Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury
Abstract
:1. Introduction
2. Results
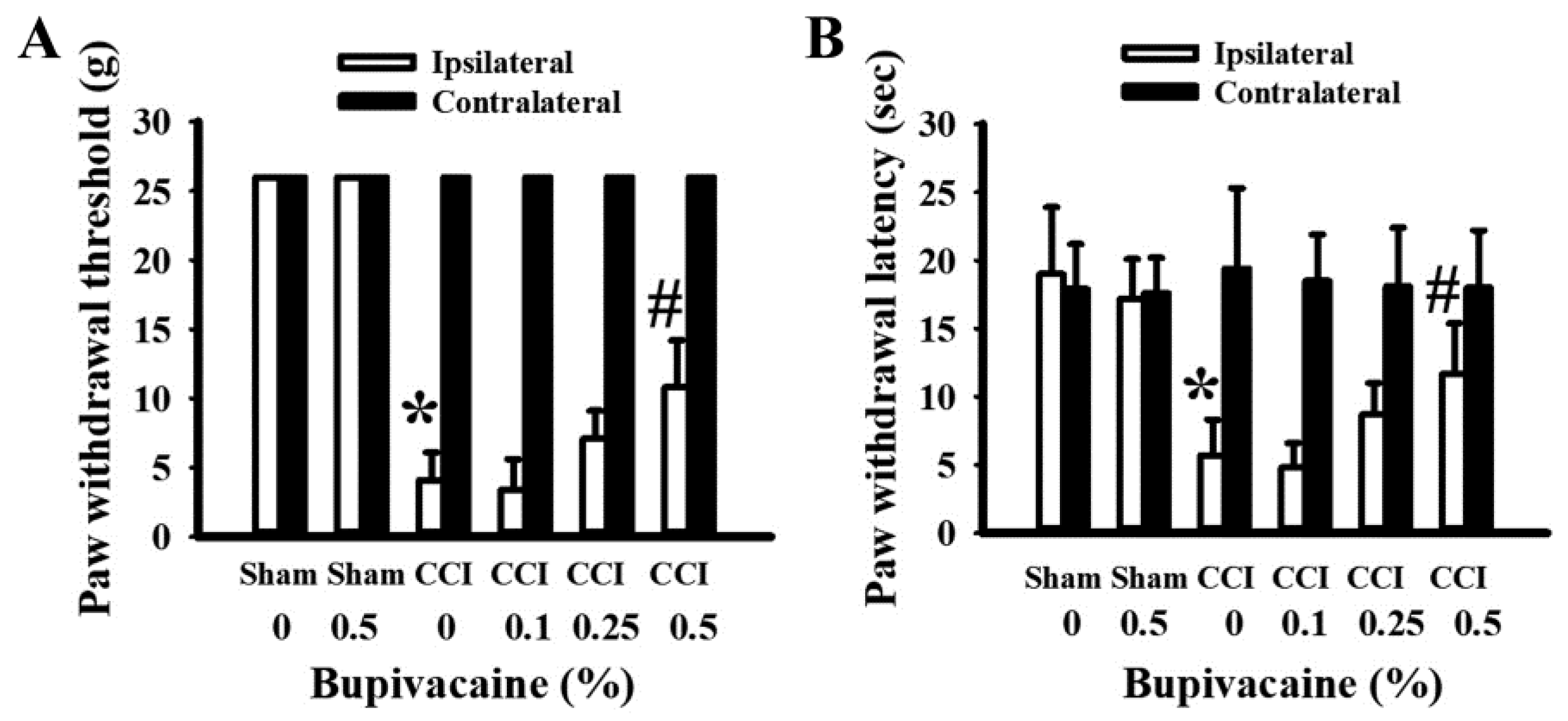
2.1. Bupivacaine Alleviated Pain Behaviors
2.2. Bupivacaine Alleviated Microglia Activation
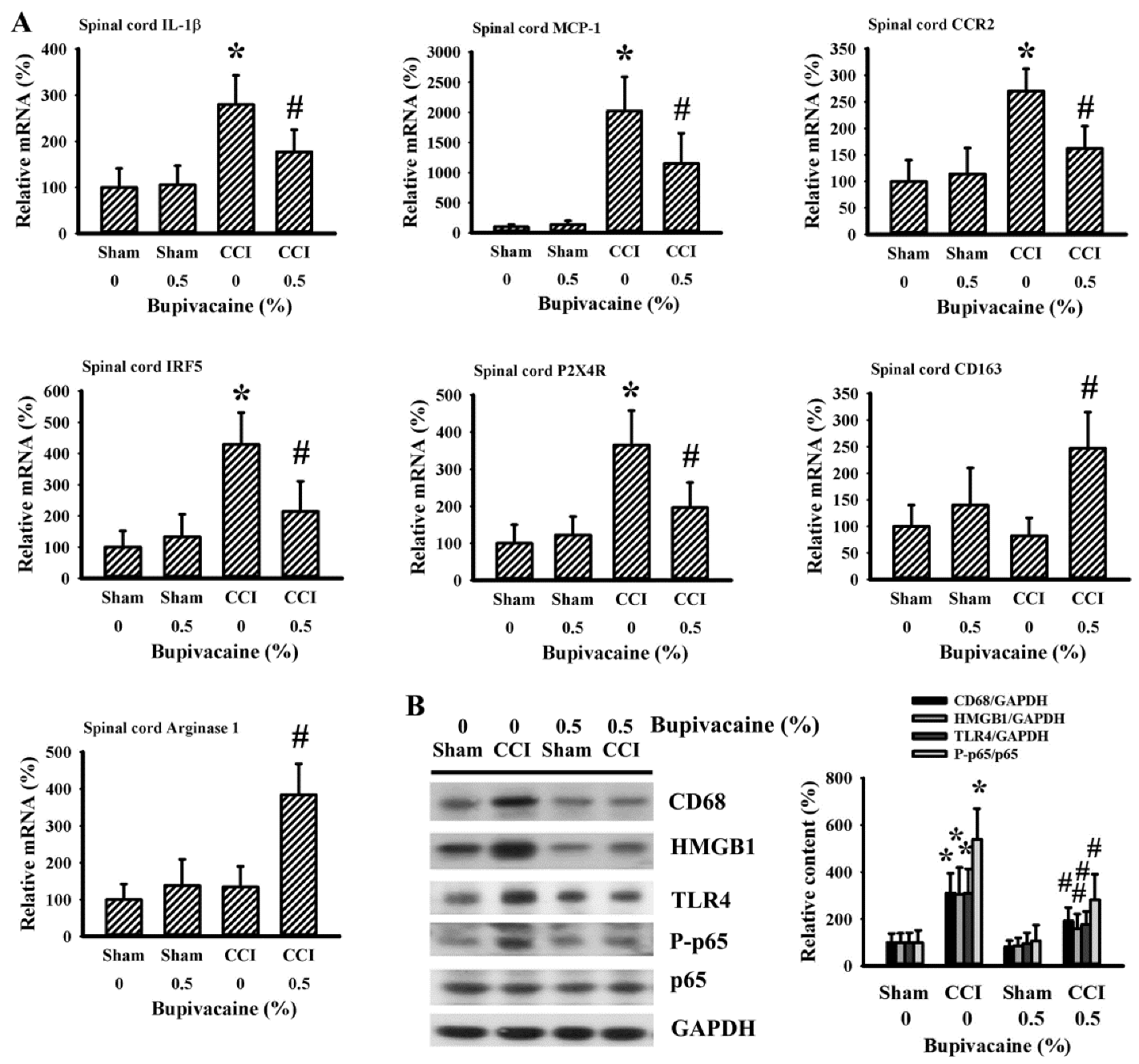
2.3. Bupivacaine Alleviated Inflammation in the Spinal Cords
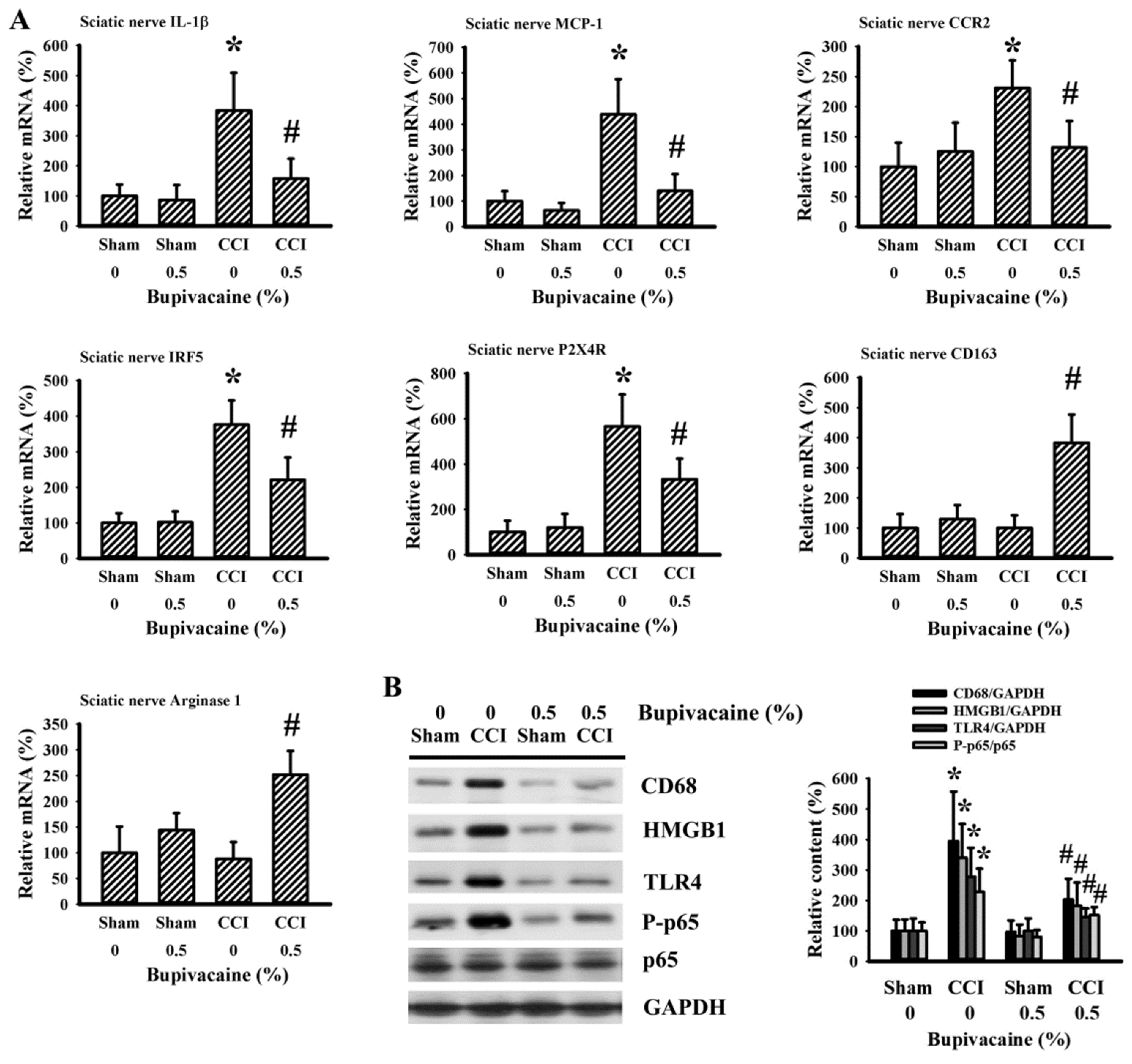
2.4. Bupivacaine Alleviated Inflammation in the Sciatic Nerve
3. Discussion
4. Materials and Methods
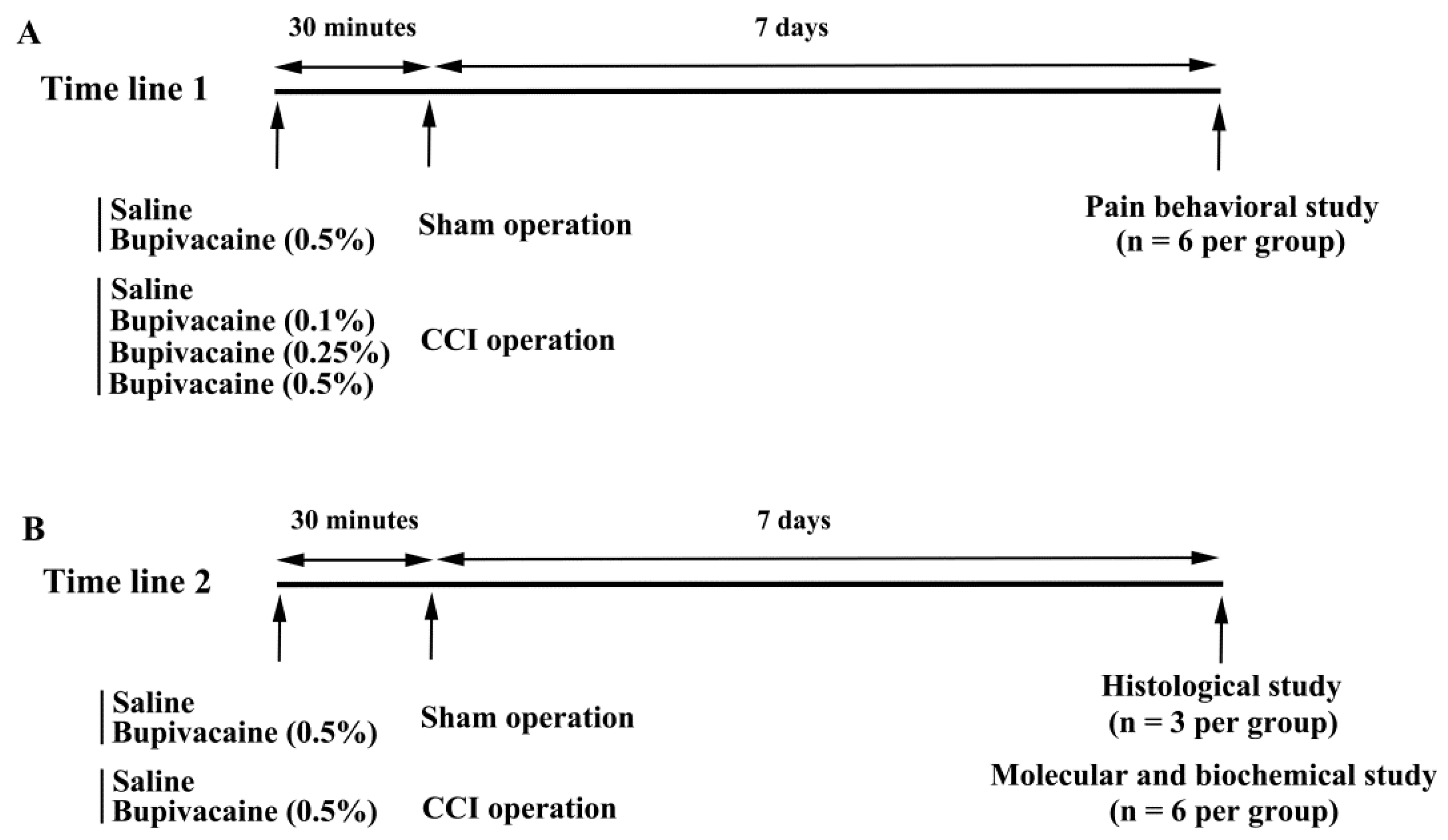
4.1. Animal Allocation and Treatments
4.2. Pain Behavioral Measurement
4.3. Immunohistochemical Examination
4.4. RNA Isolation and Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.5. Western Blot
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Descalzi, G.; Mitsi, V.; Purushothaman, I.; Gaspari, S.; Avrampou, K.; Loh, Y.E.; Shen, L.; Zachariou, V. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci. Signal. 2017, 10, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Li, X.; Zheng, Y.; Shi, H.; Zhang, Z.; Jing, B.; Chen, Z.; Qian, G.; Zhao, G. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF-κB signaling pathway. Phytother. Res. 2022, 36, 1678–1691. [Google Scholar] [CrossRef]
