Genetic LGALS1 Variants Are Associated with Heterogeneity in Galectin-1 Serum Levels in Patients with Early Arthritis
Abstract
:1. Introduction
2. Results
2.1. Study of Genetic Variability in the Gal1 Promoter and Gene
2.2. Effect of rs929039, rs9622682, and rs4820293 in Gal1 Serum Levels along the Follow-Up
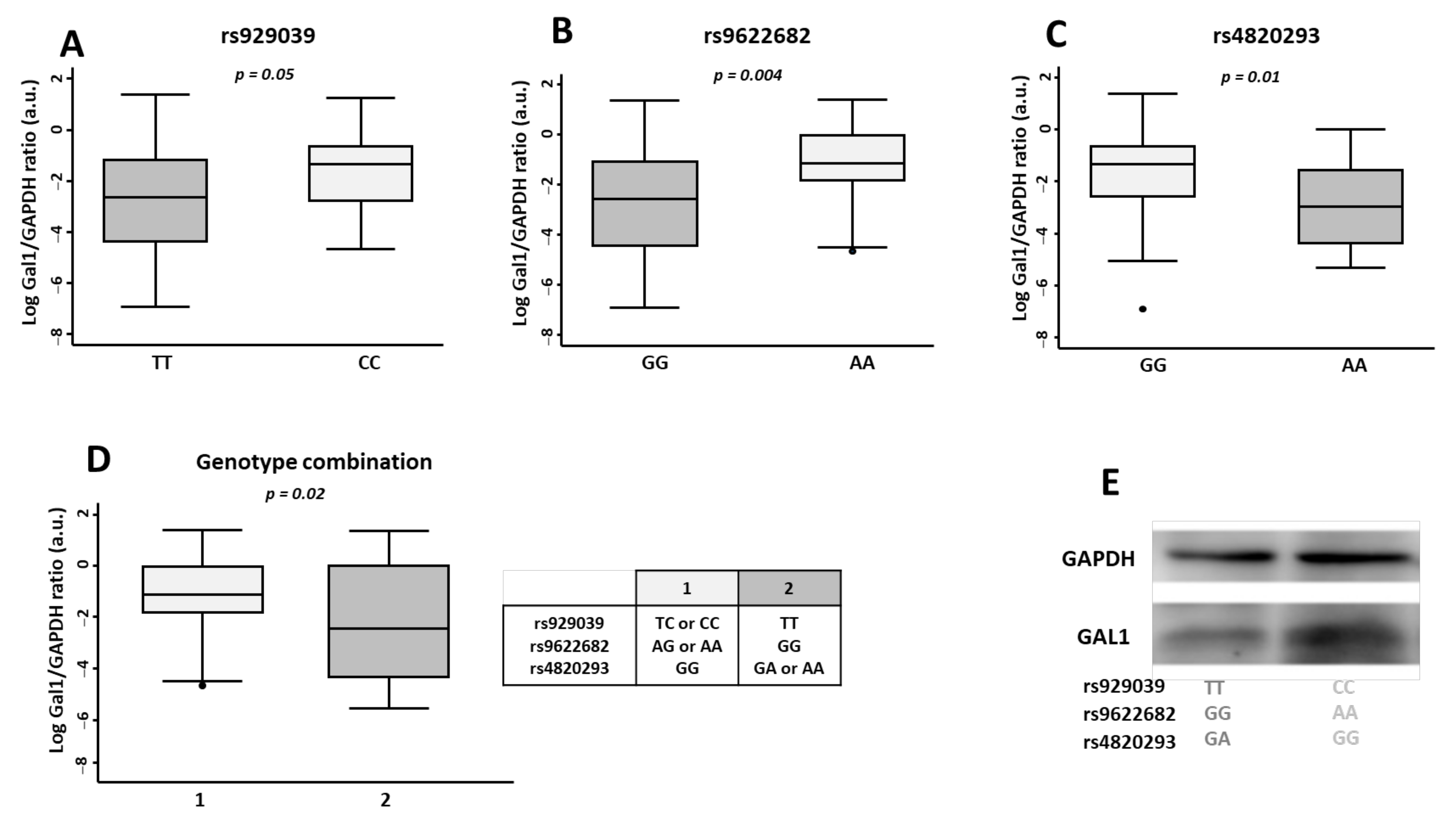
2.3. rs929039, rs9622682, and rs4820293 Genotypes Are Associated with Gal1 Expression in Peripheral Blood Lymphocytes
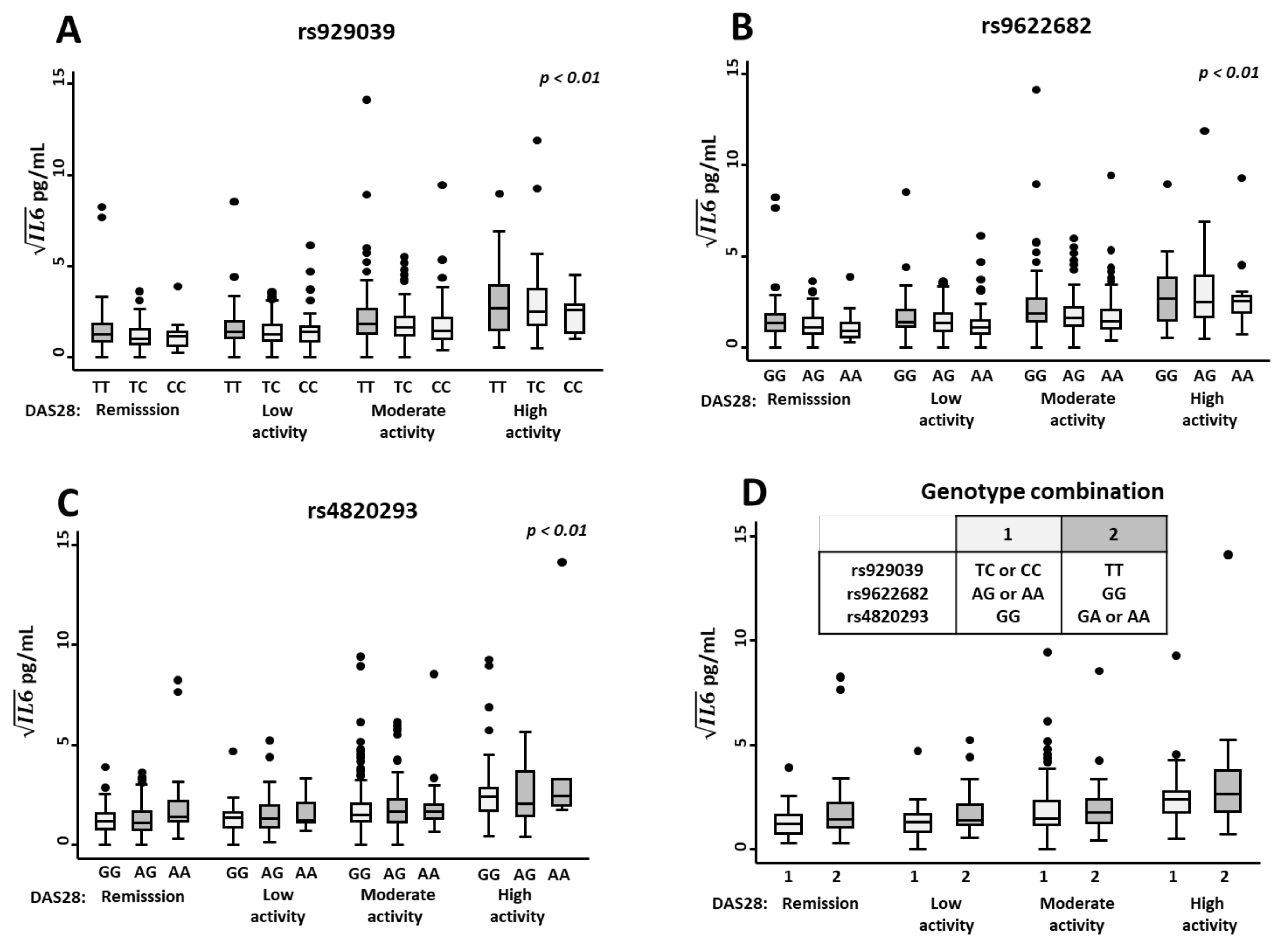
2.4. The Genotypes rs929039, rs9622682, and rs4820293 Are Associated with IL-6 Serum Levels in EA Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. DNA Isolation and LGALS1 Sequencing
4.3. rs9622682, rs929039, and rs4820293 Genotyping
4.4. Galectin-1 Assessment through Western-Blot
4.5. Measure of Gal1 Serum and IL-6 in EA Population
4.6. Statistical Analysis
4.7. Multivariate Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Kirino, Y.; Remmers, E.F. Genetic Architectures of Seropositive and Seronegative Rheumatic Diseases. Nat. Rev. Rheumatol. 2015, 11, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of Rheumatoid Arthritis Contributes to Biology and Drug Discovery. Nature 2014, 506, 376–381. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and Function of a Large Family of Animal Lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef]
