The Role of Microglia in the (Mal)adaptive Response to Traumatic Experience in an Animal Model of PTSD
Abstract
1. Introduction
2. Results
2.1. Effects of Predator Scent-Stress (PSS) Exposure on Microglia
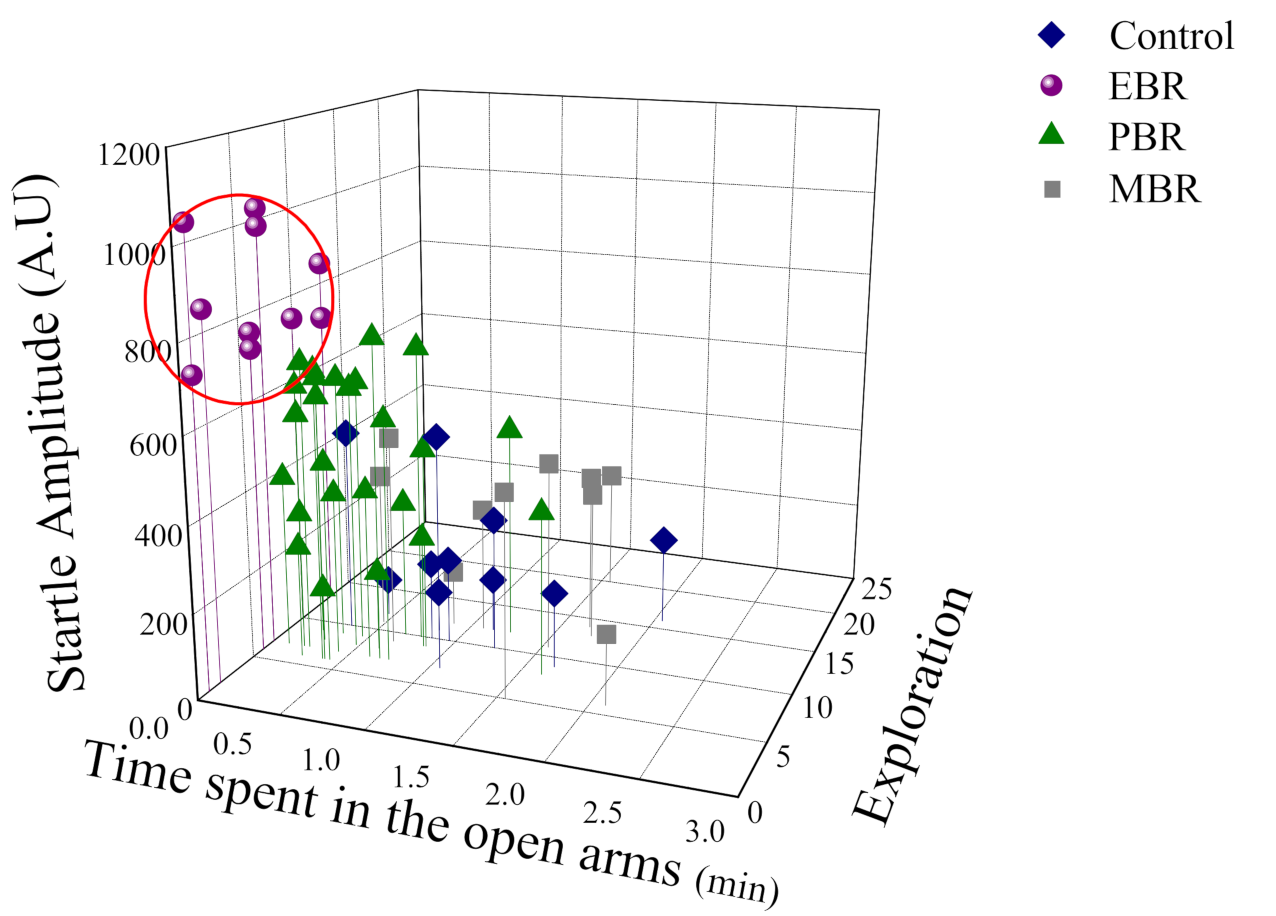
Effects of Predator Scent-Stress Exposure on Behavioral Responses at Day 7 Post Exposure
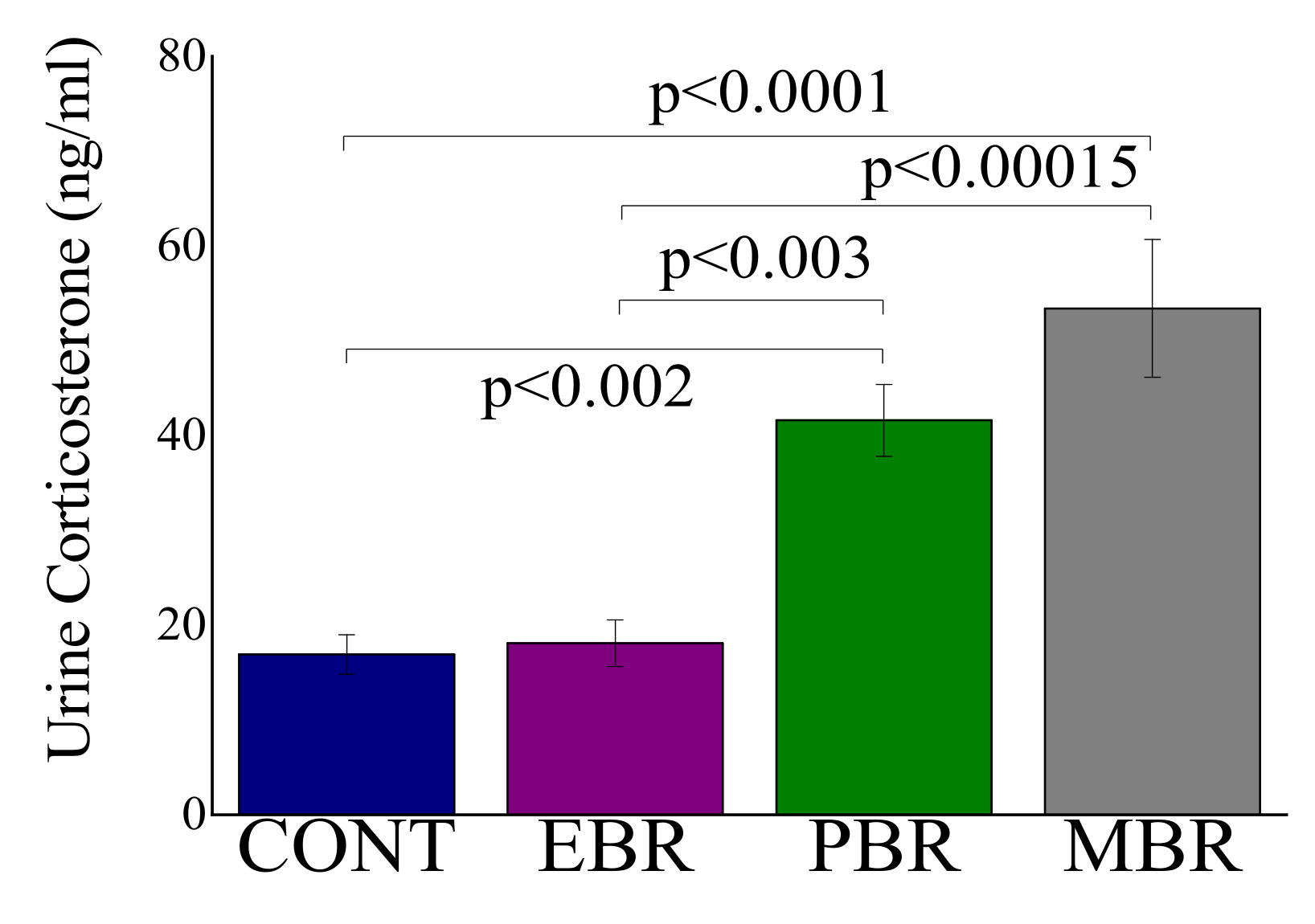
2.2. Effects of Predator Scent-Stress Exposure on Corticosterone Levels at 1–2 h after Exposure
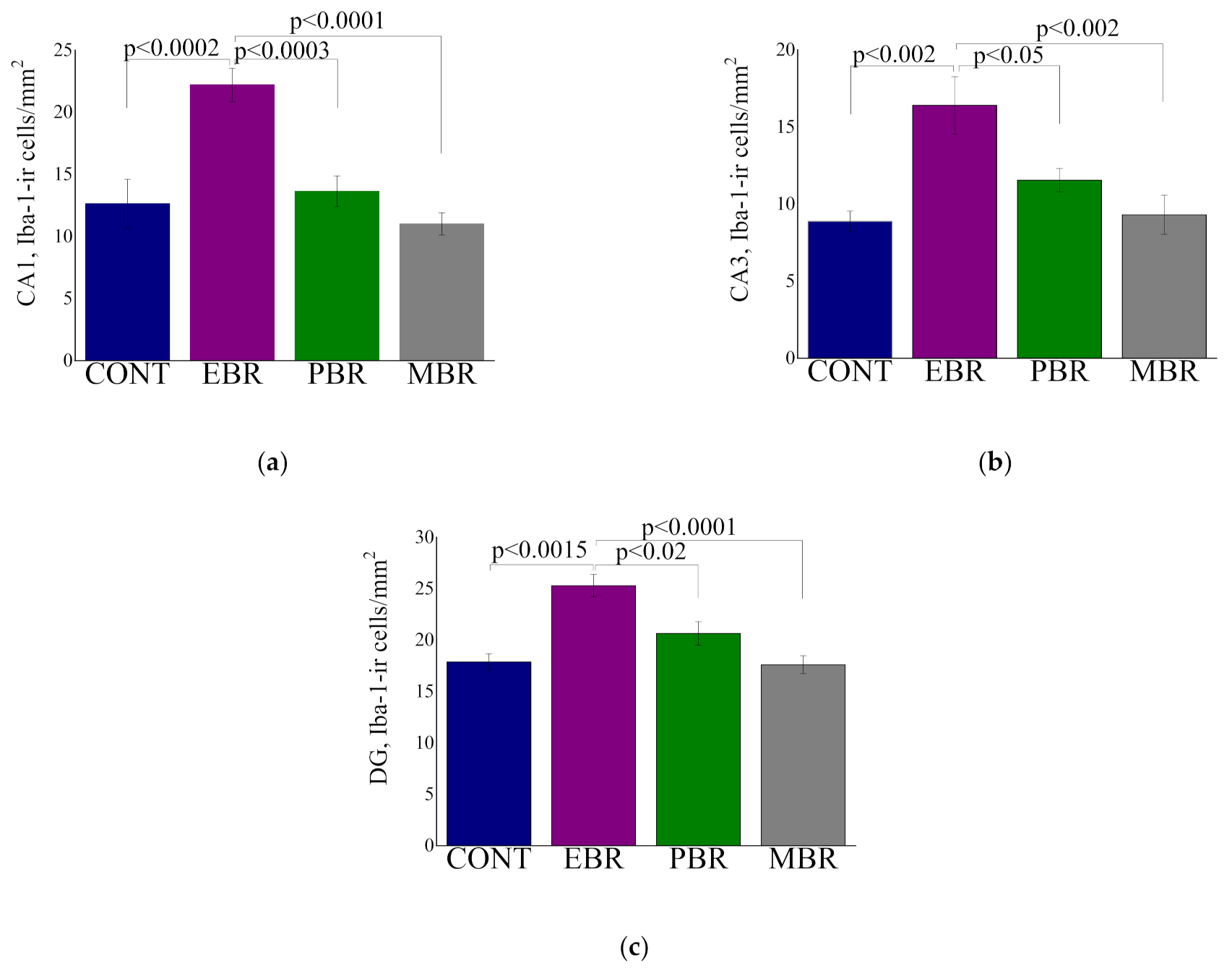
2.3. Iba-1 Positive Cell Counts in Hippocampal Subregions at Day 8 Post PSS-Exposure
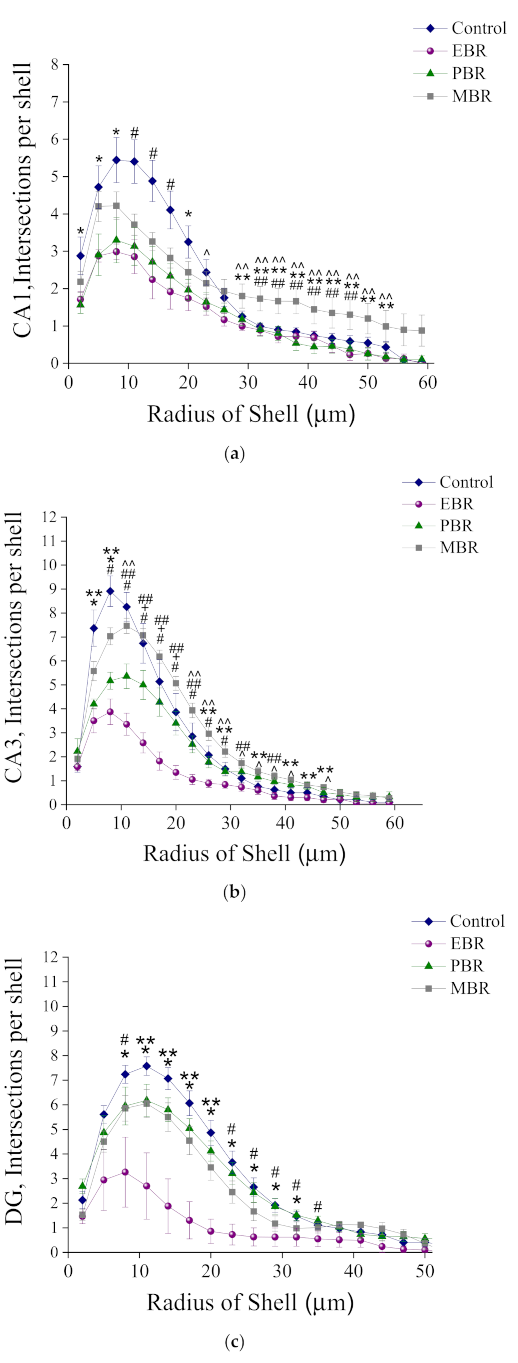
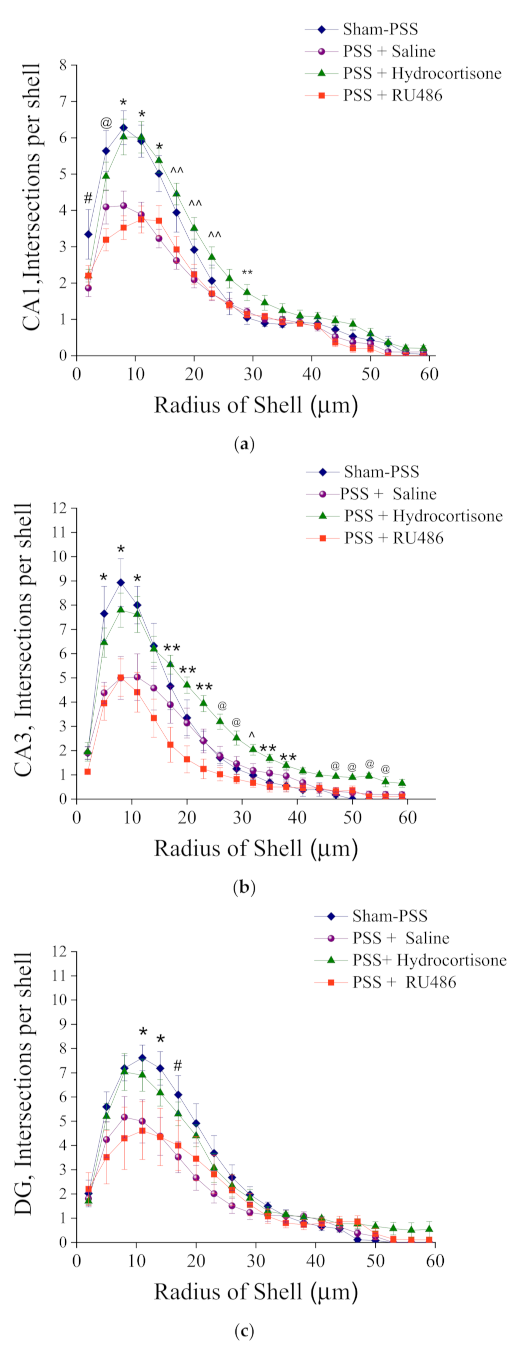
2.4. Morphological Analysis of Iba-1 in CA1, CA3 and DG Hippocampal Subregions at Day 8 Post PSS-Exposure
Sholl Analysis of Iba-1 in CA1, CA3 and DG Hippocampal Subregions at Day 8 Post PSS-Exposure
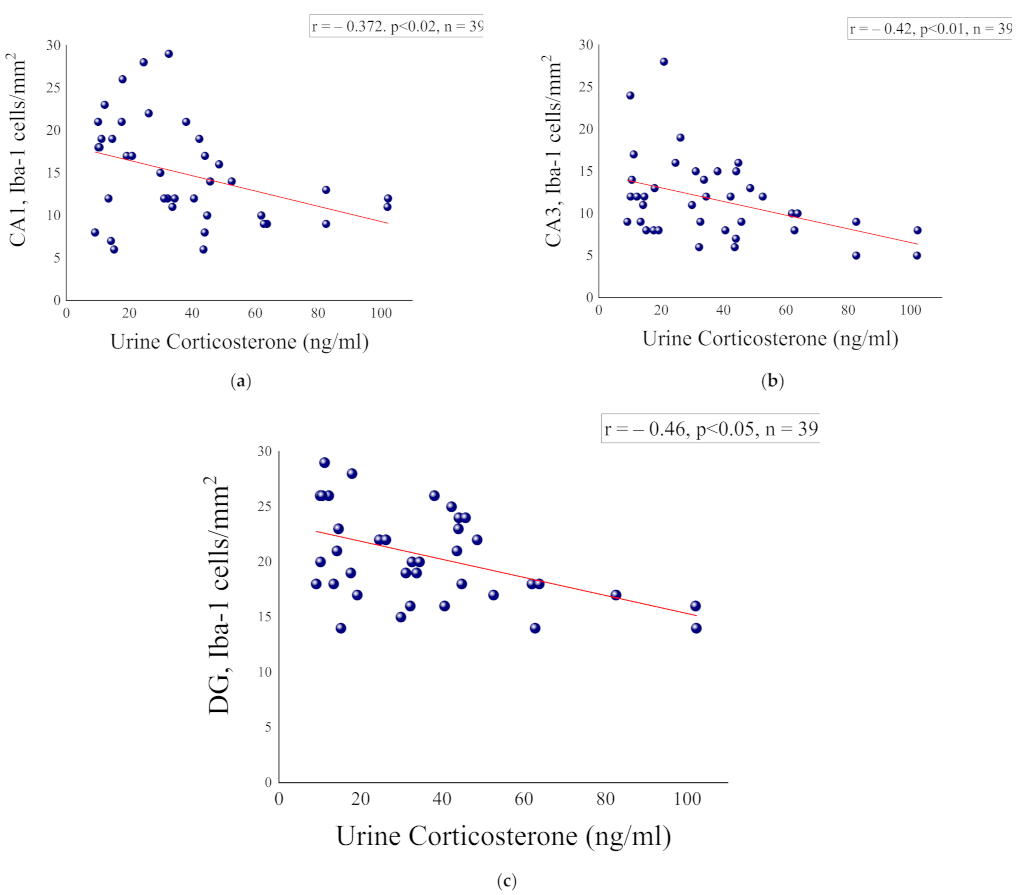
2.5. Correlation Analysis between Corticosterone Levels at the Initial PSS Response and Microglia Measures at Day 8 Post Exposure
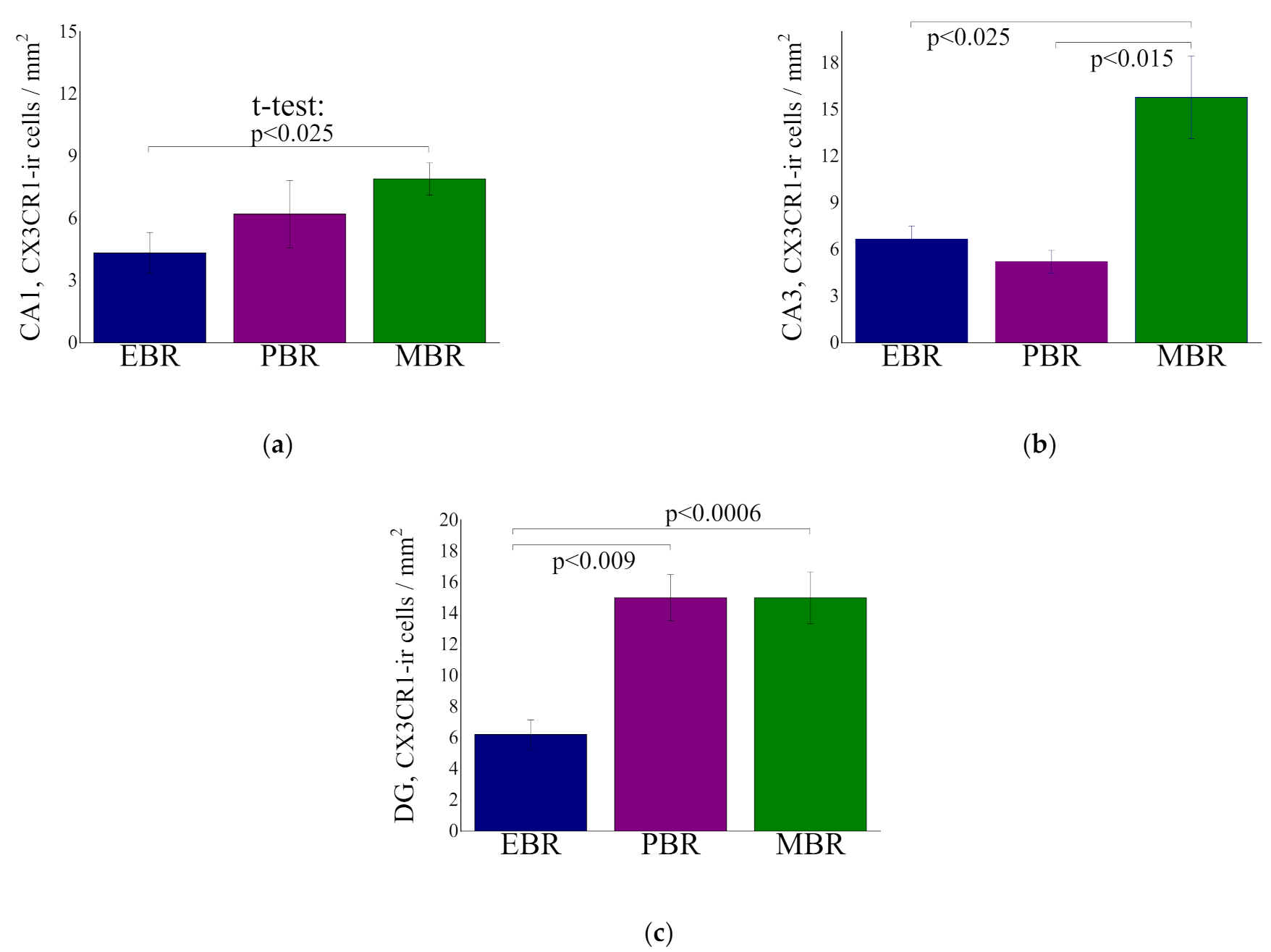
2.6. CX3CR1-ir Cells in Hippocampal Subregion at Day 8 Post PSS-Exposure
2.7. Effects of High-Dose Hydrocortisone, RU486 (GR Receptor Antagonist) or Saline Immediately Post-Exposure on Behavioral Stress Responses at Day 7 Post-Exposure
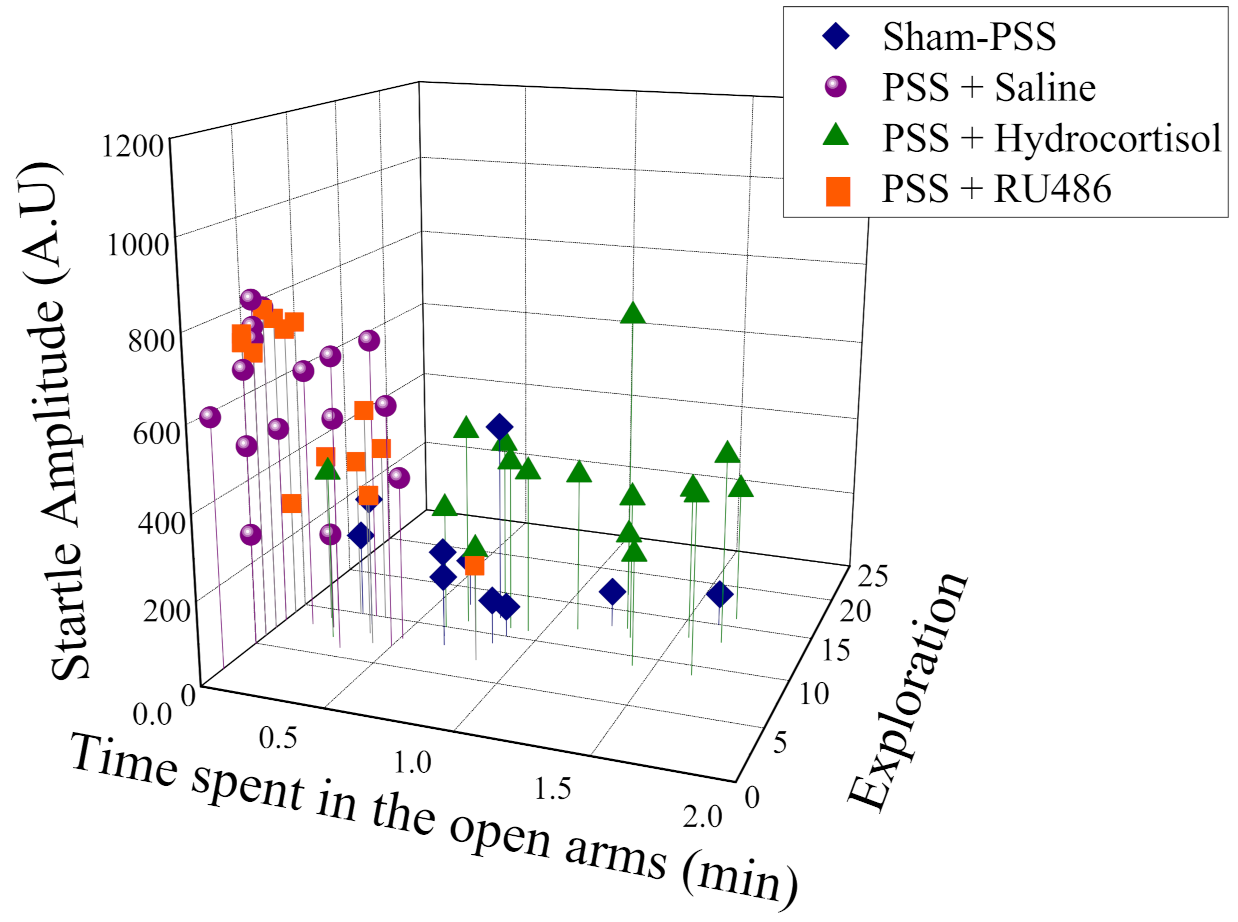
2.8. Effects of High-Dose Hydrocortisone, Saline or RU486 Early Post-Exposure on Behavioral Stress Responses at Day 7 Post-Exposure
2.9. Effects of High-Dose Hydrocortisone, Saline or RU486 Early Post-Exposure on Iba-1 Positive Cell Counts in Hippocampus
2.10. Effects of High-Dose Hydrocortisone, Saline or RU486 Immediately Post-Exposure on Iba-1 Morphology
3. Discussion
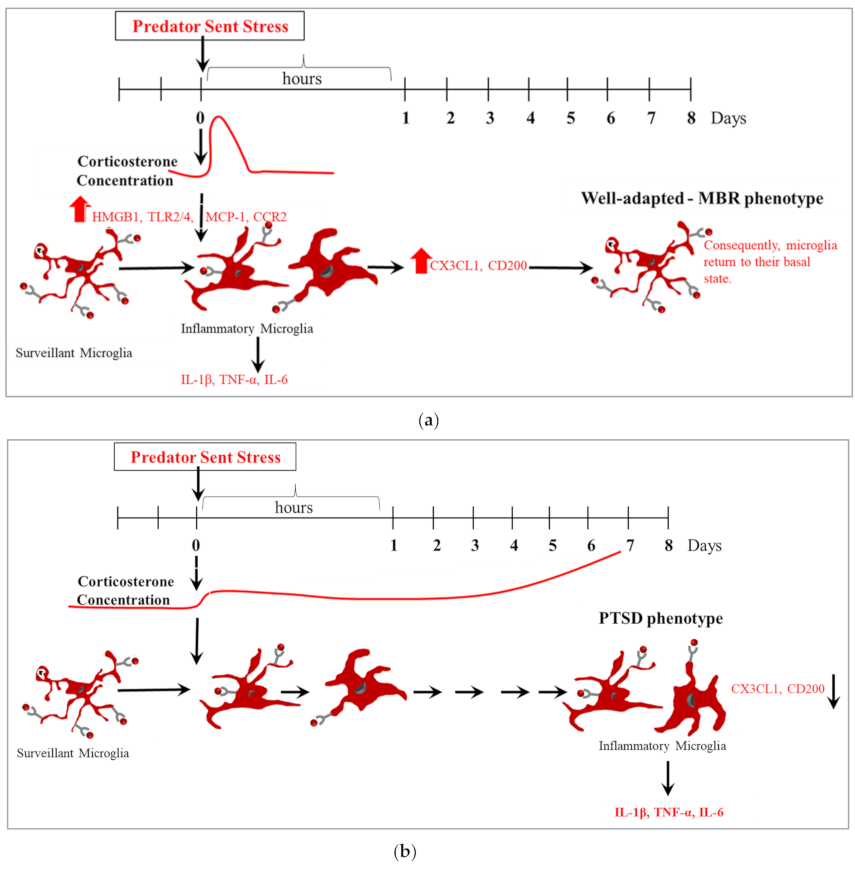
3.1. Microglia Proliferation
3.2. Microglia Morphology
3.3. CX3XR1 Levels
3.4. Urine Corticosterone Levels Immediately after PSS Exposure
3.5. High-Dose Hydrocortisone Immediately Post-Exposure Changed Expression of Microglia Morphology
4. Materials and Methods
4.1. Animals
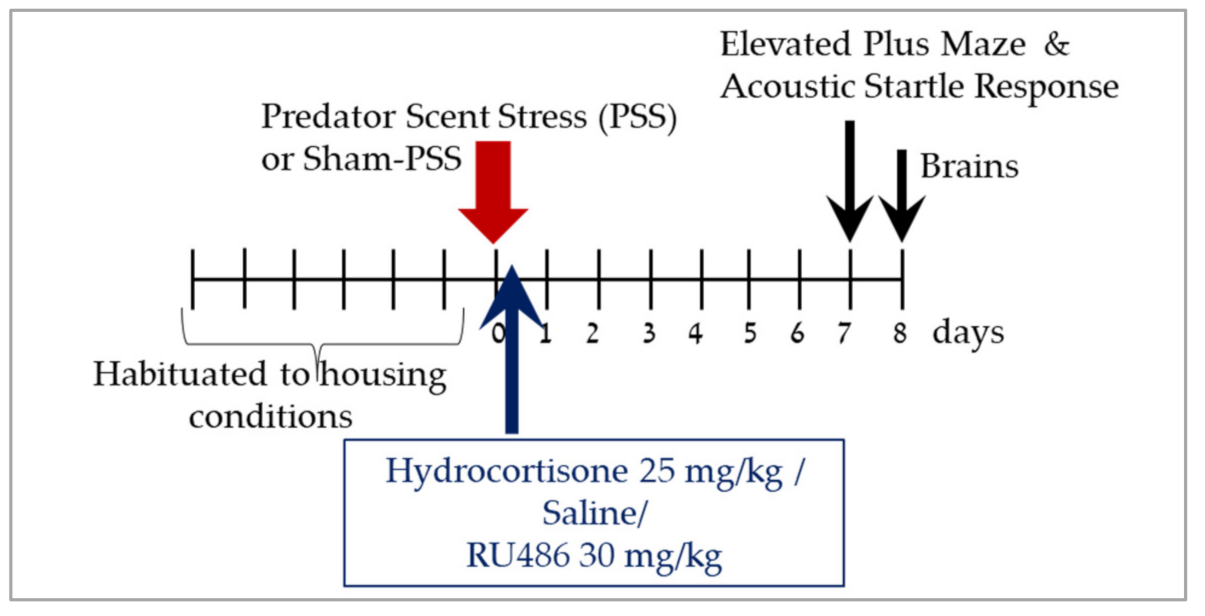
4.2. Experimental Design
4.3. Predator Scent Stress
4.4. Drugs
4.5. Behavioral Assessments
4.5.1. Elevated-Plus Maze (EPM)
4.5.2. Acoustic Startle Response (ASR)
4.6. Cut-Off Behavioral Criteria Model
4.7. Urine Corticosterone Sampling
4.8. Immunohistochemistry
4.8.1. Staining
4.8.2. Quantification
4.8.3. Morphological Analysis of Microglia Cells
4.8.4. Region of Interest
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CA | Cornu Ammonis |
| CNS | Central nervous system |
| CX3CR1 | CX3C chemokine receptor 1 |
| DG | Dentate gyrus |
| EBR | Extreme behavioral response |
| EPM | Elevated plus-maze |
| GC | Glucocorticoid |
| GR | Glucocorticoid receptor |
| HPA | Hypothalamus-pituitary-adrenal |
