Isocyanonaphthol Derivatives: Excited-State Proton Transfer and Solvatochromic Properties
Abstract
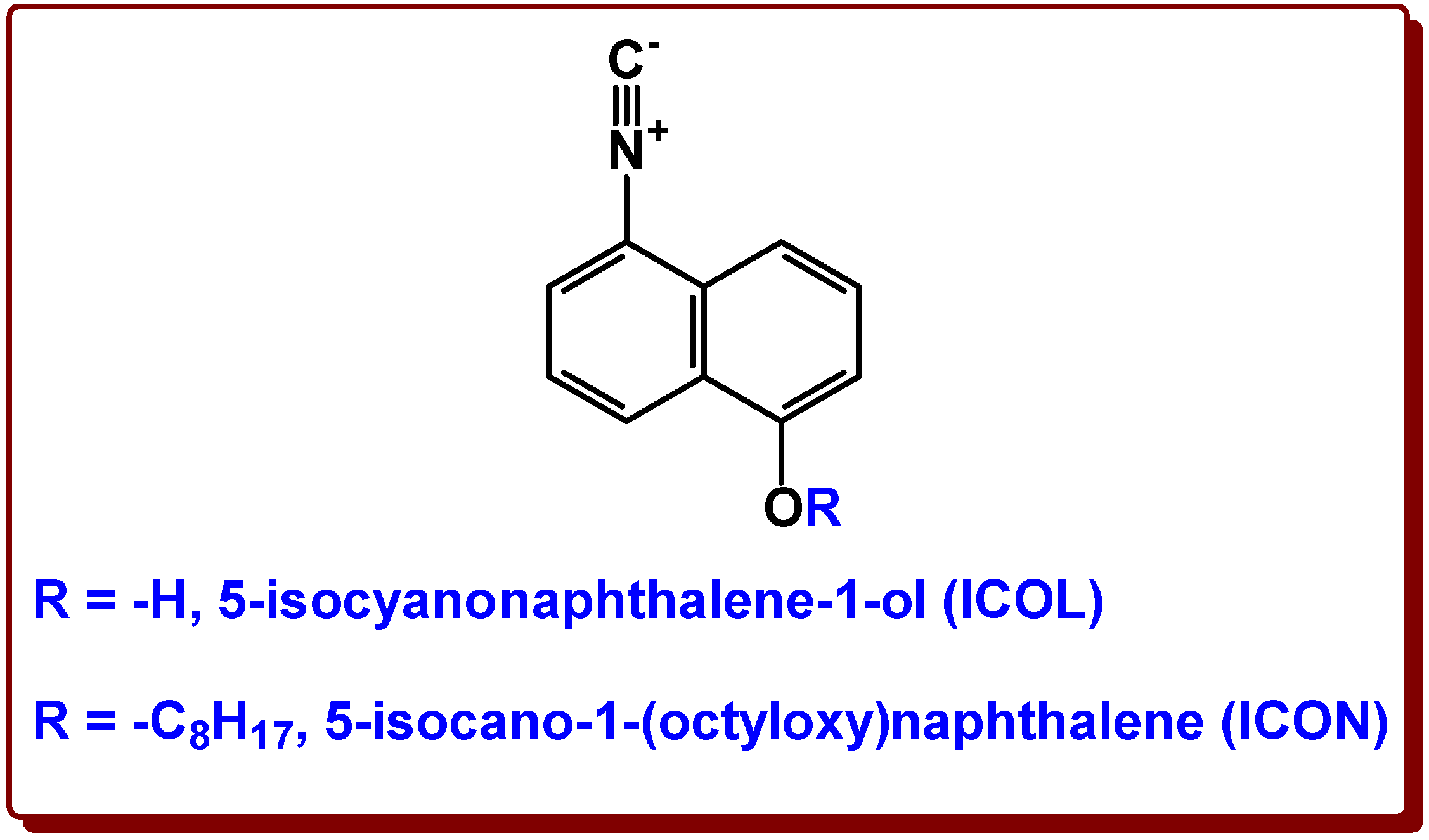
:1. Introduction
2. Result and Discussion
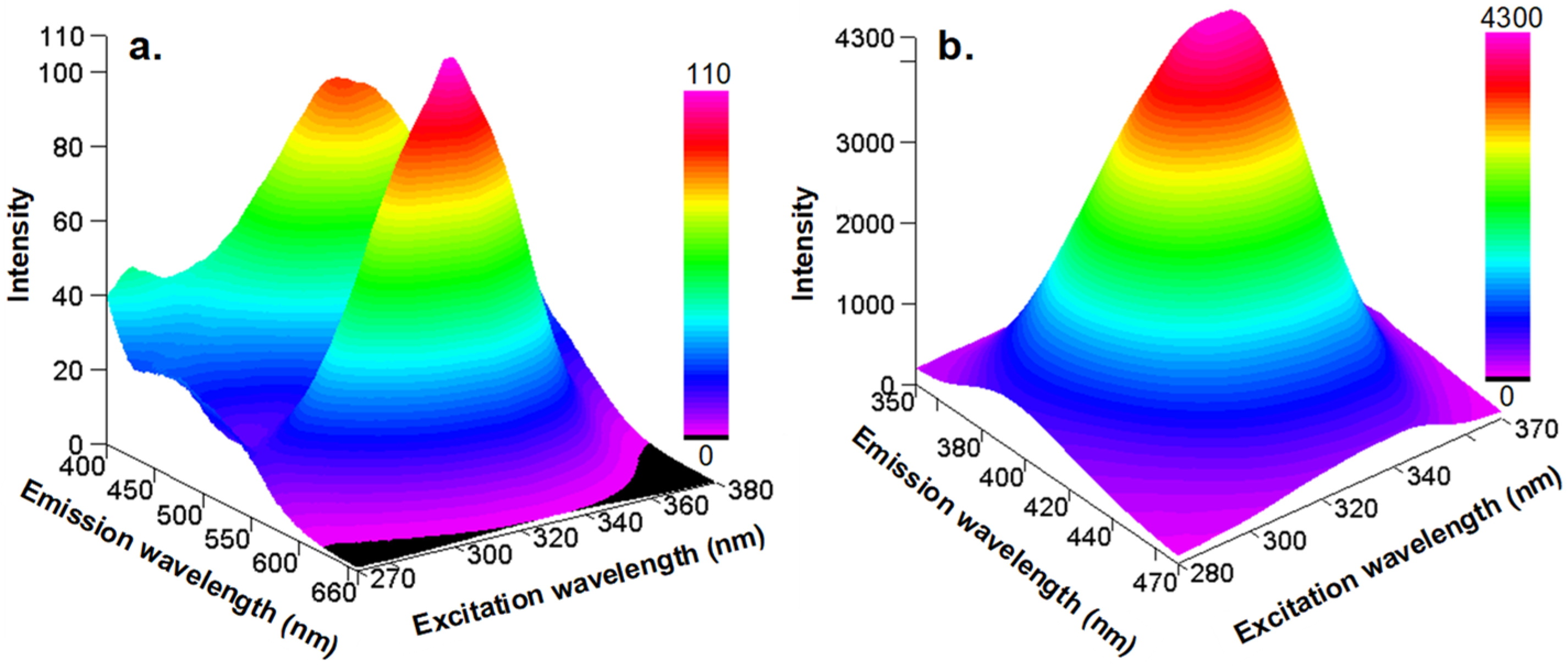
2.1. UV–Vis and Fluorescence Properties of isocyanonaphthalene-1-ol (ICOL) and 5-isocyano-1-(octyloxy)naphthalene (ICON)
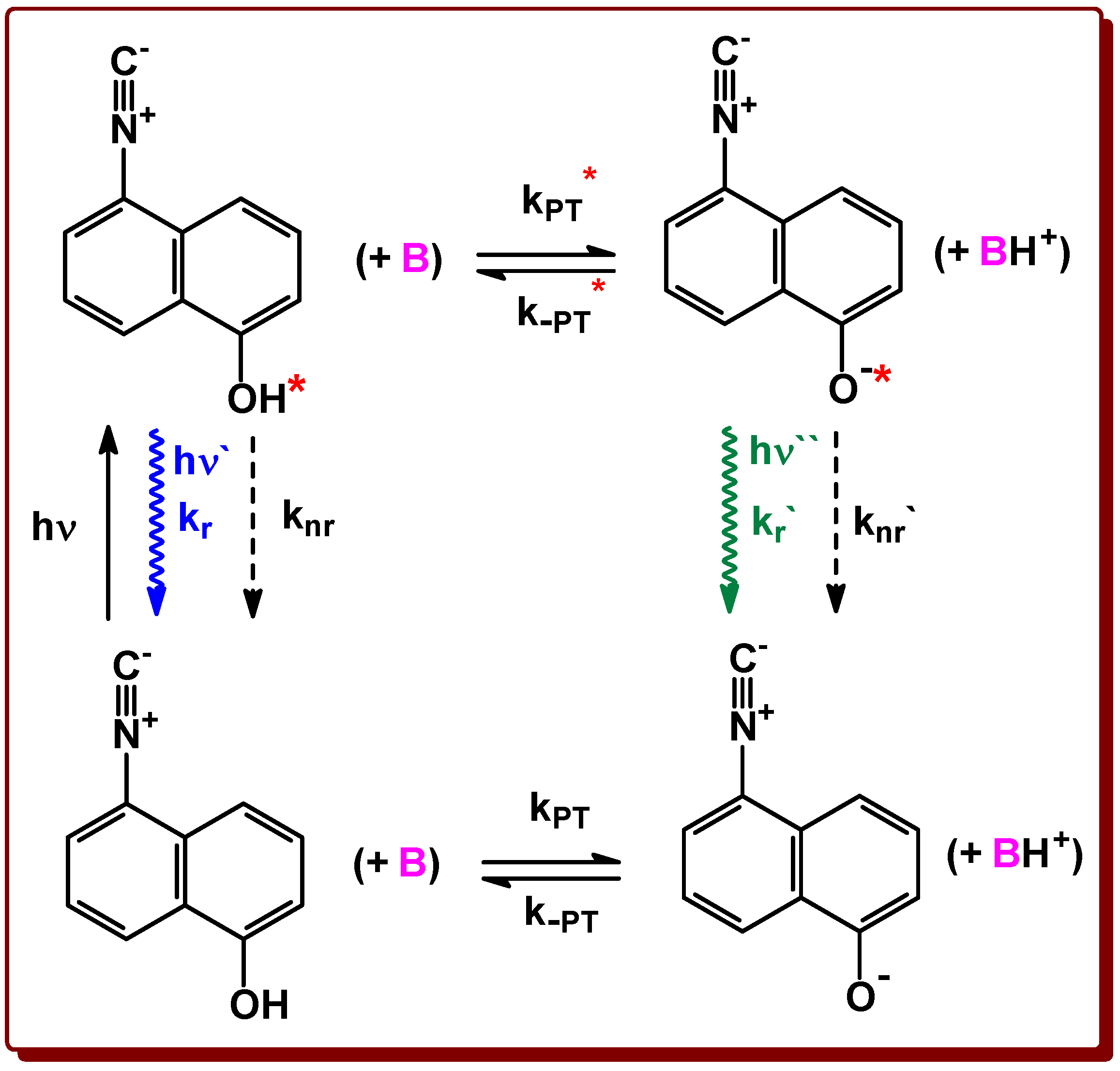
2.2. Acidity and Photoacidity of ICOL Derivative
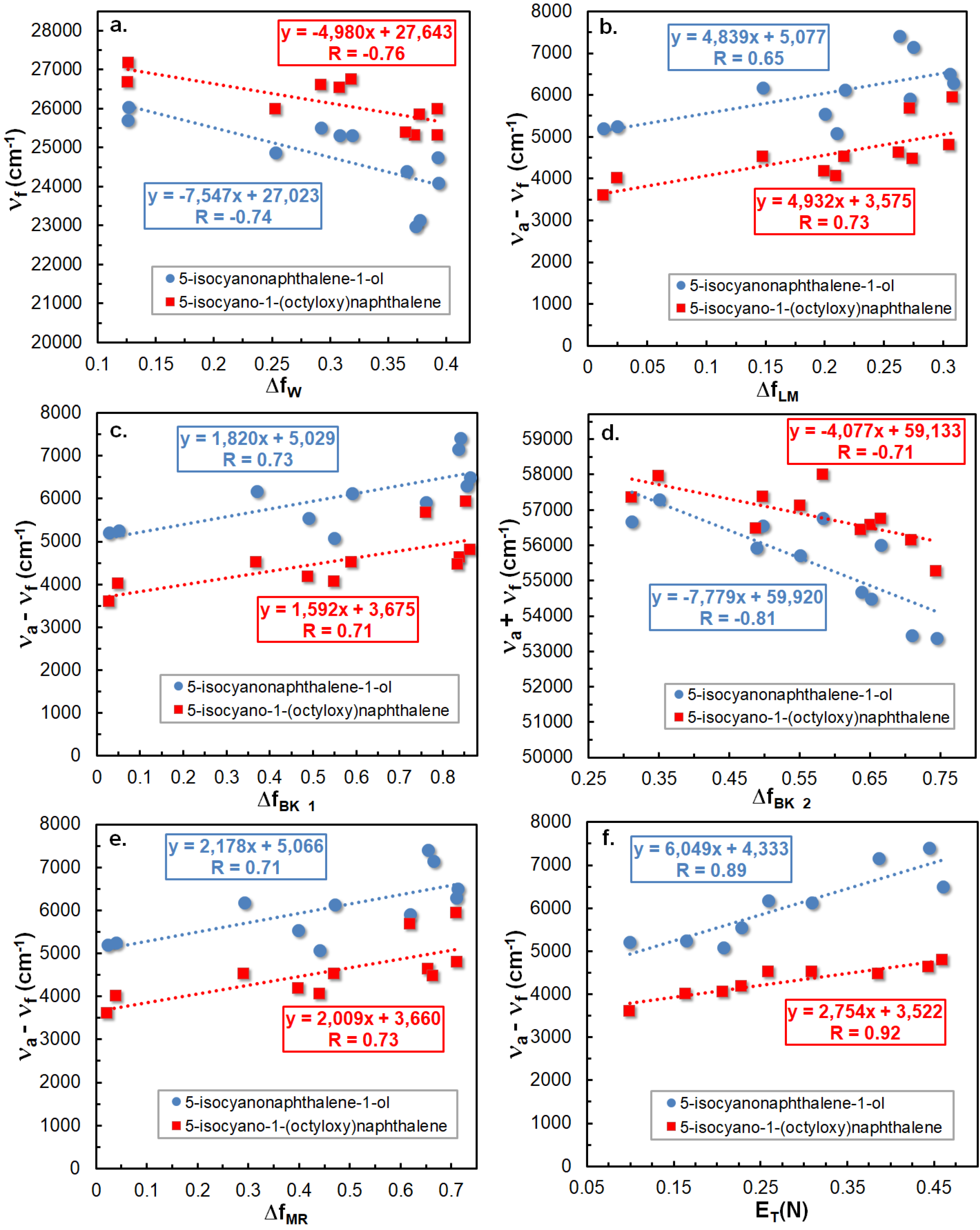
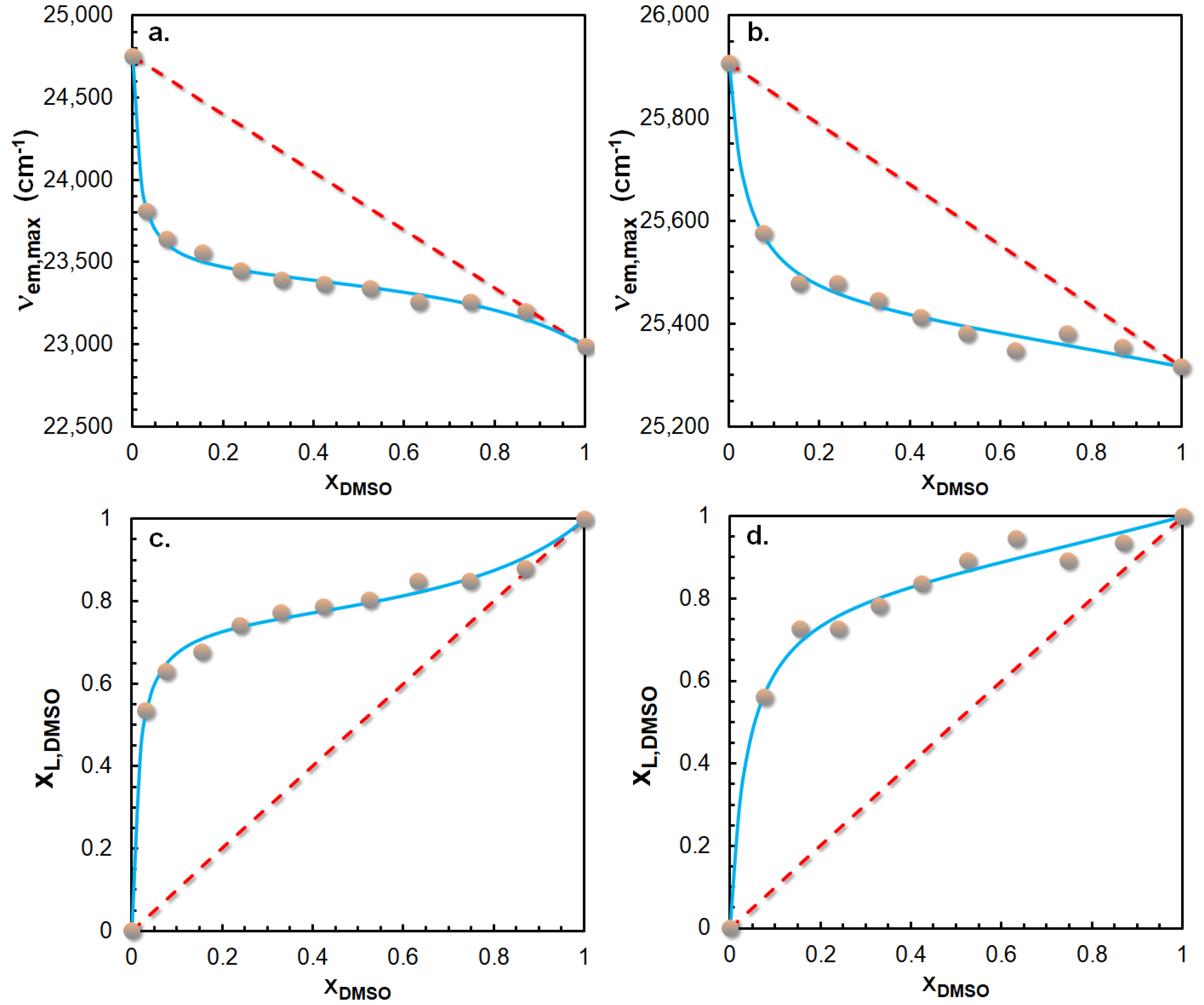
2.3. Preferential Solvation of ICOL and ICON Derivatives
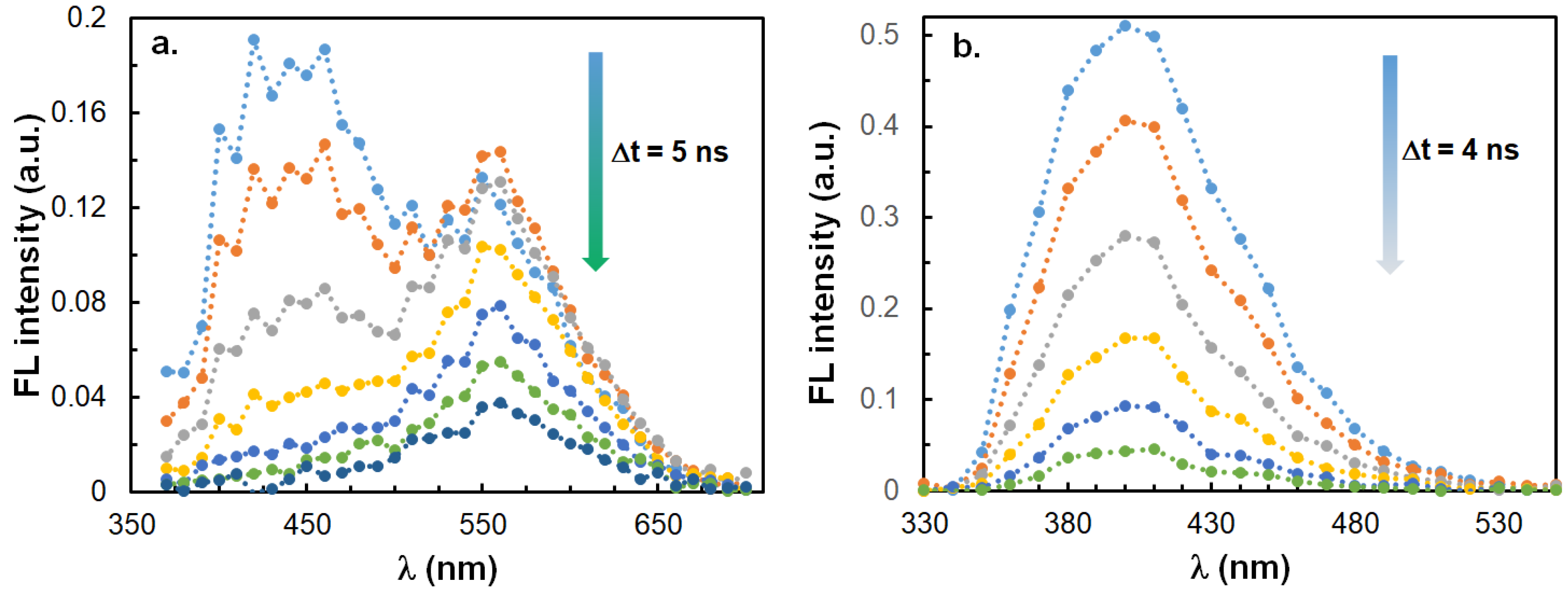
2.4. Time-Resolved Emission Properties of ICOL and ICON Derivatives
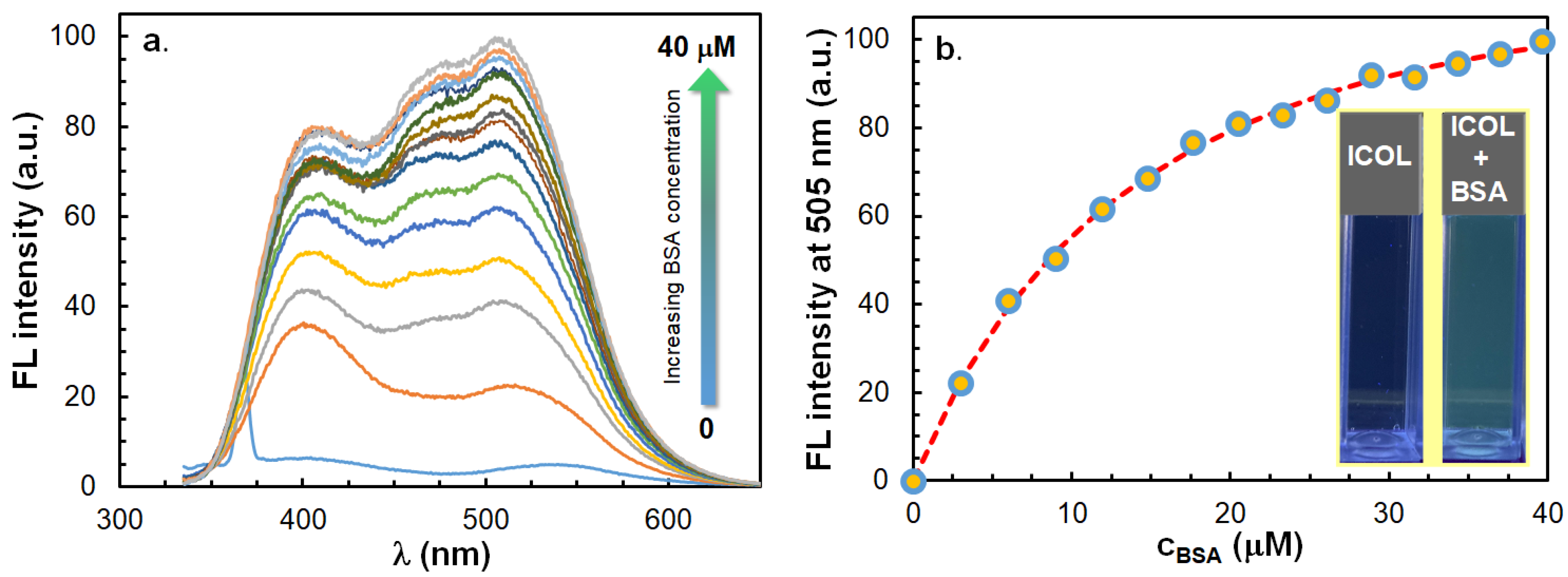
2.5. Fluorescence Properties of ICOL and ICON in the Presence of Surfactants and Bovine Serum Albumin (BSA)
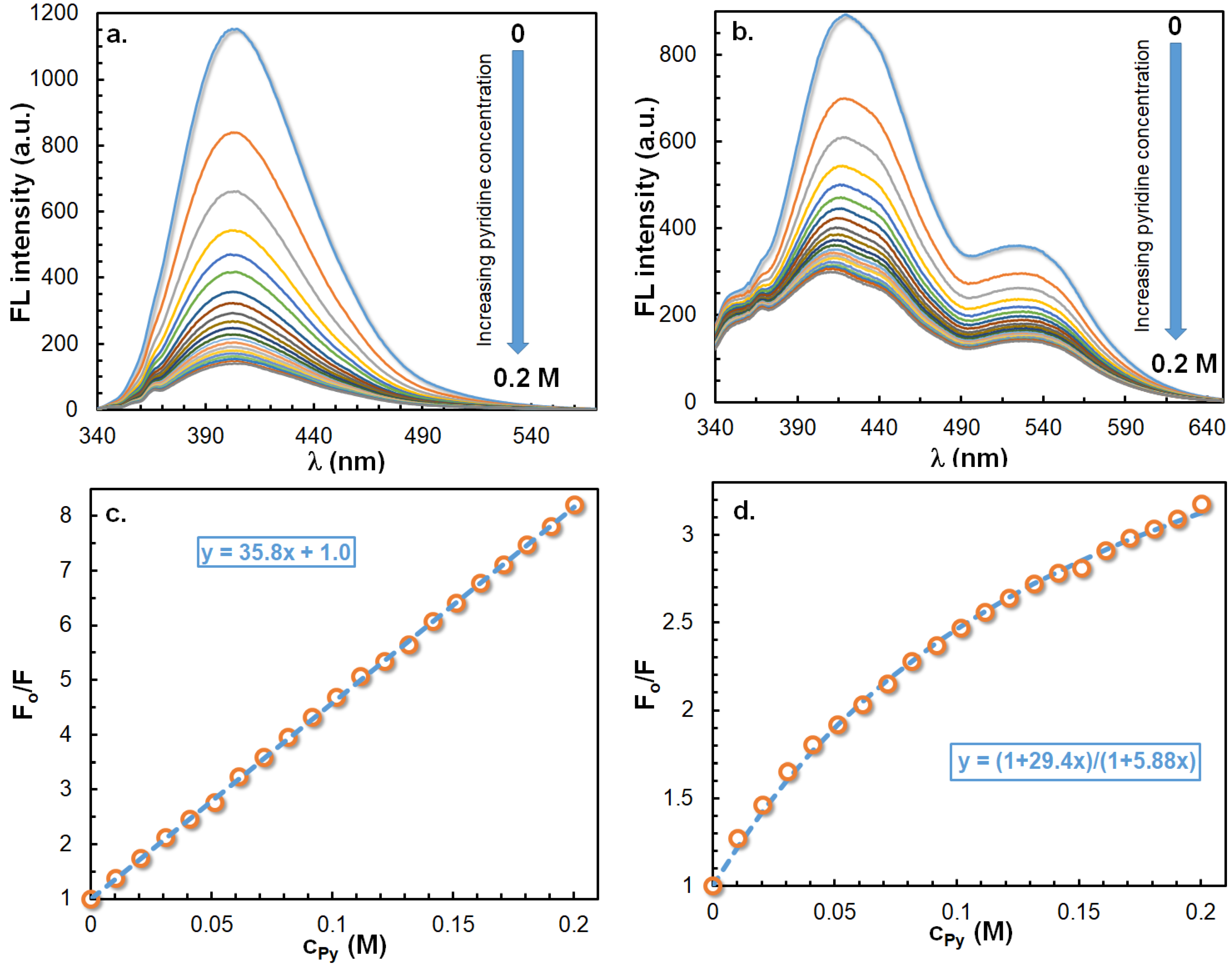
2.6. Fluorescence Quenching by Pyridine
3. Experimental
3.1. Chemicals
3.2. Synthesis of 5-isocyanonaphthalene-1-ol (ICOL) and 5-isocyano-1-(octyloxy)naphthalene (ICON)
3.3. Methods and Instrumentation
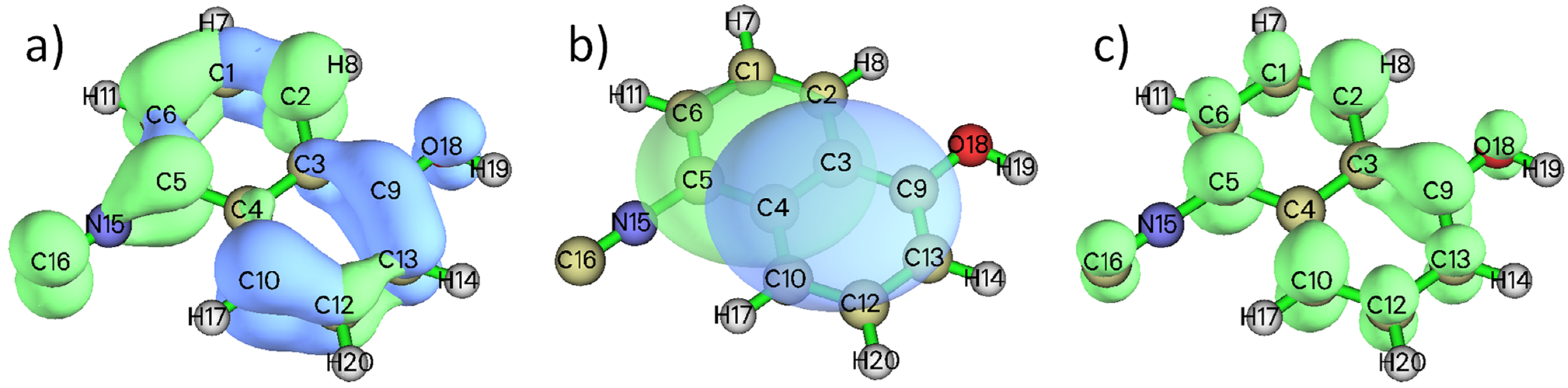
3.4. Time-Dependent Density-Functional-Theory (TD-DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Y.J.; Wei, W.L.; Qu, X.G. Colorimetric Biosensing Using Smart Materials. Adv. Mater. 2011, 23, 4215–4236. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlucker, S. SERS Microscopy: Nanoparticle Probes and Biomedical Applications. Chemphyschem 2009, 10, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as polarity sensitive fluorescence membrane probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Mohapatra, M.; Mishra, A.K. Excited state proton transfer based fluorescent molecular probes and their application in studying lipid bilayer membranes. Photochem. Photobiol. Sci. 2019, 18, 2830–2848. [Google Scholar] [CrossRef]
