Noncatalytic Domains in DNA Glycosylases
Abstract
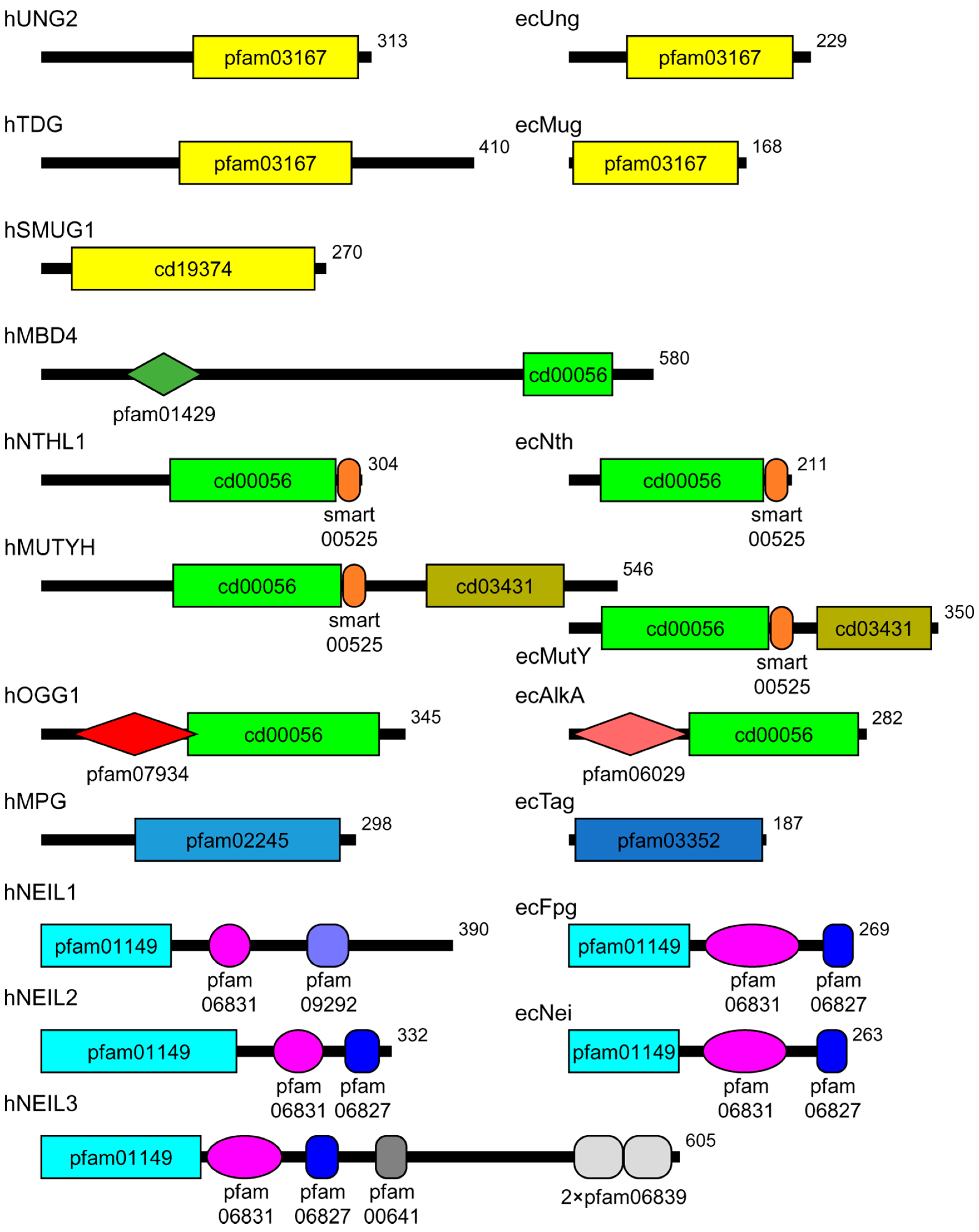
1. Introduction
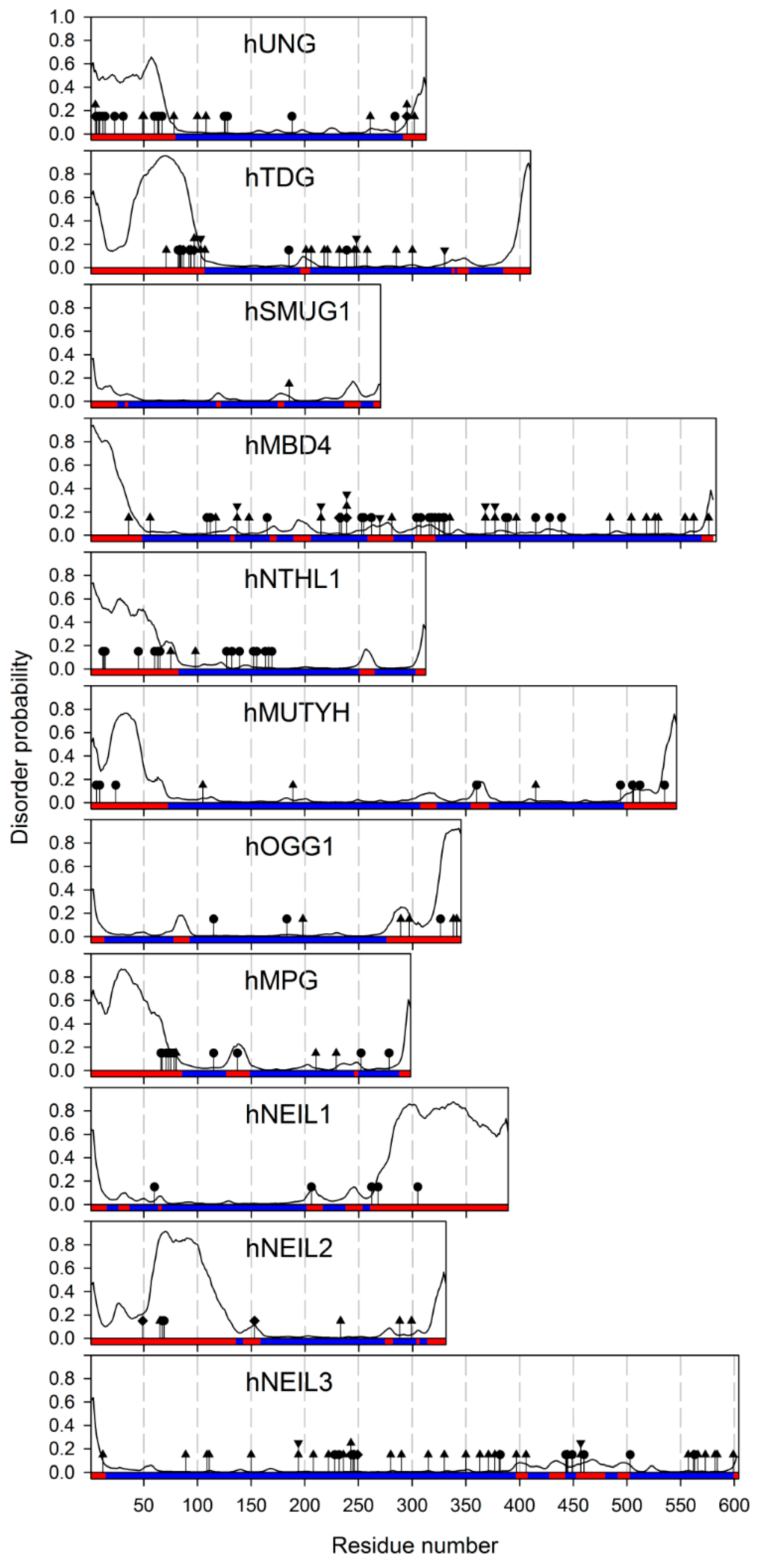
2. Unstructured Tails and Loops
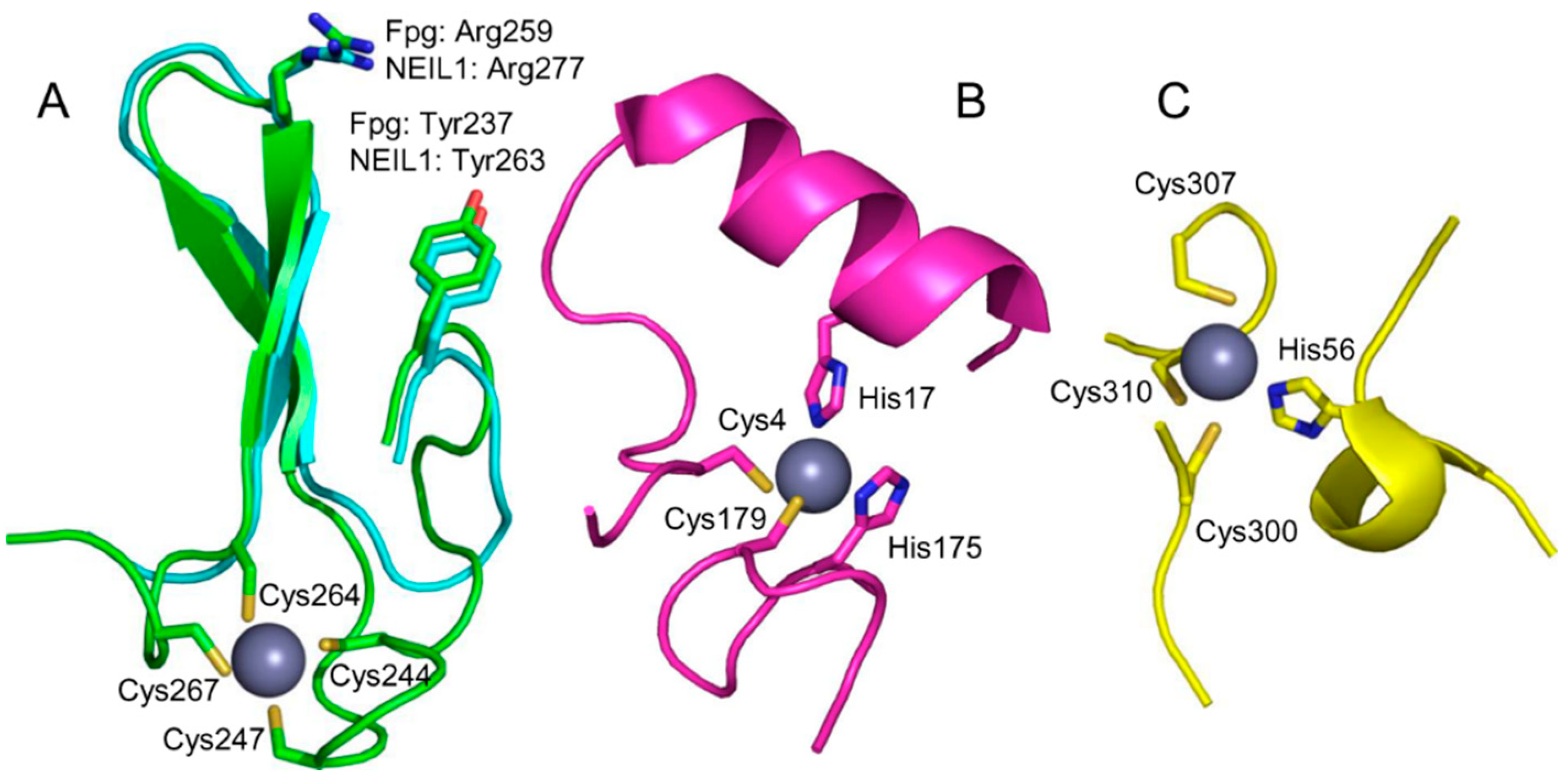
3. Zinc-Binding Structural Motifs
4. Iron–Sulfur Clusters
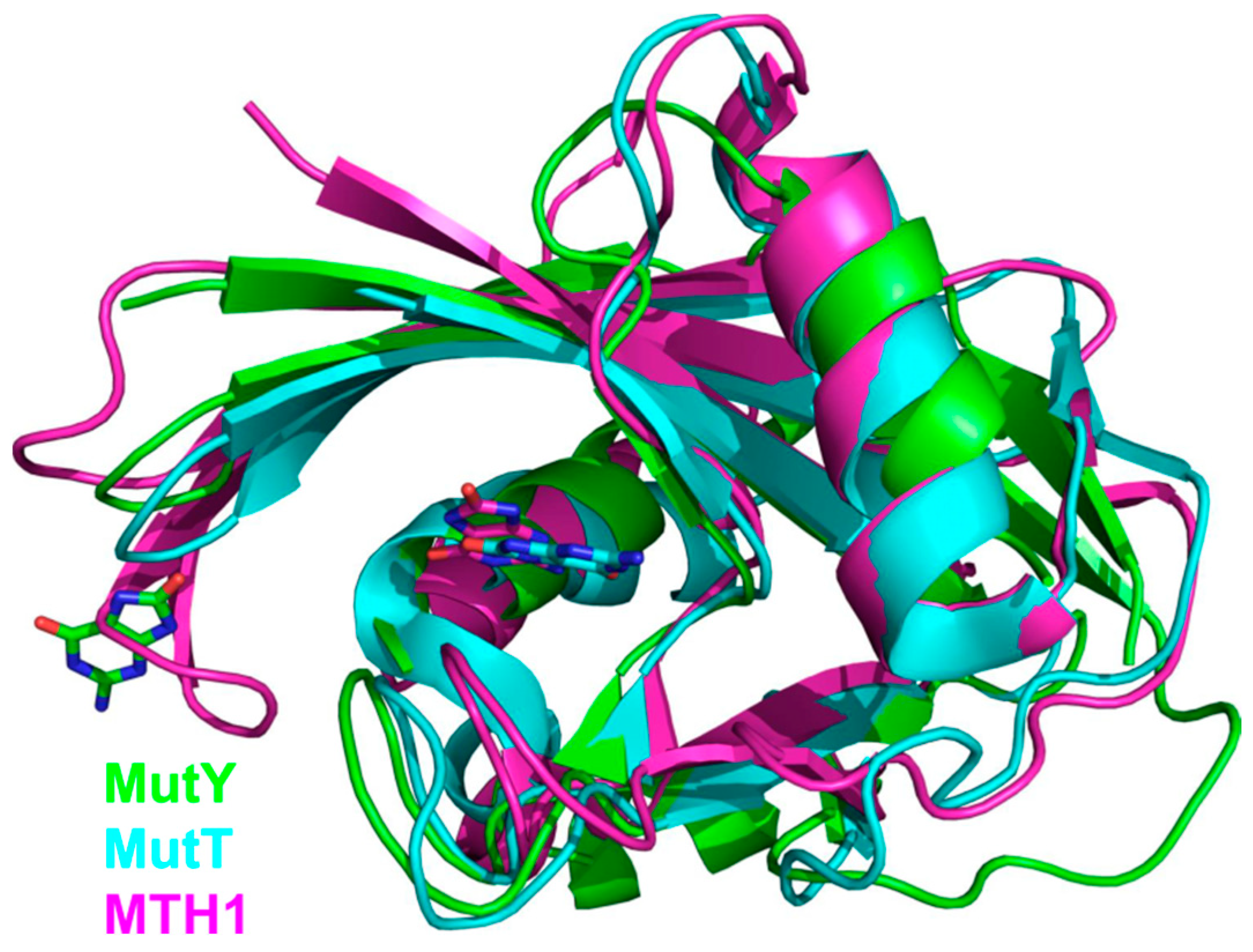
5. NUDIX Domain
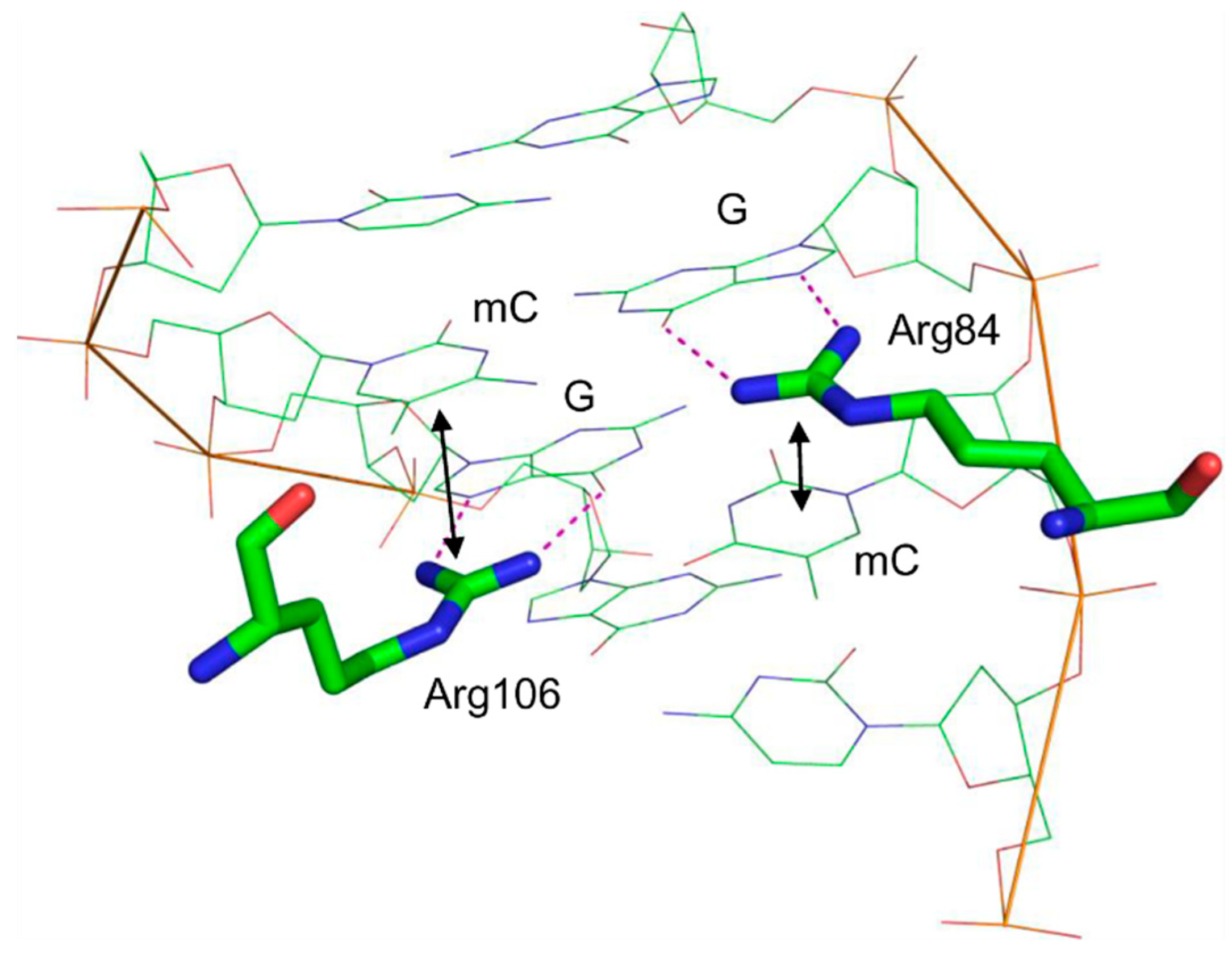
6. Methyl-Binding Domains
7. RNA-Binding Elements
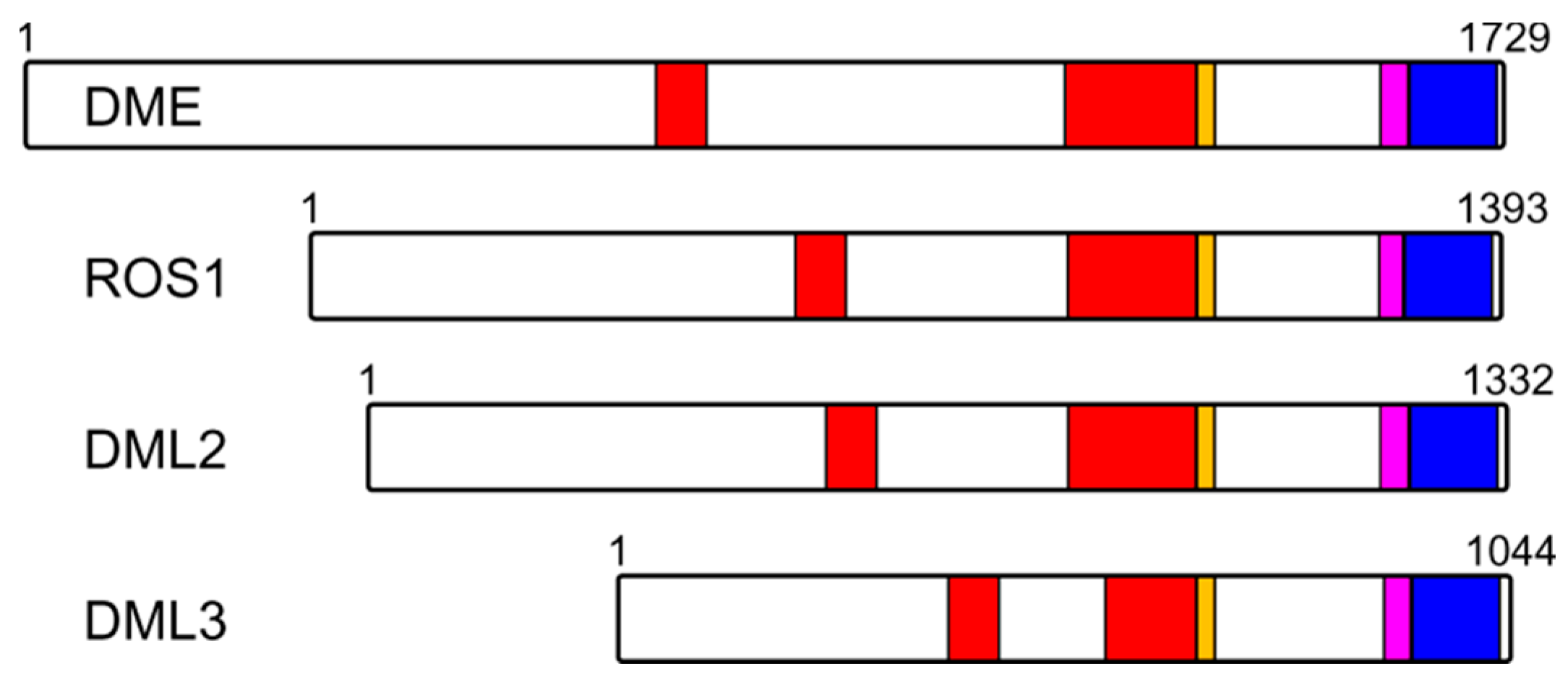
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apic, G.; Gough, J.; Teichmann, S.A. Domain combinations in archaeal, eubacterial and eukaryotic proteomes. J. Mol. Biol. 2001, 310, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Kolodny, R.; Pereyaslavets, L.; Samson, A.O.; Levitt, M. On the universe of protein folds. Annu. Rev. Biophys. 2013, 42, 559–582. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis; ASM Press: Washington, DC, USA, 2006; 1118p. [Google Scholar]
- Zharkov, D.O. Base excision DNA repair. Cell. Mol. Life Sci. 2008, 65, 1544–1565. [Google Scholar] [CrossRef]
- Hitomi, K.; Iwai, S.; Tainer, J.A. The intricate structural chemistry of base excision repair machinery: Implications for DNA damage recognition, removal, and repair. DNA Repair 2007, 6, 410–428. [Google Scholar] [CrossRef]
- Zharkov, D.O.; Shoham, G.; Grollman, A.P. Structural characterization of the Fpg family of DNA glycosylases. DNA Repair 2003, 2, 839–862. [Google Scholar] [CrossRef]
- Prakash, A.; Doublié, S.; Wallace, S.S. The Fpg/Nei family of DNA glycosylases: Substrates, structures, and search for damage. Prog. Mol. Biol. Transl. Sci. 2012, 110, 71–91. [Google Scholar] [CrossRef]
- Bruner, S.D.; Norman, D.P.G.; Verdine, G.L. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 2000, 403, 859–866. [Google Scholar] [CrossRef]
- Walsh, I.; Martin, A.J.M.; Di Domenico, T.; Tosatto, S.C.E. ESpritz: Accurate and fast prediction of protein disorder. Bioinformatics 2012, 28, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Andersen, S.; Heine, T.; Sneve, R.; König, I.; Krokan, H.E.; Epe, B.; Nilsen, H. Incorporation of dUMP into DNA is a major source of spontaneous DNA damage, while excision of uracil is not required for cytotoxicity of