| Iba-1 | Ionized calcium-binding adaptor molecule 1 |
| IL-1β | Interleukin-1β |
| Ir | Immunoreactive |
| MBR | Minimal behavioral response |
| NF-κB | Nuclear factor-κB |
| PBR | Partial behavioral response |
| PSS | Predator scent stress |
| PTE | Potentially traumatic experience |
| PTSD | Posttraumatic stress disorder |
| TFs | Transcription factors |
| TNF-α | Tumor necrosis factor α |
| URA | Upstream regulator analysis |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlingon, VA, USA, 2013. [Google Scholar]
- Cohen, H.; Kozlovsky, N.; Alona, C.; Matar, M.A.; Joseph, Z. Animal model for PTSD: From clinical concept to translational research. Neuropharmacology 2012, 62, 715–724. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal models of post-traumatic stress disorder. Curr. Protoc. Neurosci. 2013, 9, 45. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.A.; Zohar, J. Maintaining the clinical relevance of animal models in translational studies of post-traumatic stress disorder. ILAR J. 2014, 55, 233–245. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Matar, M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol. Psychiatry 2003, 53, 463–473. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Matar, M.A.; Kaplan, Z.; Geva, A.B. Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol. Psychiatry 2005, 58, 640–650. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Matar, M.A.; Zeev, K.; Loewenthal, U.; Richter-Levin, G. Setting apart the affected: The use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004, 29, 1962–1970. [Google Scholar] [CrossRef]
- Adamec, R. Transmitter systems involved in neural plasticity underlying increased anxiety and defense--implications for understanding anxiety following traumatic stress. Neurosci. Biobehav. Rev 1997, 21, 755–765. [Google Scholar] [CrossRef]
- Adamec, R.E.; Shallow, T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol. Behav. 1993, 54, 101–109. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C.; Rodgers, J.; Weiss, S.M. The characterization and modelling of antipredator defensive behavior. Neurosci. Biobehav. Rev. 1990, 14, 463–472. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Griebel, G.; Henrie, J.A.; Blanchard, D.C. Differentiation of anxiolytic and panicolytic drugs by effects on rat and mouse defense test batteries. Neurosci. Biobehav. Rev. 1997, 21, 783–789. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Nikulina, J.N.; Sakai, R.R.; McKittrick, C.; McEwen, B.; Blanchard, D.C. Behavioral and endocrine change following chronic predatory stress. Physiol. Behav. 1998, 63, 561–569. [Google Scholar] [CrossRef]
- Cohen, H.; Benjamin, J.; Kaplan, Z.; Kotler, M. Administration of high-dose ketoconazole, an inhibitor of steroid synthesis, prevents posttraumatic anxiety in an animal model. Eur. Neuropsychopharmacol. 2000, 10, 429–435. [Google Scholar] [CrossRef]