- Hofstetter, S.; Denter, C.; Winter, R.; McMullen, L.M.; Ganzle, M.G. Use of the fluorescent probe LAURDAN to label and measure inner membrane fluidity of endospores of Clostridium spp. J. Microbiol. Meth. 2012, 91, 93–100. [Google Scholar] [CrossRef]
- Perochon, E.; Tocanne, J.F. Synthesis and phase properties of phosphatidylcholine labeled with 8-(2-anthroyl) octanoic acid, a solvatochromic fluorescent probe. Chem. Phys. Lipids 1991, 58, 7–17. [Google Scholar] [CrossRef]
- Diwu, Z.; Lu, Y.; Zhang, C.; Kalubert, D.H.; Haugland, R.P. Fluorescent molecular probes, II: The synthesis, spectral properties and use of fluorescent solvatochromic dapoxyl TM dyes. Photochem. Photobiol. 1957, 66, 424–431. [Google Scholar] [CrossRef]
- Suppan, P. Solvatochromic shifts: The influence of the medium on the energy of electronic states. J. Photochem. Photobiol. A Chem. 1990, 50, 293–330. [Google Scholar] [CrossRef]
- Fukuda, R.; Chidthong, R.; Cammi, R.; Ehara, M. Optical absorption and fluorescence of PRODAN in solution: Quantum chemical study based on the symmetry-adapted cluster–configuration interaction method. Chem. Phys. Lett. 2012, 552, 53–57. [Google Scholar] [CrossRef]
- Amdursky, N.; Rashid, M.H.; Stevens, M.M.; Yarovsky, I. Exploring the binding sites and proton diffusion on insulin amyloid fibril surfaces by naphthol-based photoacid fluorescence and molecular simulations. Sci. Rep. 2017, 7, 6245. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N. Excited State Proton Transfer of Carbazole. A Convenient Way to Study Microheterogeneous Environments. Int. J. Mol. Sci. 2003, 4, 460–480. [Google Scholar] [CrossRef]