fluoropyrimidines in mouse embryonic fibroblasts. Carcinogenesis 2005, 26, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kavli, B.; Otterlei, M.; Slupphaug, G.; Krokan, H.E. Uracil in DNA—General mutagen, but normal intermediate in acquired immunity. DNA Repair 2007, 6, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Kavli, B.; Slupphaug, G.; Krokan, H.E. Genomic uracil in biology, immunity and cancer. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; Royal Society of Chemistry: London, UK, 2021; Volume 1, pp. 220–248. [Google Scholar] [CrossRef]
- Boldinova, E.O.; Khairullin, R.F.; Makarova, A.V.; Zharkov, D.O. Isoforms of base excision repair enzymes produced by alternative splicing. Int. J. Mol. Sci. 2019, 20, 3279. [Google Scholar] [CrossRef]
- Nilsen, H.; Otterlei, M.; Haug, T.; Solum, K.; Nagelhus, T.A.; Skorpen, F.; Krokan, H.E. Nuclear and mitochondrial uracil-DNA glycosylases are generated by alternative splicing and transcription from different positions in the UNG gene. Nucleic Acids Res. 1997, 25, 750–755. [Google Scholar] [CrossRef]
- Otterlei, M.; Haug, T.; Nagelhus, T.A.; Slupphaug, G.; Lindmo, T.; Krokan, H.E. Nuclear and mitochondrial splice forms of human uracil-DNA glycosylase contain a complex nuclear localisation signal and a strong classical mitochondrial localisation signal, respectively. Nucleic Acids Res. 1998, 26, 4611–4617. [Google Scholar] [CrossRef]
- Nagelhus, T.A.; Haug, T.; Singh, K.K.; Keshav, K.F.; Skorpen, F.; Otterlei, M.; Bharati, S.; Lindmo, T.; Benichou, S.; Benarous, R.; et al. A sequence in the N-terminal region of human uracil-DNA glycosylase with homology to XPA interacts with the C-terminal part of the 34-kDa subunit of replication protein A. J. Biol. Chem. 1997, 272, 6561–6566. [Google Scholar] [CrossRef]
- Otterlei, M.; Warbrick, E.; Nagelhus, T.A.; Haug, T.; Slupphaug, G.; Akbari, M.; Aas, P.A.; Steinsbekk, K.; Bakke, O.; Krokan, H.E. Post-replicative base excision repair in replication foci. EMBO J. 1999, 18, 3834–3844. [Google Scholar] [CrossRef]
- Lu, X.; Bocangel, D.; Nannenga, B.; Yamaguchi, H.; Appella, E.; Donehower, L.A. The p53-induced oncogenic phosphatase PPM1D interacts with uracil DNA glycosylase and suppresses base excision repair. Mol. Cell 2004, 15, 621–634. [Google Scholar] [CrossRef]