- Cohen, H.; Friedberg, S.; Michael, M.; Kotler, M.; Zeev, K. Interaction of CCK-4 induced anxiety and post-cat exposure anxiety in rats. Depress Anxiety 1996, 4, 144–145. [Google Scholar] [CrossRef]
- Cohen, H.; Kaplan, Z.; Kotler, M. CCK-antagonists in a rat exposed to acute stress: Implication for anxiety associated with post-traumatic stress disorder. Depress Anxiety 1999, 10, 8–17. [Google Scholar] [CrossRef]
- File, S.E.; Zangrossi Jr, H.; Sanders, F.L.; Mabbutt, P.S. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol. Behav. 1993, 54, 1109–1111. [Google Scholar] [CrossRef]
- Breslau, N.; Davis, G.C.; Andreski, P.; Peterson, E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch. Gen. Psychiatry 1991, 48, 216–222. [Google Scholar] [CrossRef]
- Breslau, N.; Kessler, R.C.; Chilcoat, H.D.; Schultz, L.R.; Davis, G.C.; Andreski, P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Arch. Gen. Psychiatry 1998, 55, 626–632. [Google Scholar] [CrossRef]
- Cohen, H.; Geva, A.B.; Matar, M.A.; Zohar, J.; Kaplan, Z. Post-traumatic stress behavioural responses in inbred mouse strains: Can genetic predisposition explain phenotypic vulnerability? Int. J. Neuropsychopharmacol. 2008, 11, 331–349. [Google Scholar] [CrossRef]
- Cohen, H.; Kaplan, Z.; Matar, M.; Loewenthal, U.; Kozlovsky, N.; Zohar, J. Anisomycin, a protein synthesis inhibitor, disrupts traumatic memory consolidation and attenuates post traumatic stress response in rats. Biol. Psychiatry 2006, 60, 767–776. [Google Scholar] [CrossRef]
- Cohen, H.; Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Zohar, J. Mapping the brain pathways of traumatic memory: Inactivation of protein kinase M zeta in different brain regions disrupts traumatic memory processes and attenuates traumatic stress responses in rats. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2010, 20, 253–271. [Google Scholar] [CrossRef]
- Cohen, H.; Kozlovsky, N.; Matar, M.A.; Zohar, J.; Kaplan, Z. The characteristic long-term upregulation of hippocampal NF-kappaB complex in PTSD-like behavioral stress response is normalized by high-dose corticosterone and pyrrolidine dithiocarbamate administered immediately after exposure. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 2286–2302. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Kozlovsky, N.; Matar, M.A.; Zohar, J.; Kaplan, Z. Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2014, 24, 1925–1944. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathe, A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Maayan, R.; Touati-Werner, D.; Kaplan, Z.; Matar, M.; Loewenthal, U.; Kozlovsky, N.; Weizman, R. Decreased circulatory levels of neuroactive steroids in behaviorally more extremely affected rats subsequent to exposure to a potentially traumatic experience. Int. J. Neuropsychopharmacol. 2007, 10, 203–209. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.; Buskila, D.; Kaplan, Z.J.Z. Early post-stressor intervention with high dose corticosterone attenuates post traumatic stress response in an animal model of PTSD. Biol. Psychiatry 2008, 15, 708–717. [Google Scholar] [CrossRef]