- Cutine, A.M.; Bach, C.A.; Veigas, F.; Merlo, J.P.; Laporte, L.; Manselle Cocco, M.N.; Massaro, M.; Sarbia, N.; Perrotta, R.M.; Mahmoud, Y.D.; et al. Tissue-Specific Control of Galectin-1-Driven Circuits during Inflammatory Responses. Glycobiology 2021, 31, 891–907. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Croci, D.O. Regulatory Circuits Mediated by Lectin-Glycan Interactions in Autoimmunity and Cancer. Immunity 2012, 36, 322–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovich, G.A.; Daly, G.; Dreja, H.; Tailor, H.; Riera, C.M.; Hirabayashi, J.; Chernajovsky, Y. Recombinant Galectin-1 and Its Genetic Delivery Suppress Collagen-Induced Arthritis via T Cell Apoptosis. J. Exp. Med. 1999, 190, 385–398. [Google Scholar] [CrossRef]
- Mendez-Huergo, S.P.; Hockl, P.F.; Stupirski, J.C.; Maller, S.M.; Morosi, L.G.; Pinto, N.A.; Berón, A.M.; Musuruana, J.L.; Nasswetter, G.G.; Cavallasca, J.A.; et al. Clinical Relevance of Galectin-1 and Galectin-3 in Rheumatoid Arthritis Patients: Differential Regulation and Correlation With Disease Activity. Front. Immunol. 2018, 9, 3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triguero-Martínez, A.; de la Fuente, H.; Montes, N.; Ortiz, A.M.; Roy-Vallejo, E.; Castañeda, S.; González-Alvaro, I.; Lamana, A. Validation of Galectin-1 as Potential Diagnostic Biomarker of Early Rheumatoid Arthritis. Sci. Rep. 2020, 10, 17799. [Google Scholar] [CrossRef]
- Mehrabian, M.; Gitt, M.A.; Sparkes, R.S.; Leffler, H.; Barondes, S.H.; Lusis, A.J. Two Members of the S-Lac Lectin Gene Family, LGALS1 and LGALS2, Reside in Close Proximity on Human Chromosome 22q12-Q13. Genomics 1993, 15, 418–420. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Cheng, Z.; Yang, S.; Chu, H.; Fan, Y.; Li, C.; Wong, B.H.