- Weiser, B.P.; Stivers, J.T.; Cole, P.A. Investigation of N-terminal phospho-regulation of uracil DNA glycosylase using protein semisynthesis. Biophys. J. 2017, 113, 393–401. [Google Scholar] [CrossRef]
- Muller-Weeks, S.; Mastran, B.; Caradonna, S. The nuclear isoform of the highly conserved human uracil-DNA glycosylase is an Mr 36,000 phosphoprotein. J. Biol. Chem. 1998, 273, 21909–21917. [Google Scholar] [CrossRef]
- Fischer, J.A.; Muller-Weeks, S.; Caradonna, S. Proteolytic degradation of the nuclear isoform of uracil-DNA glycosylase occurs during the S phase of the cell cycle. DNA Repair 2004, 3, 505–513. [Google Scholar] [CrossRef]
- Fischer, J.A.; Muller-Weeks, S.; Caradonna, S.J. Fluorodeoxyuridine modulates cellular expression of the DNA base excision repair enzyme uracil-DNA glycosylase. Cancer Res. 2006, 66, 8829–8837. [Google Scholar] [CrossRef]
- Hagen, L.; Kavli, B.; Sousa, M.M.L.; Torseth, K.; Liabakk, N.B.; Sundheim, O.; Pena-Diaz, J.; Otterlei, M.; Hørning, O.; Jensen, O.N.; et al. Cell cycle-specific UNG2 phosphorylations regulate protein turnover, activity and association with RPA. EMBO J. 2008, 27, 51–61. [Google Scholar] [CrossRef]
- Baehr, C.A.; Huntoon, C.J.; Hoang, S.-M.; Jerde, C.R.; Karnitz, L.M. Glycogen synthase kinase 3 (GSK-3)-mediated phosphorylation of uracil N-glycosylase 2 (UNG2) facilitates the repair of floxuridine-induced DNA lesions and promotes cell survival. J. Biol. Chem. 2016, 291, 26875–26885. [Google Scholar] [CrossRef]
- Buchinger, E.; Wiik, S.Å.; Kusnierczyk, A.; Rabe, R.; Aas, P.A.; Kavli, B.; Slupphaug, G.; Aachmann, F.L. Backbone 1H, 13C and 15N chemical shift assignment of full-length human uracil DNA glycosylase UNG2. Biomol. NMR Assign. 2018, 12, 15–22. [Google Scholar] [CrossRef]
- Kwon, E.; Pathak, D.; Chang, H.W.; Kim, D.Y. Crystal structure of mimivirus uracil-DNA glycosylase. PLoS ONE 2017, 12, e0182382. [Google Scholar] [CrossRef]
- Rodriguez, G.; Orris, B.; Majumdar, A.; Bhat, S.; Stivers, J.T. Macromolecular crowding induces compaction and DNA binding in the disordered N-terminal domain of hUNG2. DNA Repair 2020, 86, 102764. [Google Scholar] [CrossRef]