- Förster, T. Elektrolytische Dissoziation angeregter Moleküle. Z. Elektrochem. Angew. Phys. Chemie. 1950, 54, 42–46. [Google Scholar]
- Kagel, H.; Frohme, M.; Glökler, J. Photoacids in biochemical applications. J. Cell. Biotech. 2018, 4, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Amdursky, N.; Simkovitch, R.; Huppert, D. Excited-State Proton Transfer of Photoacids Adsorbed on Biomaterials. J. Phys. Chem. B 2014, 118, 13859–13869. [Google Scholar] [CrossRef]
- Spry, D.B.; Goun, A.; Fayer, M.D. Deprotonation Dynamics and Stokes Shift of Pyranine (HPTS). J. Phys. Chem. A 2007, 111, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Kohse, S.; Neubauer, A.; Pazidis, A.; Lochbrunner, S.; Kragl, U. Photoswitching of Enzyme Activity by Laser-Induced pH-Jump. J. Am. Chem. Soc. 2013, 135, 9407–9411. [Google Scholar] [CrossRef]
- Peretz-Soroka, H.; Pevzner, A.; Davidi, G.; Naddaka, V.; Kwiat, M.; Huppert, D.; Patolsky, F. Manipulating and Monitoring On-Surface Biological Reactions by Light-Triggered Local pH Alterations. Nano Lett. 2015, 15, 4758–4768. [Google Scholar] [CrossRef] [PubMed]
- Förster, T.H. Die pH-abhangigkeit der fluoreszenz von naphthalinderivaten. Z. Elektrochem. Angew. Phys. Chem. 1950, 54, 531–553. [Google Scholar]
- Förster, T. Formation and dissociation of excited dimers. Pure Appl. Chem. 1963, 7, 73–78. [Google Scholar] [CrossRef]
- Weller, A. Fast reactions of excited molecules. Prog. React. Kinet. 1961, 1, 187–214. [Google Scholar]
- Weller, A. Intramolecular proton transfer in excited states. Angew. Phys. Chem. 1956, 60, 1144–1147. [Google Scholar]