-Y.; Zheng, S.; Zhu, Y.; et al. Functional Variants Regulating LGALS1 (Galectin 1) Expression Affect Human Susceptibility to Influenza A(H7N9). Sci. Rep. 2015, 5, 8517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovich, G.A.; Ariel, A.; Hershkoviz, R.; Hirabayashi, J.; Kasai, K.I.; Lider, O. Specific Inhibition of T-Cell Adhesion to Extracellular Matrix and Proinflammatory Cytokine Secretion by Human Recombinant Galectin-1. Immunology 1999, 97, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chávez, F.; Castro-Leyva, V.; Espejel-Núñez, A.; Zamora-Mendoza, R.G.; Rosas-Vargas, H.; Cancino-Díaz, J.C.; Cancino-Díaz, M.E.; Estrada-Gutierrez, G.; Rodríguez-Martínez, S. Galectin-1 Reduced the Effect of LPS on the IL-6 Production in Decidual Cells by Inhibiting LPS on the Stimulation of IκBζ. J. Reprod. Immunol. 2015, 112, 46–52. [Google Scholar] [CrossRef]
- Municio, C.; Dominguez-Soto, Á.; Fuentelsaz-Romero, S.; Lamana, A.; Montes, N.; Cuevas, V.D.; Campos, R.G.; Pablos, J.L.; González-Álvaro, I.; Puig-Kröger, A. Methotrexate Limits Inflammation through an A20-Dependent Cross-Tolerance Mechanism. Ann. Rheum. Dis. 2018, 77, 752–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamana, A.; Balsa, A.; Rueda, B.; Ortiz, A.M.; Nuño, L.; Miranda-Carus, M.E.; Gonzalez-Escribano, M.F.; Lopez-Nevot, M.A.; Pascual-Salcedo, D.; Martin, J.; et al. The TT Genotype of the STAT4 Rs7574865 Polymorphism Is Associated with High Disease Activity and Disability in Patients with Early Arthritis. PLoS ONE 2012, 7, e43661. Available online: https://pubmed.ncbi.nlm.nih.gov/22937072/ (accessed on 13 April 2022).