- Wibley, J.E.A.; Waters, T.R.; Haushalter, K.; Verdine, G.L.; Pearl, L.H. Structure and specificity of the vertebrate anti-mutator uracil-DNA glycosylase SMUG1. Mol. Cell 2003, 11, 1647–1659. [Google Scholar] [CrossRef]
- Popov, A.V.; Grin, I.R.; Dvornikova, A.P.; Matkarimov, B.T.; Groisman, R.; Saparbaev, M.; Zharkov, D.O. Reading targeted DNA damage in the active demethylation pathway: Role of accessory domains of eukaryotic AP endonucleases and thymine-DNA glycosylases. J. Mol. Biol. 2020, 432, 1747–1768. [Google Scholar] [CrossRef]
- Tini, M.; Benecke, A.; Um, S.-J.; Torchia, J.; Evans, R.M.; Chambon, P. Association of CBP/p300 acetylase and thymine DNA glycosylase links DNA repair and transcription. Mol. Cell 2002, 9, 265–277. [Google Scholar] [CrossRef]
- Mohan, R.D.; Litchfield, D.W.; Torchia, J.; Tini, M. Opposing regulatory roles of phosphorylation and acetylation in DNA mispair processing by thymine DNA glycosylase. Nucleic Acids Res. 2010, 38, 1135–1148. [Google Scholar] [CrossRef]
- Smet-Nocca, C.; Wieruszeski, J.-M.; Chaar, V.; Leroy, A.; Benecke, A. The thymine-DNA glycosylase regulatory domain: Residual structure and DNA binding. Biochemistry 2008, 47, 6519–6530. [Google Scholar] [CrossRef]
- Coey, C.T.; Malik, S.S.; Pidugu, L.S.; Varney, K.M.; Pozharski, E.; Drohat, A.C. Structural basis of damage recognition by thymine DNA glycosylase: Key roles for N-terminal residues. Nucleic Acids Res. 2016, 44, 10248–10258. [Google Scholar] [CrossRef]
- Kohno, T.; Shinmura, K.; Tosaka, M.; Tani, M.; Kim, S.-R.; Sugimura, H.; Nohmi, T.; Kasai, H.; Yokota, J. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene 1998, 16, 3219–3225. [Google Scholar] [CrossRef]
- Nishioka, K.; Ohtsubo, T.; Oda, H.; Fujiwara, T.; Kang, D.; Sugimachi, K.; Nakabeppu, Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol. Biol. Cell 1999, 10, 1637–1652. [Google Scholar] [CrossRef]
- Hashiguchi, K.; Stuart, J.A.; de Souza-Pinto, N.C.; Bohr, V.A. The C-terminal αO helix of human Ogg1 is essential for 8-oxoguanine DNA glycosylase activity: The mitochondrial β-Ogg1 lacks this domain and does not have glycosylase activity. Nucleic Acids Res. 2004, 32, 5596–5608. [Google Scholar] [CrossRef]