- Cohen, H.; Yehuda, R. Gender differences in animal models of posttraumatic stress disorder. Dis. Markers 2011, 30, 141–150. [Google Scholar] [CrossRef]
- Cohen, H.; Ziv, Y.; Cardon, M.; Kaplan, Z.; Matar, M.A.; Gidron, Y.; Schwartz, M.; Kipnis, J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J. Neurobiol. 2006, 66, 552–563. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Gidron, Y.; Matar, M.A.; Belkind, D.; Loewenthal, U.; Kozlovsky, N.; Kaplan, Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry 2006, 59, 1208–1218. [Google Scholar] [CrossRef]
- Koresh, O.; Kaplan, Z.; Zohar, J.; Matar, M.A.; Geva, A.B.; Cohen, H. Distinctive cardiac autonomic dysfunction following stress exposure in both sexes in an animal model of PTSD. Behav. Brain Res. 2016, 308, 128–142. [Google Scholar] [CrossRef]
- Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Kotler, M.; Zohar, J.; Cohen, H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int. J. Neuropsychopharmacol. 2007, 10, 741–758. [Google Scholar] [CrossRef]
- Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. A distinct pattern of intracellular glucocorticoid-related responses is associated with extreme behavioral response to stress in an animal model of post-traumatic stress disorder. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2009, 19, 759–771. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Cohen, H.; Cai, G.; Buxbaum, J.D.; Yehuda, R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc. Natl. Acad. Sci. USA 2014, 111, 13529–13534. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Cohen, H.; Nievergelt, C.M.; Baker, D.G.; Buxbaum, J.D.; Russo, S.J.; Yehuda, R. New translational perspectives for blood-based biomarkers of PTSD: From glucocorticoid to immune mediators of stress susceptibility. Exp. Neurol. 2016, 284, 133–140. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Sun, B.; Cole, S.; Rempel, H.; Lenoci, M.; Pulliam, L.; Neylan, T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis. Markers 2011, 30, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhaes, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Neylan, T.C.; Sun, B.; Rempel, H.; Ross, J.; Lenoci, M.; O’Donovan, A.; Pulliam, L. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav. Immun. 2011, 25, 524–531. [Google Scholar] [CrossRef]