- Saway, J.; Salem, Z.M.; Badillo, J.J. Recent Advances in Photoacid Catalysis for Organic Synthesis. Synth. Stuttg. 2021, 53, 489–497. [Google Scholar]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on push–pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Rácz, D.; Nagy, M.; Mándi, A.; Zsuga, M.; Kéki, S. Solvatochromic properties of a new isocyanonaphthalene based fluorophore. J. Photochem. Photobiol. A Chem. 2013, 270, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Nagy, M.; Rácz, D.; Lázár, L.; Purgel, M.; Ditrói, T.; Zsuga, M.; Kéki, S. Solvatochromic Study of Highly Fluorescent Alkylated Isocyanonaphthalenes, Their -Stacking, Hydrogen-Bonding Complexation, and Quenching with Pyridine. ChemPhysChem 2014, 15, 3614–3625. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Fehér, P.P.; Kalmár, J.; Fábián, I.; Kiss, A.; Zsuga, M.; Kéki, S. Solvatochromic isocyanonaphthalene dyes as ligands for silver(I) complexes, their applicability in silver(I) detection and background reduction in biolabelling. Sens. Actuators B Chem. 2018, 255, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Fehér, P.P.; Kovács, S.L.; Bankó, C.; Bacsó, Z.; Kiss, A.; Zsuga, M.; Kéki, S. Amino-isocyanoacridines: Novel, Tunable Solvatochromic Fluorophores as Physiological pH Probes. Sci. Rep. 2019, 9, 8250. [Google Scholar] [CrossRef]
- Kovács, S.L.; Nagy, M.; Fehér, P.P.; Zsuga, M.; Kéki, S. Effect of the Substitution Position on the Electronic and Solvatochromic Properties of Isocyanoaminonaphthalene (ICAN) Fluorophores. Molecules 2019, 24, 2434. [Google Scholar] [CrossRef] [Green Version]
- Tolbert, L.M.; Haubrich, J.E. Enhanced Photoacidities of Cyanonaphthols. J. Am. Chem. Soc. 1990, 112, 8163–8165. [Google Scholar] [CrossRef]
- Tolbert, L.M.; Haubricht, J.E. Photoexcited Proton Transfer from Enhanced Photoacids. J. Am. Chem. Soc. 1994, 116, 10593–10600. [Google Scholar] [CrossRef]
- Harris, C.M.; Selinger, B.K. Acid-Base Properties of l-Naphthol. Proton-Induced Fluorescence Quenching. J. Phys. Chem. 1980, 84, 1300–1371. [Google Scholar] [CrossRef]