- Su, Y.-H.; Lee, Y.-L.; Chen, S.-F.; Lee, Y.-P.; Hsieh, Y.-H.; Tsai, J.-H.; Hsu, J.-L.; Tian, W.-T.; Huang, W. Essential role of β-human 8-oxoguanine DNA glycosylase 1 in mitochondrial oxidative DNA repair. Environ. Mol. Mutagen. 2013, 54, 54–64. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, J. Mitochondrial nucleoid: Shield and switch of the mitochondrial genome. Oxid. Med. Cell. Longev. 2017, 2017, 8060949. [Google Scholar] [CrossRef]
- Hegde, M.L.; Tsutakawa, S.E.; Hegde, P.M.; Holthauzen, L.M.F.; Li, J.; Oezguen, N.; Hilser, V.J.; Tainer, J.A.; Mitra, S. The disordered C-terminal domain of human DNA glycosylase NEIL1 contributes to its stability via intramolecular interactions. J. Mol. Biol. 2013, 425, 2359–2371. [Google Scholar] [CrossRef]
- Prakash, A.; Moharana, K.; Wallace, S.S.; Doublié, S. Destabilization of the PCNA trimer mediated by its interaction with the NEIL1 DNA glycosylase. Nucleic Acids Res. 2017, 45, 2897–2909. [Google Scholar] [CrossRef]
- Sharma, N.; Chakravarthy, S.; Longley, M.J.; Copeland, W.C.; Prakash, A. The C-terminal tail of the NEIL1 DNA glycosylase interacts with the human mitochondrial single-stranded DNA binding protein. DNA Repair 2018, 65, 11–19. [Google Scholar] [CrossRef]
- Das, A.; Boldogh, I.; Lee, J.W.; Harrigan, J.A.; Hegde, M.L.; Piotrowski, J.; de Souza Pinto, N.; Ramos, W.; Greenberg, M.M.; Hazra, T.K.; et al. The human Werner syndrome protein stimulates repair of oxidative DNA base damage by the DNA glycosylase NEIL1. J. Biol. Chem. 2007, 282, 26591–26602. [Google Scholar] [CrossRef]
- Dou, H.; Theriot, C.A.; Das, A.; Hegde, M.L.; Matsumoto, Y.; Boldogh, I.; Hazra, T.K.; Bhakat, K.K.; Mitra, S. Interaction of the human DNA glycosylase NEIL1 with proliferating cell nuclear antigen: The potential for replication-associated repair of oxidized bases in mammalian genomes. J. Biol. Chem. 2008, 283, 3130–3140. [Google Scholar] [CrossRef]
- Hegde, M.L.; Theriot, C.A.; Das, A.; Hegde, P.M.; Guo, Z.; Gary, R.K.; Hazra, T.K.; Shen, B.; Mitra, S. Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease 1. J. Biol. Chem. 2008, 283, 27028–27037. [Google Scholar] [CrossRef]