- Smith, A.K.; Conneely, K.N.; Kilaru, V.; Mercer, K.B.; Weiss, T.E.; Bradley, B.; Tang, Y.; Gillespie, C.F.; Cubells, J.F.; Ressler, K.J. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2011, 156, 700–708. [Google Scholar] [CrossRef]
- Uddin, M.; Aiello, A.E.; Wildman, D.E.; Koenen, K.C.; Pawelec, G.; de Los Santos, R.; Goldmann, E.; Galea, S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA 2010, 107, 9470–9475. [Google Scholar] [CrossRef]
- van Zuiden, M.; Heijnen, C.J.; Maas, M.; Amarouchi, K.; Vermetten, E.; Geuze, E.; Kavelaars, A. Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: A prospective study. Psychoneuroendocrinology 2012, 37, 1822–1836. [Google Scholar] [CrossRef]
- Walker, F.R.; Nilsson, M.; Jones, K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets 2013, 14, 1262–1276. [Google Scholar] [CrossRef]
- Hinwood, M.; Tynan, R.J.; Charnley, J.L.; Beynon, S.B.; Day, T.A.; Walker, F.R. Chronic stress induced remodeling of the prefrontal cortex: Structural re-organization of microglia and the inhibitory effect of minocycline. Cereb. Cortex 2013, 23, 1784–1797. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Bonneau, R.H. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J. Neuroimmunol. 2006, 171, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Diz-Chaves, Y.; Astiz, M.; Bellini, M.J.; Garcia-Segura, L.M. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav. Immun. 2013, 28, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Diz-Chaves, Y.; Pernia, O.; Carrero, P.; Garcia-Segura, L.M. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J. Neuroinflamm. 2012, 9, 71. [Google Scholar] [CrossRef]
- Sugama, S.; Fujita, M.; Hashimoto, M.; Conti, B. Stress induced morphological microglial activation in the rodent brain: Involvement of interleukin-18. Neuroscience 2007, 146, 1388–1399. [Google Scholar] [CrossRef]
- Sugama, S.; Takenouchi, T.; Fujita, M.; Kitani, H.; Hashimoto, M. Cold stress induced morphological microglial activation and increased IL-1beta expression in astroglial cells in rat brain. J. Neuroimmunol. 2011, 233, 29–36. [Google Scholar] [CrossRef]
- Tynan, R.J.; Naicker, S.; Hinwood, M.; Nalivaiko, E.; Buller, K.M.; Pow, D.V.; Day, T.A.; Walker, F.R. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010, 24, 1058–1068. [Google Scholar] [CrossRef]
- Frank, M.G.; Baratta, M.V.; Sprunger, D.B.; Watkins, L.R.; Maier, S.F. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 2007, 21, 47–59. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain Behav. Immun. 2018, 69, 62–73. [Google Scholar] [CrossRef]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2012, 26, 337–345. [Google Scholar] [CrossRef]