- Gui, X.; Wang, H.; Wu, L.; Tian, S.; Wang, X.; Zheng, H.; Wu, W. Botulinum toxin type A promotes microglial M2 polarization and suppresses chronic constriction injury-induced neuropathic pain through the P2X7 receptor. Cell. Biosci. 2020, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. Blockade of P2X4 receptors inhibits neuropathic pain-related behavior by preventing MMP-9 activation and, consequently, pronociceptive interleukin release in a rat model. Front. Pharmacol. 2017, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Guo, Q.; Ye, Z.; Wang, E.; Zou, W.; Sun, Z.; He, Z.; Zhong, T.; Weng, Y.; Pan, Y. PPAR γ prevents neuropathic pain by down-regulating CX3CR1 and attenuating M1 activation of microglia in the spinal cord of rats using a sciatic chronic constriction injury model. Front. Neurosci. 2021, 15, 620525. [Google Scholar] [CrossRef]
- Masuda, T.; Iwamoto, S.; Yoshinaga, R.; Tozaki-Saitoh, H.; Nishiyama, A.; Mak, T.W.; Tamura, T.; Tsuda, M.; Inoue, K. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat. Commun. 2014, 5, 3771. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Slusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Direct and indirect pharmacological modulation of CCL2/CCR2 pathway results in attenuation of neuropathic pain—In Vivo and In Vitro evidence. J. Neuroimmunol. 2016, 297, 9–19. [Google Scholar] [CrossRef]