- Lippert, E. Spectroscopic determination of the dipole moment of aromatic compounds in the first excited singlet state. Z. Elektrochem. 1957, 61, 962–975. [Google Scholar]
- Mataga, N.; Kaifu, N.; Koizumi, M. Solvent effects upon fluorescence spectra and the dipole moments of excited molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Bilot, L.; Kawski, A. Zur Theorie des einflusses von losungsmitteln auf die elektronenspektren der molecule. Z. Natuforsch. 1962, 17, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Bilot, L.; Kawski, A. Der EinfluB des losungsmittels auf die elektronenspektren lumineszierender molecule. Z. Natuforsch. 1963, 18, 10–15. [Google Scholar] [CrossRef] [Green Version]
- McRae, E.G. Theory of solvent effects on molecular electronic spectra Frequency shifts. J. Phys. Chem. 1957, 61, 562–572. [Google Scholar] [CrossRef]
- Reichardt, C. Empirical parameters of solvent polarity as linear free-energy relationships. Angew. Chem. 1979, 18, 98–110. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The alpha-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, pi*, alpha, and beta, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Skwierczynski, R.D.; Connors, K.A. Solvent effects on chemical processes. Part 7. Quantitative description of the composition dependence of the solvent polarity measure ET(30) in binary aqueous-organic solvent mixtures. J. Chem. Soc. Perkin Trans. 1994, 2, 467–472. [Google Scholar] [CrossRef]
- Silva, M.A.R.; Silva, D.C.; Machado, V.G.; Longhinotti, E.; Frescura, V.L.A. Preferential solvation of a hydrophobic probe in binary mixtures comprised of a nonprotic and a hydroxylic solvent: A view of solute–solvent and solvent–solvent interactions. J. Phys. Chem. A 2002, 106, 8820–8826. [Google Scholar] [CrossRef]