- Frank, M.G.; Watkins, L.R.; Maier, S.F. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav. Immun. 2013, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Fenn, A.M.; Pacenta, A.M.; Powell, N.D.; Sheridan, J.F.; Godbout, J.P. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 2012, 37, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Hanke, M.L.; Corona, A.W.; Powell, N.D.; Stiner, L.M.; Bailey, M.T.; Nelson, R.J.; Godbout, J.P.; Sheridan, J.F. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 6277–6288. [Google Scholar] [CrossRef]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, Z.; Lei, Y.; Liu, Y.; Lu, C.; Rong, H.; Sun, Y.; Zhang, W.; Ma, Z.; Gu, X. Hippocampal activation of microglia may underlie the shared neurobiology of comorbid posttraumatic stress disorder and chronic pain. Mol. Pain 2016, 12, 1744806916679166. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Pan, Z.; Hou, Z.; Huang, C.; Li, W.; Zhao, B. Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Res. Bull. 2012, 88, 471–476. [Google Scholar] [CrossRef]
- Lee, S.; Varvel, N.H.; Konerth, M.E.; Xu, G.; Cardona, A.E.; Ransohoff, R.M.; Lamb, B.T. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. J. Pathol. 2010, 177, 2549–2562. [Google Scholar] [CrossRef]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Andoh, M.; Koyama, R. Microglia regulate synaptic development and plasticity. Dev. Neurobiol. 2021, 81, 568–590. [Google Scholar] [CrossRef] [PubMed]
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex 2012, 22, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.L.; Wick, D.; Herman, J.P. Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol. Behav. 2013, 122, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Fenchel, D.; Kaplan, Z.; Zohar, J.; Cohen, H. Early post-stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 124–132. [Google Scholar] [CrossRef]
- Wilson, C.B.; McLaughlin, L.D.; Nair, A.; Ebenezer, P.J.; Dange, R.; Francis, J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE 2013, 8, e76146. [Google Scholar] [CrossRef]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Bittner, T.; Jung, C.K.; Burgold, S.; Page, R.M.; Mitteregger, G.; Haass, C.; LaFerla, F.M.; Kretzschmar, H.; Herms, J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2010, 13, 411–413. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.M.; Luo, Q.Q.; Huang, S.; Yang, Q.W.; Wang, F.X.; Ke, Y.; Qian, Z.M. CX3CL1/CX3CR1-mediated microglia activation plays a detrimental role in ischemic mice brain via p38MAPK/PKC pathway. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Gan, Y.; Liu, Q.; Yin, J.X.; Liu, Q.; Shi, J.; Shi, F.D. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J. Neuroinflamm. 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.M.; Bachstetter, A.D.; Hudson, C.E.; Gemma, C.; Bickford, P.C. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J. Neuroinflamm. 2011, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Harry, G.J. Microglia during development and aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Morganti, J.M.; Bachstetter, A.D.; Hudson, C.E.; Peters, M.M.; Grimmig, B.A.; Weeber, E.J.; Bickford, P.C.; Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 16241–16250. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef]
