3 | 43.0 ± 2.0 |
| 30 | ![Ijms 23 07178 i001]() | 170.0 ± 3.0 | 145.0 ± 7.0 | 153.0 ± 5.0 | NT |
| 31 | | 10.0 ± 0.4 | 16.0 ± 0.2 | 16.0 ± 0.1 | 26.0 ± 1.0 |
| 32 | 14.0 ± 0.5 | 16.0 ± 0.5 | 14.0 ± 0.5 | 28.0 ± 1.0 |
| 33 | 19.0 ± 0.5 | 26.0 ± 1.0 | 25.0 ± 1.0 | 41.0 ± 2.0 |
| 34 | 18.0 ± 1.0 | 12.0 ± 0.5 | 24.0 ± 2.0 | 41.0 ± 1.0 |
| 35 | 14.0 ± 0.7 | 16.0 ± 1.0 | 16.0 ± 1.0 | 32.0 ± 2.0 |
| 36 | 12.0 ± 0.1 | 13.0 ± 0.1 | 12.0 ± 0.1 | 28.0 ± 1.0 |
| 37 | 18.0 ± 0.5 | 19.0 ± 1.0 | 24.0 ± 1.0 | 37.0 ± 2.0 |
| 38 | 15.0 ± 0.5 | 28.0 ± 1.0 | 19.0 ± 1.0 | 35.0 ± 1.0 |
| 39 | 15.0 ± 1.0 | 17.0 ± 1.0 | 18.0 ± 1.0 | 37.0 ± 2.0 |
| 40 | 21.0 ± 0.5 | 28.0 ± 2.0 | 23.0 ± 1.0 | 45.0 ± 3.0 |
| 41 | 21.0 ± 1.0 | 46.0 ± 1.0 | 39.0 ± 2.0 | 51.0 ± 3.0 |
| 42 | 31.0 ± 1.0 | 31.0 ± 2.0 | 30.0 ± 1.5 | 61.0 ± 4.0 |
| 43 | 17.0 ± 1.0 | 22.0 ± 0.5 | 19.0 ± 0.5 | 43.0 ± 2.0 |
| 44 | 14.0 ± 0.5 | 16.0 ± 0.5 | 14.0 ± 0.5 | 28.0 ± 1.0 |
| cisplatin | 3.8 ± 0.1 | 3.0 ± 0.1 | 2.2 ± 0.1 | 7.7 ± 0.2 |
Table 2.
Cytotoxicity of N-(1-benzylimidazolidin-2-ylidene)benzenesulfonamides 45–73 and N-(1-naphthylimidazolidin-2-ylidene)benzenesulfonamides 74–81 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
Table 2.
Cytotoxicity of N-(1-benzylimidazolidin-2-ylidene)benzenesulfonamides 45–73 and N-(1-naphthylimidazolidin-2-ylidene)benzenesulfonamides 74–81 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
| Compd | Substituent | IC50 [µM] |
|---|
| HCT-116 | MCF-7 | HeLa | HaCaT |
|---|
| 45 | | 19.0 ± 0.4 | 22.0 ± 0.2 | 34.0 ± 1.0 | 43.0 ± 2.0 |
| 46 | 21.0 ± 0.2 | 23.0 ± 0.7 | 37.0 ± 0.4 | NT |
| 47 | 19.0 ± 0.2 | 20.0 ± 0.4 | 34.0 ± 0.3 | 45.0 ± 2.0 |
| 48 | 6.0 ± 0.1 | 7.0 ± 0.2 | 25.0 ± 0.3 | 33.0 ± 1.0 |
| 49 | 14.0 ± 0.1 | 17.0 ± 0.3 | 24.0 ± 0.5 | 38.0 ± 1.0 |
| 50 | 18.0 ± 0.2 | 43.0 ± 1.0 | 35.0 ± 2.0 | 51.0 ± 2.0 |
| 51 | 19.0 ± 0.6 | 10.0 ± 0.5 | 15.0 ± 1.0 | 220.0 ± 12.0 |