- Mandal, A.B.; Nair, B.U.; Ramaswamy, D. Determination of the Critical Micelle Concentration of Surfactants and the Partition Coefficient of an Electrochemical Probe by Using Cyclic Voltammetry. Langmuir 1988, 4, 736–739. [Google Scholar] [CrossRef]
- Tummino, P.J.; Gafni, A. Determination of the aggregation number of detergent micelles using steady-state fluorescence quenching. Biophys. J. 1993, 64, 1580–1587. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Non-covalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Func-tionals and 12 Other Functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Fehér, P.P.; Madarász, Á.; Stirling, A. Multiscale Modeling of Electronic Spectra Including Nuclear Quantum Effects. J. Chem. Theory Comput. 2021, 17, 6340–6352. [Google Scholar] [CrossRef]
- Guido, C.A.; Cortona, P.; Mennucci, B.; Adamo, C. On the Metric of Charge Transfer Molecular Excitations: A Simple Chemical Descriptor. J. Chem. Theory Comput. 2013, 9, 3118–3126. [Google Scholar] [CrossRef]




| 5-isocyanonaphthalene-1-ol | ||||||
|---|---|---|---|---|---|---|
| No. | Solvent | λem (nm) | λex (nm) | Δν (cm−1) | ε (M−1cm−1) | QF |
| 1 | Toluene | 384 | 320 | 5208 | 6005 | 0.44 |
| 2 | Dioxane | 389 | 323 | 5253 | 5540 | 0.60 |
| 3 | Ethyl acetate | 392 | 322 | 5546 | 6420 | 0.43 |
| 4 | Dichloromethane | 395 | 318 | 6130 | 6150 | 0.70 |
| 5 | Tetrahydrofuran | 395 | 329 | 5079 | 6160 | 0.69 |
| 6 | Chloroform | 402 | 322 | 6180 | 5510 | 0.51 |
| 7 | Acetonitrile | 404 | 320 | 6498 | 5900 | 0.47 |
| 8 | 2-propanol | 410 | 330 | 5913 | 5330 | 0.12 |
| 9 | Methanol | 415 | 329 | 6299 | 6220 | 0.04 |
| 10 | N,N-dimethyformamide | 432 | 330 | 7155 | 6200 | 0.07 |