- Theriot, C.A.; Hegde, M.L.; Hazra, T.K.; Mitra, S. RPA physically interacts with the human DNA glycosylase NEIL1 to regulate excision of oxidative DNA base damage in primer-template structures. DNA Repair 2010, 9, 643–652. [Google Scholar] [CrossRef]
- Hegde, M.L.; Hegde, P.M.; Arijit, D.; Boldogh, I.; Mitra, S. Human DNA glycosylase NEIL1’s interactions with downstream repair proteins is critical for efficient repair of oxidized DNA base damage and enhanced cell survival. Biomolecules 2012, 2, 564–578. [Google Scholar] [CrossRef]
- Hegde, M.L.; Banerjee, S.; Hegde, P.M.; Bellot, L.J.; Hazra, T.K.; Boldogh, I.; Mitra, S. Enhancement of NEIL1 protein-initiated oxidized DNA base excision repair by heterogeneous nuclear ribonucleoprotein U (hnRNP-U) via direct interaction. J. Biol. Chem. 2012, 287, 34202–34211. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Fitzpatrick, M.; Kompaniez, K.; Jacob, K.D.; Moore, B.R.; Nagle, J.; Barnes, J.; Lohani, A.; Evans, M.K. Coordination of DNA repair by NEIL1 and PARP-1: A possible link to aging. Aging 2012, 4, 674–685. [Google Scholar] [CrossRef]
- Hegde, P.M.; Dutta, A.; Sengupta, S.; Mitra, J.; Adhikari, S.; Tomkinson, A.E.; Li, G.-M.; Boldogh, I.; Hazra, T.K.; Mitra, S.; et al. The C-terminal domain (CTD) of human DNA glycosylase NEIL1 is required for forming BERosome repair complex with DNA replication proteins at the replicating genome: Dominant negative function of the CTD. J. Biol. Chem. 2015, 290, 20919–20933. [Google Scholar] [CrossRef]
- Sengupta, S.; Yang, C.; Hegde, M.L.; Hegde, P.M.; Mitra, J.; Pandey, A.; Dutta, A.; Datarwala, A.T.; Bhakat, K.K.; Mitra, S. Acetylation of oxidized base repair-initiating NEIL1 DNA glycosylase required for chromatin-bound repair complex formation in the human genome increases cellular resistance to oxidative stress. DNA Repair 2018, 66–67, 1–10. [Google Scholar] [CrossRef]
- Eckenroth, B.E.; Cao, V.B.; Averill, A.M.; Dragon, J.A.; Doublié, S. Unique structural features of mammalian NEIL2 DNA glycosylase prime its activity for diverse DNA substrates and environments. Structure 2021, 29, 29–42. [Google Scholar] [CrossRef]
- Zhdanova, P.V.; Ishchenko, A.A.; Chernonosov, A.A.; Zharkov, D.O.; Koval, V.V. Dynamics and conformational changes in human NEIL2 DNA glycosylase analyzed by hydrogen/deuterium exchange mass spectrometry. J. Mol. Biol. 2022, 434, 167334. [Google Scholar] [CrossRef]