- Cheng, F.; Qin, W.; Yang, A.X.; Yan, F.F.; Chen, Y.; Ma, J.X. Propofol alleviates neuropathic pain in chronic constriction injury rat models via the microRNA-140-3p/Jagged-1 peptide/Notch signaling pathway. Synapse 2021, 75, e22219. [Google Scholar] [CrossRef]
- Shin, J.W.; Pancaro, C.; Wang, C.F.; Gerner, P. Low-dose systemic bupivacaine prevents the development of allodynia after thoracotomy in rats. Anesth. Analg. 2008, 107, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.R.; Berta, T.; Gao, Y.J.; Decosterd, I.; Ji, R.R. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: Different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain 2009, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Strong, J.A.; Zhang, J.M. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 2009, 160, 847–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Deng, X. Bupivacaine effectively relieves inflammation-induced pain by suppressing activation of the NF-κB signalling pathway and inhibiting the activation of spinal microglia and astrocytes. Exp. Ther. Med. 2017, 13, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Deruddre, S.; Combettes, E.; Estebe, J.P.; Duranteau, J.; Benhamou, D.; Beloeil, H.; Mazoit, J.X. Effects of a bupivacaine nerve block on the axonal transport of Tumor Necrosis Factor-alpha (TNF-alpha) in a rat model of carrageenan-induced inflammation. Brain Behav. Immun. 