| 11 | Dimethyl sulfoxide | 435 | 329 | 7407 | 5200 | 0.05 |
| 5-isocyano-1-(octyloxy)naphthalene | ||||||
|---|---|---|---|---|---|---|
| No. | Solvent | λem (nm) | λex (nm) | Δν (cm−1) | ε (M−1cm−1) | QF |
| 1 | Toluene | 368 | 325 | 3595 | 10,890 | 0.36 |
| 2 | Dioxane | 375 | 326 | 4008 | 11,900 | 0.50 |
| 3 | Ethyl acetate | 376 | 325 | 4173 | 11,920 | 0.33 |
| 4 | Dichloromethane | 374 | 320 | 4512 | 11,190 | 0.17 |
| 5 | Tetrahydrofuran | 377 | 327 | 4056 | 12,020 | 0.41 |
| 6 | Chloroform | 385 | 328 | 4514 | 10,870 | 0.07 |
| 7 | Acetonitrile | 385 | 325 | 4795 | 10,850 | 0.38 |
| 8 | 2-propanol | 394 | 322 | 5675 | 10,020 | 0.42 |
| 9 | Methanol | 395 | 320 | 5934 | 9690 | 0.33 |
| 10 | N,N-dimethyformamide | 387 | 330 | 4463 | 11,800 | 0.50 |
| 11 | Dimethyl sulfoxide | 395 | 334 | 4624 | 11,820 | 0.68 |
| 5-isocyanonaphthalene-1-ol (ICOL) | ||||||
|---|---|---|---|---|---|---|
| W | LM | BK | MR | R | TD-DFT | |
| μG (D) | - | - | 5.6 ± 0.4 | - | - | 5.3 |
| μE (D) | 6.9 ± 1.0 | - | 9.0 ± 0.5 | - | - | 9.3 |
| Δμ (D) | - | 5.5 ± 1.1 | 3.4 ± 0.9 | 3.7 ± 0.6 | 3.4 ± 0.3 | 4.0 |
| 5-isocyano-1-(octyloxy)naphthalene (ICON) | ||||||
| μG (D) | - | - | 2.5 ± 0.4 | - | - | 5.8 |
| μE (D) | 5.6 ± 0.8 | - | 5.7 ± 0.5 | - | - | 10.4 |
| Δμ (D) | - | 5.6 ± 0.9 | 3.2 ± 0.9 | 3.6 ± 0.6 | 2.3 ± 0.2 | 4.6 |
| 5-isocyanonaphthalene-1-ol (ICOL) | ||||
|---|---|---|---|---|
| Yo (cm−1) | aα (cm−1) | bβ (cm−1) | cπ* (cm−1) | |
| νf (cm−1) | 28,320 ± 2890 | −615 ± 245 | −1730 ± 310 | −4180 ± 445 |
| Δν (cm−1) | 3250 ± 380 | 555 ± 320 | 315 ± 210 | 4000 ± 580 |
| 5-isocyano-1-(octyloxy)naphthalene (ICON) | ||||
| νf (cm−1) | 28,100 ± 190 | −1050 ± 130 | −1305 ± 235 | −1735 ± 250 |
| Δν (cm−1) | 3730 ± 1020 | 2520 ± 700 | −2540 ± 1270 | 2980 ± 1360 |