| 52 | 37.0 ± 0.5 | 64.0 ± 2.0 | 61.0 ± 2.0 | 92.0 ± 3.0 |
| 53 | 10.0 ± 0.5 | 11.0 ± 0.5 | 85.0 ± 2.0 | 81.0 ± 4.0 |
| 54 | 11.0 ± 0.1 | 12.0 ± 0.1 | 16.0 ± 0.2 | 28.0 ± 1.0 |
| 55 | 7.0 ± 0.1 | 10.0 ± 0.2 | 13.0 ± 0.1 | 39.0 ± 2.0 |
| 56 | 22.0 ± 1.0 | 54.0 ± 1.0 | 37.0 ± 1.0 | 58.0 ± 2.0 |
| 57 | ![Ijms 23 07178 i002]() | 8.0 ± 0.1 | 8.0 ± 0.2 | 102.0 ± 2.0 | 48.0 ± 2.0 |
| 58 | | 11.0 ± 0.3 | 17.0 ± 1.0 | 14.0 ± 2.0 | 29.0 ± 1.0 |
| 59 | 13.0 ± 0.3 | 18.0 ± 1.0 | 15.0 ± 1.0 | 30.0 ± 1.0 |
| 60 | 8.0 ± 0.3 | 14.0 ± 0.3 | 9.0 ± 0.5 | 23.0 ± 1.0 |
| 61 | 6.5 ± 0.1 | 9.0 ± 0.3 | 10.0 ± 0.2 | 21.0 ± 1.0 |
| 62 | 6.0 ± 0.1 | 7.0 ± 0.1 | 107.0 ± 1.0 | 32.0 ± 1.0 |
| 63 | 7.0 ± 0.1 | 9.0 ± 0.5 | 12.0 ± 0.5 | 24.0 ± 1.0 |
| 64 | 11.0 ± 0.4 | 14.0 ± 0.5 | 15.0 ± 1.0 | 28.0 ± 1.0 |
| 65 | 7.0 ± 0.2 | 11.0 ± 0.5 | 11.0 ± 0.3 | 23.0 ± 1.0 |
| 66 | 12.0 ± 0.4 | 29.0 ± 1.0 | 20.0 ± 1.0 | 36.0 ± 2.0 |
| 67 | 168.0 ± 8.0 | 153.0 ± 5.0 | 140.0 ± 7.0 | NT |
| 68 | 16.0 ± 0.5 | 16.0 ± 0.5 | 14.0 ± 0.5 | 32.0 ± 2.0 |
| 69 | 66.0 ± 2.0 | 122.0 ± 7.0 | 100.0 ± 6.0 | 128.0 ± 5.0 |
| 70 | 300.0 ± 18.0 | 490.0 ± 10.0 | 550.0 ± 21.0 | NT |
| 71 | 155.0 ± 6.0 | 205.0 ± 4.0 | 265.0 ± 8.0 | 27.0 ± 1.0 |
| 72 | 6.5 ± 0.1 | 9.0 ± 0.5 | 17.0 ± 0.5 | 28.0 ± 1.0 |
| 73 | 5.2 ± 0.2 | 7.5 ± 0.3 | 14.0 ± 0.3 | 29.0 ± 1.0 |
| 74 | 44.0 ± 2.0 | 12.0 ± 0.6 | 13.0 ± 0.5 | 81.0 ± 4.0 |
| 75 | 30.0 ± 2.0 | 8.0 ± 0.5 | 77.0 ± 5.0 | 110.0 ± 5.0 |
| 76 | ![Ijms 23 07178 i003]() | 46.0 ± 1.0 | 25.0 ± 1.0 | 28.0 ± 2.0 | 99.0 ± 4.0 |
| 77 | | 40.0 ± 1.0 | 29.0 ± 1.0 | 110.0 ± 5.0 | 220.0 ± 11.0 |
| 78 | 19.5 ± 0.6 | 11.0 ± 0.5 | 130.0 ± 8.0 | 280.0 ± 14.0 |
| 79 | 94.0 ± 2.0 | 98.0 ± 5.0 | 88.0 ± 3.0 | NT |
| 80 | 79.0 ± 2.0 | 89.0 ± 4.0 | 98.0 ± 5.0 | NT |
| 81 | 8.4 ± 0.3 | 8.0 ± 0.5 | 63.0 ± 1.0 | 240.0 ± 12.0 |
| cisplatin | 3.8 ± 0.1 | 3.0 ± 0.1 | 2.2 ± 0.1 | 7.7 ± 0.2 |
Table 3.