- Udeochu, J.C.; Shea, J.M.; Villeda, S.A. Microglia communication: Parallels between aging and Alzheimer’s disease. Clin. Exp. Neuroimmunol. 2016, 7, 114–125. [Google Scholar] [CrossRef]
- McFarlane, A.C.; Barton, C.A.; Yehuda, R.; Wittert, G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology 2011, 36, 720–727. [Google Scholar] [CrossRef]
- Yehuda, R.; McFarlane, A.C.; Shalev, A.Y. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatry 1998, 44, 1305–1313. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J. An animal model of posttraumatic stress disorder: The use of cut-off behavioral criteria. Ann. N. Y. Acad. Sci. 2004, 1032, 167–178. [Google Scholar] [CrossRef]
- Young, E.A.; Abelson, J.; Lightman, S.L. Cortisol pulsatility and its role in stress regulation and health. Front. Neuroendocr. 2004, 25, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, A.; Ram, O.; Ifergane, G.; Matar, M.A.; Kaplan, Z.; Hoffman, J.R.; Sadot, O.; Cohen, H. Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J. Neurotrauma 2019, 36, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 2388–2404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pitman, R.K.; Milad, M.R.; Igoe, S.A.; Vangel, M.G.; Orr, S.P.; Tsareva, A.; Gamache, K.; Nader, K. Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behav. Neurosci. 2011, 125, 632–638. [Google Scholar] [CrossRef]
- Sapolsky, R.M. A mechanism for glucocorticoid toxicity in the hippocampus: Increased neuronal vulnerability to metabolic insults. J. Neurosci. Off. J. Soc. Neurosci. 1985, 5, 1228–1232. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Johnson, J.D.; O’Connor, K.A.; Deak, T.; Stark, M.; Watkins, L.R.; Maier, S.F. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav. Immun. 2002, 16, 461–476. [Google Scholar] [CrossRef]
- Frank, M.G.; Wieseler-Frank, J.L.; Watkins, L.R.; Maier, S.F. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. J. Neurosci. Methods 2006, 151, 121–130. [Google Scholar] [CrossRef]

| Control N = 8 I | EBR N = 10 II | PBR N = 11 III | MRB N = 10 IV | Statistics | |
|---|---|---|---|---|---|
| CA1: | |||||
| Number of branches | 11.25 ± 0.9 | 6.5 ± 0.85 | 7.5 ± 1.3 | 8.6 ± 0.8 | I ≠ II |
| Branches Length | 169.3 ± 12.6 | 97.8 ± 13.7 | 115.2 ± 14.3 | 130.3 ± 10.4 | I ≠ II, III |
| Soma Area | 30.1 ± 1.8 | 39.4 ± 2.9 | 28.6 ± 2.5 | 29.6 ± 2.4 | II ≠ III, IV |
| CA1 reconstructions of the branches tree |  |  | 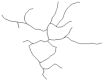 | 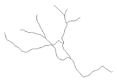 | |
| CA3: | |||||