- Zharkov, D.O.; Grollman, A.P. The DNA trackwalkers: Principles of lesion search and recognition by DNA glycosylases. Mutat. Res. 2005, 577, 24–54. [Google Scholar] [CrossRef]
- Mechetin, G.V.; Zharkov, D.O. The mechanism of substrate search by base excision repair enzymes. Dokl. Biochem. Biophys. 2011, 437, 94–97. [Google Scholar] [CrossRef]
- Rodriguez, G.; Esadze, A.; Weiser, B.P.; Schonhoft, J.D.; Cole, P.A.; Stivers, J.T. Disordered N-terminal domain of human uracil DNA glycosylase (hUNG2) enhances DNA translocation. ACS Chem. Biol. 2017, 12, 2260–2263. [Google Scholar] [CrossRef]
- Dey, P.; Bhattacherjee, A. Mechanism of facilitated diffusion of DNA repair proteins in crowded environment: Case study with human uracil DNA glycosylase. J. Phys. Chem. B 2019, 123, 10354–10364. [Google Scholar] [CrossRef]
- Weiser, B.P.; Rodriguez, G.; Cole, P.A.; Stivers, J.T. N-terminal domain of human uracil DNA glycosylase (hUNG2) promotes targeting to uracil sites adjacent to ssDNA–dsDNA junctions. Nucleic Acids Res. 2018, 46, 7169–7178. [Google Scholar] [CrossRef]
- Hedglin, M.; O’Brien, P.J. Human alkyladenine DNA glycosylase employs a processive search for DNA damage. Biochemistry 2008, 47, 11434–11445. [Google Scholar] [CrossRef]
- Vuzman, D.; Azia, A.; Levy, Y. Searching DNA via a “monkey bar” mechanism: The significance of disordered tails. J. Mol. Biol. 2010, 396, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Vuzman, D.; Levy, Y. Intrinsically disordered regions as affinity tuners in protein–DNA interactions. Mol. Biosyst. 2012, 8, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hedglin, M.; Zhang, Y.; O’Brien, P.J. Isolating contributions from intersegmental transfer to DNA searching by alkyladenine DNA glycosylase. J. Biol. Chem. 2013, 288, 24550–24559. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Üren, A.; Roy, R. N-terminal extension of N-methylpurine DNA glycosylase is required for turnover in hypoxanthine excision reaction. J. Biol. Chem. 2007, 282, 30078–30084. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Roy, R. Truncation of amino-terminal tail stimulates activity of human endonuclease III (hNTH1). J. Mol. Biol. 2002, 321, 265–276. [Google Scholar] [CrossRef]