Cytotoxicity of N-[1-(4-fluoro or bromo)benzylimidazolidin-2-ylidene]benzenesulfonamides 82–111 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
Table 3.
Cytotoxicity of N-[1-(4-fluoro or bromo)benzylimidazolidin-2-ylidene]benzenesulfonamides 82–111 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
| Compd | Substituent | IC50 [µM] |
|---|
| HCT-116 | MCF-7 | HeLa | HaCaT |
|---|
| 82 | | 19.0 ± 0.8 | 12.0 ± 0.1 | 30.0 ± 1.0 | 54.0 ± 2.0 |
| 83 | 10.0 ± 0.5 | 14.0 ± 1.0 | 12.0 ± 3.0 | 32.0 ± 1.0 |
| 84 | 9.0 ± 0.5 | 7.0 ± 0.1 | 15.0 ± 0.6 | 27.0 ± 1.0 |
| 85 | 15.0 ± 0.5 | 23.0 ± 1.0 | 21.0 ± 4.0 | 38.0 ± 1.0 |
| 86 | 13.0 ± 0.5 | 15.0 ± 0.2 | 16.0 ± 0.2 | 35.0 ± 2.0 |
| 87 | 10.0 ± 0.4 | 7.0 ± 0.3 | 15.0 ± 1.0 | 34.0 ± 1.0 |
| 88 | 17.0 ± 0.5 | 31.0 ± 1.0 | 25.0 ± 2.0 | 43.0 ± 2.0 |
| 89 | 14.0 ± 0.7 | 18.0 ± 1.0 | 17.0 ± 0.6 | 31.0 ± 1.0 |
| 90 | 12.0 ± 0.2 | 17.0 ± 1.0 | 15.0 ± 0.6 | 30.0 ± 2.0 |
| 91 | ![Ijms 23 07178 i004]() | 20.0 ± 1.0 | 13.0 ± 0.4 | 34.0 ± 2.0 | 54.0 ± 2.0 |
| 92 | | 7.0 ± 0.3 | 8.0 ± 0.4 | 8.0 ± 0.5 | 18.0 ± 1.0 |
| 93 | 7.0 ± 0.3 | 7.0 ± 0.5 | 8.0 ± 0.4 | 18.0 ± 1.0 |
| 94 | 9.0 ± 0.2 | 14.0 ± 0.5 | 12.0 ± 1.0 | 25.0 ± 1.0 |
| 95 | 7.0 ± 0.3 | 7.0 ± 0.1 | 8.0 ± 0.4 | 18.0 ± 1.0 |
| 96 | 6.0 ± 0.3 | 6.0 ± 0.2 | 9.0 ± 0.3 | 22.0 ± 1.0 |
| 97 | 6.0 ± 0.2 | 7.0 ± 0.4 | 9.0 ± 0.3 | 24.0 ± 1.0 |
| 98 | 7.0 ± 0.1 | 7.0 ± 0.4 | 8.0 ± 0.4 | 18.0 ± 1.0 |
| 99 | 17.0 ± 1.0 | 17.0 ± 1.0 | 18.0 ± 0.5 | 30.0 ± 1.0 |
| 100 | 8.0 ± 0.2 | 7.0 ± 0.1 | 8.0 ± 0.5 | 18.0 ± 1.0 |
| 101 | 12.0 ± 0.5 | 11.0 ± 0.2 | 10.0 ± 0.6 | 21.0 ± 1.0 |
| 102 | 8.0 ± 0.4 | 7.5 ± 0.4 | 17.0 ± 1.0 | 33.0 ± 1.0 |
| 103 | 7.0 ± 0.1 | 6.5 ± 0.3 | 16.0 ± 0.5 | 28.0 ± 1.0 |
| 104 | 17.0 ± 1.0 | 13.0 ± 0.4 | 25.0 ± 1.0 | 44.0 ± 2.0 |
| 105 | ![Ijms 23 07178 i005]() | 13.0 ± 0.8 | 10.0 ± 0.2 | 17.0 ± 0.5 | 35.0 ± 2.0 |
| 106 | | 8.5 ± 0.1 | 6.5 ± 0.4 | 82.0 ± 4.0 | 110.0 ± 4.0 |
| 107 | 8.5 ± 0.3 | 7.0 ± 0.2 | 58.0 ± 2.0 | 76.0 ± 4.0 |
| 108 | 10.5 ± 0.5 | 11.0 ± 0.5 | 11.0 ± 0.5 | 24.0 ± 1.0 |
| 109 | 23.0 ± 1.0 | 22.0 ± 0.5 | 36.0 ± 2.0 | 55.0 ± 2.0 |
| 110 | 20.0 ± 0.6 | 23.0 ± 1.0 | 85.0 ± 5.0 | 123.0 ± 6.0 |
| 111 | 5.6 ± 0.3 | 7.0 ± 0.2 | 7.5 ± 0.3 | 18.0 ± 1.0 |
| cisplatin | 3.8 ± 0.1 | 3.0 ± 0.1 | 2.2 ± 0.1 | 7.7 ± 0.2 |
Table 4.