- Hulkkonen, J.; Pertovaara, M.; Antonen, J.; Pasternack, A.; Hurme, M. Elevated Interleukin-6 Plasma Levels Are Regulated by the Promoter Region Polymorphism of the IL6 Gene in Primary Sjögren’s Syndrome and Correlate with the Clinical Manifestations of the Disease. Rheumatology 2001, 40, 656–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbers, C.; Monhasery, N.; Aparicio-Siegmund, S.; Lokau, J.; Baran, P.; Nowell, M.A.; Jones, S.A.; Rose-John, S.; Scheller, J. The Interleukin-6 Receptor Asp358Ala Single Nucleotide Polymorphism Rs2228145 Confers Increased Proteolytic Conversion Rates by ADAM Proteases. Biochim. Biophys. Acta 2014, 1842, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Montoro, M.; Cañadas-Garre, M.; González-Utrilla, A.; Ángel Calleja-Hernández, M. Influence of IL6R Gene Polymorphisms in the Effectiveness to Treatment with Tocilizumab in Rheumatoid Arthritis. Pharm. J. 2018, 18, 167–172. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Freitag, D.F.; Cutler, A.J.; Howson, J.M.M.; Rainbow, D.B.; Smyth, D.J.; Kaptoge, S.; Clarke, P.; Boreham, C.; Coulson, R.M.; et al. Functional IL6R 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases. PLoS Genet. 2013, 9, e1003444. [Google Scholar] [CrossRef]
- Seoane, I.V.; Martínez, C.; García-Vicuña, R.; Ortiz, A.M.; Juarranz, Y.; Talayero, V.C.; González-Álvaro, I.; Gomariz, R.P.; Lamana, A. Vasoactive Intestinal Peptide Gene Polymorphisms, Associated with Its Serum Levels, Predict Treatment Requirements in Early Rheumatoid Arthritis. Sci. Rep. 2018, 8, 2035. [Google Scholar] [CrossRef]
- Lamana, A.; Villares, R.; Seoane, I.V.; Andrés, N.; Lucas, P.; Emery, P.; Vital, E.M.; Triguero-Martínez, A.; Marquez, A.; Ortiz, A.M.; et al. Identification of a Human SOCS1 Polymorphism That Predicts Rheumatoid Arthritis Severity. Front. Immunol. 2020, 11, 1336. [Google Scholar] [CrossRef]
- Cerliani, J.P.; Blidner, A.G.; Toscano, M.A.; Croci, D.O.; Rabinovich, G.A. Translating the “Sugar Code” into Immune and Vascular Signaling Programs. Trends Biochem. Sci. 2017, 42, 255–273. [Google Scholar] [CrossRef]
- Lei, T.; Moos, S.; Klug, J.; Aslani, F.; Bhushan, S.; Wahle, E.; Fröhlich, S.; Meinhardt, A.; Fijak, M. Galectin-1 Enhances TNFα-Induced Inflammatory Responses in Sertoli Cells through Activation of MAPK Signalling. Sci. Rep. 2018, 8, 3741. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, J.A.; Chang, M.H.; Wang, S.; Almazan, T.; Hashimi, S.T.; Eriksson, A.U.; Wen, X.; Pang, M.; Baum, L.G.; Singh, R.R.; et al. Galectin-1 Co-Clusters CD43/CD45 on Dendritic Cells and Induces Cell Activation and Migration through Syk and Protein Kinase C Signaling. J. Biol. Chem. 2009, 284, 26860–26870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulcher, J.A.; Hashimi, S.T.; Levroney, E.L.; Pang, M.; Gurney, K.B.; Baum, L.G.; Lee, B. Galectin-1-Matured Human Monocyte-Derived Dendritic Cells Have Enhanced Migration through Extracellular Matrix. J. Immunol. 2006, 177, 216–226. [Google Scholar] [CrossRef] [Green Version]
- De F. Zanon, C.; Sonehara, N.M.; Girol, A.P.; Gil, C.D.; Oliani, S.M. Protective Effects of the Galectin-1 Protein on in Vivo and in Vitro Models of Ocular Inflammation. Mol. Vis. 2015, 21, 1036–1050. [Google Scholar]