| Number of branches | 15.4 ± 1.7 | 5.4 ± 0.7 | 11.2 ± 0.8 | 23.7 ± 1.4 | II ≠ I, III, IV; IV ≠ I, III |
| Branches Length | 210.9 ± 19.7 | 63.9 ± 9.2 | 166.9 ± 11.9 | 286.8 ± 13.7 | II ≠ I, III, IV; IV ≠ I, III |
| Soma Area | 31.3 ± 1.4 | 35.3 ± 3.8 | 32.4 ± 1.7 | 28.4 ± 1.9 | NS |
| Reconstructions of the branches tree | 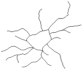 |  | 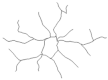 |  | |
| DG: | |||||
| Number of branches | 24.9 ± 1.9 | 10.0 ± 2.8 | 17.1 ± 1.3 | 14.8 ± 2.1 | I ≠ II, IV |
| Branches Length | 256.1 ± 17.0 | 130.7 ± 28.6 | 213.5 ± 15.8 | 188.6 ± 31.6 | I ≠ II |
| Soma Area | 25.1 ± 1.3 | 36.8 ± 3.0 | 31.4 ± 4.1 | 28.3 ± 2.4 | NS |
| Reconstructions of the branches tree | 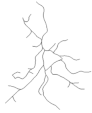 | 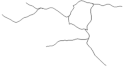 | 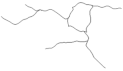 | 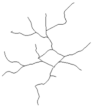 |
| Sham-PSS N = 10 I | PSS + Saline N = 16 II | PSS + Hydrocortisone N = 16 III | PSS + RU486 N = 14 IV | Statistics | |
|---|---|---|---|---|---|
| CA1: Number of branches 1 Branches Length 2 Soma Area 3 | 12.9 ± 1.0 185.85 ± 13.1 28.9 ± 1.7 | 7.8 ± 1.0 111.6 ± 11.9 35.3 ± 2.0 | 14.2 ± 0.7 190.4 ± 9.2 30.4 ± 1.4 | 8.4 ± 0.7 104.7 ± 5.2 36.1 ± 2.3 | I, III ≠ II, IV I, III ≠ II, Iv I ≠ IV |
| CA3: Number of branches 4 Branches Length 5 Soma Area 6 | 17.1 ± 2.0 204.9 ± 19.5 30.5 ± 1.4 | 9.0 ± 1.0 124.6 ± 14.9 37.4 ± 2.0 | 17.0 ± 1.1 248.6 ± 10.9 30.7 ± 1.4 | 10 ± 1.0 125.5 ± 10.6 40.8 ± 2.1 | I, III ≠ II, Iv I, III ≠ II, IV III≠II, IV; ≠IV |
| DG: Number of branches 7 Branches Length 8 Soma Area 9 | 22.1 ± 1.7 254.5 ± 16.1 27.8 ± 2.8 | 12.3 ± 1.6 153.9 ± 18.0 33.5 ± 2.9 | 18.6 ± 1.7 232.4 ± 18.1 26.1 ± 1.7 | 13.4 ± 1.3 140.7 ± 7.6 35.5 ± 2.9 | I ≠ II, IV; II ≠ III I, III ≠ II, IV III ≠ IV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahum, K.; Todder, D.; Zohar, J.; Cohen, H. The Role of Microglia in the (Mal)adaptive Response to Traumatic Experience in an Animal Model of PTSD. Int. J. Mol. Sci. 2022, 23, 7185. https://doi.org/10.3390/ijms23137185
Nahum K, Todder D, Zohar J, Cohen H. The Role of Microglia in the (Mal)adaptive Response to Traumatic Experience in an Animal Model of PTSD. International Journal of Molecular Sciences. 2022; 23(13):7185. https://doi.org/10.3390/ijms23137185
Chicago/Turabian StyleNahum, Kesem, Doron Todder, Joseph Zohar, and Hagit Cohen. 2022. "The Role of Microglia in the (Mal)adaptive Response to Traumatic Experience in an Animal Model of PTSD" International Journal of Molecular Sciences 23, no. 13: 7185. https://doi.org/10.3390/ijms23137185
APA StyleNahum, K., Todder, D., Zohar, J., & Cohen, H. (2022). The Role of Microglia in the (Mal)adaptive Response to Traumatic Experience in an Animal Model of PTSD. International Journal of Molecular Sciences, 23(13), 7185. https://doi.org/10.3390/ijms23137185