2010, 24, 652–659. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, L.; Xie, A.; Liao, O.; Ju, F.; Zhou, Y. Low concentration of Bupivacaine ameliorates painful diabetic neuropathy by mediating miR-23a/PDE4B axis in microglia. Eur. J. Pharmacol. 2021, 891, 173719. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.Y.; Zhang, L.Q.; Li, D.Y.; Wu, J.Y.; Gao, S.J.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. Nrf2 activation attenuates chronic constriction injury-induced neuropathic pain via induction of PGC-1α-mediated mitochondrial biogenesis in the spinal cord. Oxidative Med. Cell. Longev. 2021, 2021, 9577874. [Google Scholar] [CrossRef]
- Liao, M.F.; Hsu, J.L.; Lu, K.T.; Chao, P.K.; Cheng, M.Y.; Hsu, H.C.; Lo, A.L.; Lee, Y.L.; Hung, Y.H.; Lyu, R.K.; et al. Granulocyte colony stimulating factor (GCSF) can attenuate neuropathic pain by suppressing monocyte chemoattractant protein-1 (MCP-1) expression, through upregulating the early microRNA-122 expression in the dorsal root ganglia. Cells 2020, 9, 1669. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, J.; Ma, S.; Zhou, J. Paeoniflorin attenuates chronic constriction injury-induced neuropathic pain by suppressing spinal NLRP3 inflammasome activation. Inflammopharmacology 2020, 28, 1495–1508. [Google Scholar] [CrossRef]