- Shen, Z.; Xu, H.; Song, W.; Hu, C.; Guo, M.; Li, J.; Li, J. Galectin-1 Ameliorates Perioperative Neurocognitive Disorders in Aged Mice. CNS Neurosci. Ther. 2021, 27, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, X.; Liu, S.; Zou, Q.; Zheng, S.; Yu, F.; Chen, Y. Galectin-1 Ameliorates Influenza A H1N1pdm09 Virus-Induced Acute Lung Injury. Front. Microbiol. 2020, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R. Galectins as Pattern Recognition Receptors: Structure, Function, and Evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Toscano, M.A.; Martínez Allo, V.C.; Cutine, A.M.; Rabinovich, G.A.; Mariño, K.V. Untangling Galectin-Driven Regulatory Circuits in Autoimmune Inflammation. Trends Mol. Med. 2018, 24, 348–363. [Google Scholar] [CrossRef]
- Gatie, M.I.; Spice, D.M.; Garha, A.; McTague, A.; Ahmer, M.; Timoshenko, A.V.; Kelly, G.M. O-GlcNAcylation and Regulation of Galectin-3 in Extraembryonic Endoderm Differentiation. Biomolecules 2022, 12, 623. [Google Scholar] [CrossRef]
- Mathew, M.P.; Abramowitz, L.K.; Donaldson, J.G.; Hanover, J.A. Nutrient-Responsive O-GlcNAcylation Dynamically Modulates the Secretion of Glycan-Binding Protein Galectin 3. J. Biol. Chem. 2022, 298, 101743. [Google Scholar] [CrossRef] [PubMed]
- Tazhitdinova, R.; Timoshenko, A.V. The Emerging Role of Galectins and O-GlcNAc Homeostasis in Processes of Cellular Differentiation. Cells 2020, 9, 1792. [Google Scholar] [CrossRef] [PubMed]
- Toledano, E.; Ortiz, A.M.; Ivorra-Cortes, J.; Montes, N.; Beltran, A.; Rodríguez-Rodriguez, L.; Carmona, L.; González-Álvaro, I. Are Rheumatologists Adhering to the Concepts Window of Opportunity and Treat-to-Target? Earlier and More Intense Disease-Modifying Anti-Rheumatic Drug Treatment over Time in Patients with Early Arthritis in the PEARL Study. Clin. Exp. Rheumatol. 2018, 36, 382–388. [Google Scholar] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic Insights from the Treatment of Rheumatoid Arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [Green Version]
- González-Álvaro, I.; Ortiz, A.M.; Alvaro-Gracia, J.M.; Castañeda, S.; Díaz-Sánchez, B.; Carvajal, I.; García-Vadillo, J.A.; Humbría, A.; López-Bote, J.P.; Patiño, E.; et al. Interleukin 15 Levels in Serum May Predict a Severe Disease Course in Patients with Early Arthritis. PLoS ONE 2011, 6, e29492. [Google Scholar] [CrossRef] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheumatol. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Verpoort, K.N.; van Dongen, H.; Allaart, C.F.; Toes, R.E.M.; Breedveld, F.C.; Huizinga, T.W.J. Undifferentiated Arthritis--Disease Course Assessed in Several Inception Cohorts. Clin. Exp. Rheumatol. 2004, 22, S12–S17. [Google Scholar]
- Carlson, C.S.; Eberle, M.A.; Rieder, M.J.; Yi, Q.; Kruglyak, L.; Nickerson, D.A. Selecting a Maximally Informative Set of Single-Nucleotide Polymorphisms for Association Analyses Using Linkage Disequilibrium. Am. J. Hum. Genet. 2004, 74, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The Structure of Haplotype Blocks in the Human Genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef] [Green Version]
- Galectin-1: A Potential Biomarker Differentiating between Early Rheumatoid Arthritis and Spondyloarthritis. Available online: https://assets.researchsquare.com/files/rs-695957/v1/31f744cd-24cc-4378-b4b7-1929706e238b.pdf?c=1635106833 (accessed on 13 April 2022).