Cytotoxicity of N-[1-(3- or 4-trifluoromethyl)benzylimidazolidin-2-ylidene]benzenesulfonamides 112–143 and N-[1-(3,5-bis(trifluoromethyl)benzylimidazolidin-2-ylidene]benzenesulfonamides 144–157 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
Table 5.
Cytotoxicity of N-[1-(4-trifluoromethyl)benzyltetrahydropyrimidin-2(1H)-ylidene]benzenesulfonamides 158–162 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
Table 5.
Cytotoxicity of N-[1-(4-trifluoromethyl)benzyltetrahydropyrimidin-2(1H)-ylidene]benzenesulfonamides 158–162 toward human cancer cell lines and non-cancerous line HaCaT. Cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa), and the human keratinocyte cell line (HaCaT).
| Compd | Substituent | IC50 [µM] |
|---|
| HCT-116 | MCF-7 | HeLa | HaCaT |
|---|
| 158 | | 5.0 ± 0.3 | 8.0 ± 0.4 | 6.0 ± 0.3 | 17.0 ± 1.0 |
| 159 | ![Ijms 23 07178 i009]() | 9.0 ± 0.5 | 10.0 ± 0.3 | 15.5 ± 0.5 | 29.0 ± 1.0 |
| 160 | | 10.5 ± 0.4 | 12.0 ± 0.5 | 8.5 ± 0.3 | 23.0 ± 1.0 |
| 161 | 6.5 ± 0.1 | 6.5 ± 0.2 | 14.0 ± 0.5 | 27.0 ± 1.0 |
| 162 | 5.5 ± 0.3 | 6.0 ± 0.2 | 12.5 ± 0.5 | 25.0 ± 0.7 |
| cisplatin | 3.8 ± 0.2 | 3.0 ± 0.1 | 2.2 ± 0.1 | 7.7 ± 0.2 |
Table 6.
Selectivity indexes (SI) of selected compounds toward cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa).
Table 6.
Selectivity indexes (SI) of selected compounds toward cell lines: colon cancer (HCT-116), breast cancer (MCF-7), cervical cancer (HeLa).
| Compd | Selectivity Index (SI) |
|---|
| HCT-116 | MCF-7 | HeLa |
|---|
| 48 | 5.5 | 4.7 | 1.3 |
| 55 | 5.5 | 3.9 | 3.0 |
| 62 | 5.3 | 4.5 | 0.3 |
| 73 | 5.5 | 3.8 | 2.0 |
| 114 | 5.4 | 4.1 | 2.2 |
| 134 | 4.4 | 2.0 | 2.2 |
| 140 | 4.4 | 2.2 | 2.7 |
| 150 | 6.4 | 5.1 | 2.0 |
| 157 | 4.6 | 3.8 | 1.7 |
| 162 | 4.5 | 4.1 | 2.0 |
| cisplatin | 2.0 | 2.5 | 3.5 |
Table 7.
Summary of the QSAR equations.
Table 7.