- Jin, X.H.; Wang, L.N.; Zuo, J.L.; Yang, J.P.; Liu, S.L. P2X4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J. Neurosci. Res. 2014, 92, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Y.; Xue, M.; Wang, Y.; Huang, Z.H.; Huang, C. Electroacupuncture alleviates spared nerve injury-induced neuropathic pain and modulates HMGB1/NF-κB signaling pathway in the spinal cord. J. Pain Res. 2019, 12, 2851–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çömez, M.S.; Borazan, Y.; Özgür, T.; İşler, C.T.; Cellat, M.; Güvenç, M.; Altuğ, M.E. Effects of dexamethasone on bupivacaine-induced peripheral nerve injection injury in the rat sciatic model. J. Investig. Surg. 2021, 34, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.K.; Chang, C.Y.; Ou, Y.C.; Chen, W.Y.; Kuan, Y.H.; Pan, H.C.; Liao, S.L.; Li, G.Z.; Chen, C.J. Tetramethylpyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. Exp. Neurol. 2013, 247, 188–201. [Google Scholar] [CrossRef]
- Wu, C.C.; Chang, C.Y.; Shih, K.C.; Hung, C.Y.; Wang, Y.Y.; Lin, S.Y.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Wang, W.Y.; et al. b-Funaltrexamine displayed anti-inflammatory and neuroprotective effects in cells and rat model of stroke. Int. J. Mol. Sci. 2020, 21, 3866. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Chang, C.-Y.; Tzeng, C.-Y.; Huang, J.-H.; Hung, C.-J.; Chen, W.-Y.; Liao, S.-L.; Kuan, Y.-H.; Chen, C.-J. Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury. Int. J. Mol. Sci. 2022, 23, 7197. https://doi.org/10.3390/ijms23137197
Wu C-C, Chang C-Y, Tzeng C-Y, Huang J-H, Hung C-J, Chen W-Y, Liao S-L, Kuan Y-H, Chen C-J. Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury. International Journal of Molecular Sciences. 2022; 23(13):7197. https://doi.org/10.3390/ijms23137197
Chicago/Turabian StyleWu, Chih-Cheng, Cheng-Yi Chang, Chung-Yuh Tzeng, Jen-Hsuan Huang, Chih-Jen Hung, Wen-Ying Chen, Su-Lan Liao, Yu-Hsiang Kuan, and Chun-Jung Chen. 2022. "Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury" International Journal of Molecular Sciences 23, no. 13: 7197. https://doi.org/10.3390/ijms23137197
APA StyleWu, C.-C., Chang, C.-Y., Tzeng, C.-Y., Huang, J.-H., Hung, C.-J., Chen, W.-Y., Liao, S.-L., Kuan, Y.-H., & Chen, C.-J. (2022). Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury. International Journal of Molecular Sciences, 23(13), 7197. https://doi.org/10.3390/ijms23137197