- Pan, W. Akaike’s Information Criterion in Generalized Estimating Equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]

| LGALS1 (rs929039) | LGALS1 (rs9622682) | LGALS1 (rs4820293) | ||||
|---|---|---|---|---|---|---|
| β Coeff. (95% CI) | p Value | β Coeff. (95% CI) | p Value | β Coeff. (95% CI) | p Value | |
| DAS28 | ||||||
| Remission | Reference | − | Reference | − | Reference | − |
| Low DA | 0.19 (0.05 to 0.33) | 0.006 | 0.17 (0.03 to 0.31) | 0.01 | 0.21 (0.06 to 0.36) | 0.04 |
| Moderate DA | 0.61 (0.45 to 0.76) | <0.001 | 0.62 (0.46 to 0.78) | <0.001 | 0.66 (0.50 to 0.82) | <0.001 |
| High DA | 1.38 (1 to 1.76) | <0.001 | 1.39 (1 to 1.78) | <0.001 | 1.30 (0.93 to 1.68) | <0.001 |
| Methotrexate dose (mg) | −0.008 (−0.01 to −0.0007) | 0.03 | −0.008 (−0.015 to −0.0002) | 0.04 | −0.01 (−0.018 to −0.002) | 0.01 |
| LGALS1 (rs929039) | ||||||
| TT | Reference | − | ||||
| TC | −0.35 (−0.56 to −0.13) | 0.001 | ||||
| CC | −0.39 (−0.67 to −0.1) | 0.007 | ||||
| LGALS1 (rs9622682) | ||||||
| GG | Reference | − | ||||
| GA | −0.38 (−0.63 to −0.13) | 0.003 | ||||
| AA | −0.52 (−0.78 to −0.26) | <0.001 | ||||
| LGALS1 (rs4820293) | ||||||
| GG | Reference | − | ||||
| GA | 0.14 (−0.03 to −0.33) | 0.12 | ||||
| AA | 0.58 (0.15 to 1) | 0.007 | ||||
| Population 1 | Population 2 | p | |
|---|---|---|---|
| (n = 53) | (n = 479) | ||
| Female; n (%) | 38 (71.70) | 384 (80.17) | 0.15 |
| Age; p50 [p25–p75] | 53.62 [43.33–67.49] | 55.16 [44.23–66.50] | 0.69 |
| Disease duration (months); p50 [p25–p75] | 6.53 [4.06–8.53] | 5.0.6 [2.76–8.5] | 0.11 |
| RF positive; n (%) | 27 (50.94) | 262 (54.70) | 0.6 |
| ACPA positive; n (%) | 26 (49.06) | 243 (51.16) | 0.79 |
| DAS28; p50 [p25–p75] | 4.88 [3.85–6.05] | 4.22 [3.21–5.51] | 0.01 |
| HAQ; p50 [p25–p75] | 1.12 [0.62–1.62] | 1 [0.43–1.62] | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triguero-Martínez, A.; Roy-Vallejo, E.; Montes, N.; de la Fuente, H.; Ortiz, A.M.; Castañeda, S.; González-Álvaro, I.; Lamana, A. Genetic LGALS1 Variants Are Associated with Heterogeneity in Galectin-1 Serum Levels in Patients with Early Arthritis. Int. J. Mol. Sci. 2022, 23, 7181. https://doi.org/10.3390/ijms23137181
Triguero-Martínez A, Roy-Vallejo E, Montes N, de la Fuente H, Ortiz AM, Castañeda S, González-Álvaro I, Lamana A. Genetic LGALS1 Variants Are Associated with Heterogeneity in Galectin-1 Serum Levels in Patients with Early Arthritis. International Journal of Molecular Sciences. 2022; 23(13):7181. https://doi.org/10.3390/ijms23137181
Chicago/Turabian StyleTriguero-Martínez, Ana, Emilia Roy-Vallejo, Nuria Montes, Hortensia de la Fuente, Ana María Ortiz, Santos Castañeda, Isidoro González-Álvaro, and Amalia Lamana. 2022. "Genetic LGALS1 Variants Are Associated with Heterogeneity in Galectin-1 Serum Levels in Patients with Early Arthritis" International Journal of Molecular Sciences 23, no. 13: 7181. https://doi.org/10.3390/ijms23137181