| Fluorescence Decay Rates (kt, kr, knr) × 10−8 (s−1) | ||||||
|---|---|---|---|---|---|---|
| 5-isocyanonaphthalene-1-ol | 5-isocyano-1-(octyloxy)naphthalene | |||||
| kt | kr | knr | kt | kr | knr | |
| Toluene | 1.41 | 0.62 | 0.79 | 2.44 | 0.88 | 1.56 |
| Tetrahydrofuran | 1.25 | 0.86 | 0.39 | 2.63 | 1.08 | 1.55 |
| Dichloromethane | 0.99 | 0.69 | 0.30 | 1.96 | 0.33 | 1.63 |
| Acetonitrile | 1.23 | 0.58 | 0.65 | 2.33 | 0.89 | 1.44 |
| Methanol | 2.00 | 0.08 | 1.92 | 1.96 | 0.65 | 1.31 |
| Dimethyl sulfoxide | 1.23 *, 0.68 ** | (0.03) (0.02) | (1.2) (0.66) | 1.75 | 1.19 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamoczky, A.; Nagy, T.; Fehér, P.P.; Pardi-Tóth, V.; Kuki, Á.; Nagy, L.; Zsuga, M.; Kéki, S. Isocyanonaphthol Derivatives: Excited-State Proton Transfer and Solvatochromic Properties. Int. J. Mol. Sci. 2022, 23, 7250. https://doi.org/10.3390/ijms23137250
Adamoczky A, Nagy T, Fehér PP, Pardi-Tóth V, Kuki Á, Nagy L, Zsuga M, Kéki S. Isocyanonaphthol Derivatives: Excited-State Proton Transfer and Solvatochromic Properties. International Journal of Molecular Sciences. 2022; 23(13):7250. https://doi.org/10.3390/ijms23137250
Chicago/Turabian StyleAdamoczky, Anita, Tibor Nagy, Péter Pál Fehér, Veronika Pardi-Tóth, Ákos Kuki, Lajos Nagy, Miklos Zsuga, and Sándor Kéki. 2022. "Isocyanonaphthol Derivatives: Excited-State Proton Transfer and Solvatochromic Properties" International Journal of Molecular Sciences 23, no. 13: 7250. https://doi.org/10.3390/ijms23137250
APA StyleAdamoczky, A., Nagy, T., Fehér, P. P., Pardi-Tóth, V., Kuki, Á., Nagy, L., Zsuga, M., & Kéki, S. (2022). Isocyanonaphthol Derivatives: Excited-State Proton Transfer and Solvatochromic Properties. International Journal of Molecular Sciences, 23(13), 7250. https://doi.org/10.3390/ijms23137250