Summary of the QSAR equations.
| STEP 1 |
| Model 1 | R1 = H, compounds: 20–44 |
IC50 = −8.783228 ast_violation − 0.242646 PEOE_VSA-0 − 28.231708 rings + 212.156433
R2 = 0.88; R2adj = 0.85; Q2LOO = 0.80; F(3,11) = 26.76; p = 0.2 × 10−4; N(train) = 15; N(test) = 6 |
| STEP 2 |
| Model 2 | R1 = Bn, compounds: 45–73 |
IC50 = 100.345937 b_1rotN + 10.490686 GCUT_PEOE_0 + 0.507081 lip_acc − 0.318859 radius + 6.112894
R2 = 0.88; R2adj = 0.85; Q2LOO = 0.72; F(4,15) = 28.17; p = 0.8 × 10−6; N(train) = 20; N(test) = 7 |
| STEP 3 |
| Model 3 | R1 = 4-F-Bn, compounds: 82–101 |
IC50= 256.006020 BCUT_SMR_1 + 0.139099 Q_VSA_HYD − 0.120301 vsurf_V + 201.402742
R2 = 0.91; R2adj = 0.89; Q2LOO = 0.85; F(3,11) = 37.67; p = 0.4 × 10−5; N(train) = 15; N(test) = 5 |
| STEP 4 |
| Model 4 | R1 = 3-F3C-Bn, 4-F3C-Bn, 3,5-bis(F3C)Bn, compounds: 112–157 |
IC50 = 10^[1/(−2.44368 vsurf_CW6 + 1.59455 VDistEq − 0.02515 vsurf_DD12 + 0.24868 vsurf_IW4 − 0.11745 vsurf_IW5 − 5.49371)]
R2 = 0.84; R2adj = 0.81; Q2LOO = 0.74; F(5,25) = 26.27; p = 0.3 × 10−8; N(train) = 31; N(test) = 14 |
Table 8.
Type and chemical meaning of molecular descriptors in QSAR models.
Table 8.
Type and chemical meaning of molecular descriptors in QSAR models.
| | Descriptor | Type | Definition |
|---|
| Model 1 | ast_violation | The atom count and bond count descriptors | Number of Astex fragment-likeness violations |
| PEOE_VSA-0 | Partial charge descriptor | Sum of the van der Waals area, where the partial charge is in the range −0.05 to 0.50 |
| rings | The atom count and bond count descriptors | The number of rings in molecule |
| Model 2 | b_1rotN | The atom count and bond count descriptors | Number of rotatable single bonds. Conjugated single bonds are not included (e.g. ester and peptide bonds) |
| GCUT_PEOE_0 | Adjacency and distance matrix descriptors | The smallest GCUT descriptor using partial PEOE loads. The descriptor is related to the distance between the atoms and the charge of the atoms |
| lip_acc | The atom count and bond count descriptors | The number of oxygen and nitrogen atoms |
| radius | Adjacency and distance matrix descriptors | The descriptor is related to the distance between atoms and the surrounding atoms by other atoms |
| Model 3 | BCUT_SMR_1 | Adjacency and distance matrix descriptors | The BCUT descriptors using atomic contribution to molar refractivity (using the Wildman and Crippen SMR method) instead of partial charge. |
| Q_VSA_HYD | Partial charge descriptors | Total hydrophobic van der Waals surface area. |
| vsurf_S | Surface area, volume and shape descriptors | Interaction field surface area |
| Model 4 | vsurf_CW6 | Capacity factor | The descriptor is depend on the structure of molecule, connectivity and configuration of atoms |
| VDistEq | Adjacency and distance matrix descriptor | The descriptor VDistEq is information content index of vertex distance equality. |
| vsurf_DD12 | Adjacency and distance matrix descriptor | The descriptor quantifies the contact distance of lowest and the 2nd lowest hydrophobic energy points of a molecule |
| vsurf_IW4 | Partial charge descriptor | Hydrophilic interaction energy moment |
| vsurf_IW5 | Partial charge descriptor | Hydrophilic interaction energy moment |
Table 9.
Comparison of IC50 value for the HCT-116 and MCF-7 cell lines for compounds 31, 57, 92, 106, 131, 150, with the substituent R2 = 4-phenylpiperazin-1-yl, and compounds 25, 50, 85, 104, 122, 146, with the substituent R2 = phenylamino.
Table 9.
Comparison of IC50 value for the HCT-116 and MCF-7 cell lines for compounds 31, 57, 92, 106, 131, 150, with the substituent R2 = 4-phenylpiperazin-1-yl, and compounds 25, 50, 85, 104, 122, 146, with the substituent R2 = phenylamino.
| Compd | 31 | 57 | 92 | 106 | 131 | 150 |
|---|
| R1 = | H | Bn | 4-F-Bn | 4-Br-Bn | 4-F3C-Bn | 3,5-bis(F3C)Bn |
| R2 = | 4-Ph-piperazin-1-yl |
| | IC50 [µM] |
| HCT-116 | 45.0 | 8.0 | 7.0 | 8.5 | 7.0 | 3.6 |
| MCF-7 | 46.0 | 8.0 | 8.0 | 6.5 | 15.0 | 4.5 |
| Compd | 25 | 50 | 85 | 104 | 122 | 146 |
| R1 = | H | Bn | 4-F-Bn | 4-Br-Bn | 4-F3C-Bn | 3,5-bis(F3C)Bn |
| R2 = | PhNH |
| | IC50 [µM] |
| HCT-116 | 45.0 | 18.0 | 15.0 | 17.0 | 14.0 | 11.5 |
| MCF-7 | 46.0 | 43.0 | 23.0 | 13.0 | 20.0 | 12.5 |
Table 10.
Comparison of IC50 values for the HCT-116, MCF-7 and HeLa cell lines for compounds 34, 60, 134, with R2 = S = 4-[4-(trifluoromethyl)phenyl]piperazin-1-yl, and compounds 35, 61, 135, with R2 = T = 4-[3-(trifluoromethyl)phenyl]piperazin-1-yl.
Table 10.
Comparison of IC50 values for the HCT-116, MCF-7 and HeLa cell lines for compounds 34, 60, 134, with R2 = S = 4-[4-(trifluoromethyl)phenyl]piperazin-1-yl, and compounds 35, 61, 135, with R2 = T = 4-[3-(trifluoromethyl)phenyl]piperazin-1-yl.
| Compd | 34 | 35 | 60 | 61 | 134 | 135 |
|---|
| R1 = | H | H | Bn | Bn | 4-F3C-Bn | 4-F3C-Bn |
| R2 = | S | T | S | T | S | T |
| | IC50 [µM] |
| HCT-116 | 18.0 | 14.0 | 8.0 | 6.5 | 5.0 | 5.0 |
| MCF-7 | 12.0 | 16.0 | 14.0 | 9.0 | 11.0 | 6.5 |
| HeLa | 24.0 | 16.0 | 9.0 | 10.0 | 10.0 | 7.0 |
![Ijms 23 07178 i010]() |
Table 11.
Cytotoxicity of compounds 48, 62, 134, 138 and 140 toward ovarian cancer (A2780, SKOV-3) and breast cancer (MCF-7, T47D) cell lines.
Table 11.
Cytotoxicity of compounds 48, 62, 134, 138 and 140 toward ovarian cancer (A2780, SKOV-3) and breast cancer (MCF-7, T47D) cell lines.
| Compd | Substituent | IC50 [µM] | |
|---|
| A2780 | SKOV3 | MCF-7 | T47D | HaCaT |
|---|
| 48 | ![Ijms 23 07178 i011]() | 4.0 ± 0.1 | 8.1 ± 0.1 | 7.8 ± 0.1 | 4.8 ± 0.1 | 25.0 ± 1.0 |
| 62 | 3.9 ± 0.1 | 10.1 ± 0.1 | 10.4 ± 0.1 | 4.9 ± 0.1 | 32.0 ± 1.0 |
| 134 | ![Ijms 23 07178 i012]() | 3.9 ± 0.1 | 5.7 ± 0.1 | 7.8 ± 0.1 | 3.2 ± 0.1 | 22.0 ± 1.0 |
| 138 | 2.2 ± 0.1 | 5.8 ± 0.1 | 8.2 ± 0.1 | 4.4 ± 0.1 | 22.0 ± 1.0 |
| 140 | 4.5 ± 0.1 | 6.6 ± 0.1 | 6.9 ± 0.1 | 5.3 ± 0.1 | 22.0 ± 1.0 |
| cisplatin | 1.4 ± 0.1 | 71.0 ± 0.1 | 3.0 ± 0.1 | 3.1 ± 0.1 | 7.7 ± 0.2 |