- Liu, X.; Choudhury, S.; Roy, R. In vitro and in vivo dimerization of human endonuclease III stimulates its activity. J. Biol. Chem. 2003, 278, 50061–50069. [Google Scholar] [CrossRef]
- Liu, X.; Roy, R. Mutation at active site lysine 212 to arginine uncouples the glycosylase activity from the lyase activity of human endonuclease III. Biochemistry 2001, 40, 13617–13622. [Google Scholar] [CrossRef]
- O’Connor, T.R.; Graves, R.J.; de Murcia, G.; Castaing, B.; Laval, J. Fpg protein of Escherichia coli is a zinc finger protein whose cysteine residues have a structural and/or functional role. J. Biol. Chem. 1993, 268, 9063–9070. [Google Scholar] [CrossRef]
- Tchou, J.; Michaels, M.L.; Miller, J.H.; Grollman, A.P. Function of the zinc finger in Escherichia coli Fpg protein. J. Biol. Chem. 1993, 268, 26738–26744. [Google Scholar] [CrossRef]
- Gilboa, R.; Zharkov, D.O.; Golan, G.; Fernandes, A.S.; Gerchman, S.E.; Matz, E.; Kycia, J.H.; Grollman, A.P.; Shoham, G. Structure of formamidopyrimidine-DNA glycosylase covalently complexed to DNA. J. Biol. Chem. 2002, 277, 19811–19816. [Google Scholar] [CrossRef]
- Zharkov, D.O.; Golan, G.; Gilboa, R.; Fernandes, A.S.; Gerchman, S.E.; Kycia, J.H.; Rieger, R.A.; Grollman, A.P.; Shoham, G. Structural analysis of an Escherichia coli endonuclease VIII covalent reaction intermediate. EMBO J. 2002, 21, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.S.; Majumdar, I.; Grishin, N.V. Structural classification of zinc fingers. Nucleic Acids Res. 2003, 31, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Rajagopalan, L.; Mathura, V.S.; Rigby, S.J.; Mitra, S.; Hazra, T.K. Identification of a zinc finger domain in the human NEIL2 (Nei-like-2) protein. J. Biol. Chem. 2004, 279, 47132–47138. [Google Scholar] [CrossRef] [PubMed]
- Doublié, S.; Bandaru, V.; Bond, J.P.; Wallace, S.S. The crystal structure of human endonuclease VIII-like 1 (NEIL1) reveals a zincless finger motif required for glycosylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10284–10289. [Google Scholar] [CrossRef]
- Duclos, S.; Aller, P.; Jaruga, P.; Dizdaroglu, M.; Wallace, S.S.; Doublié, S. Structural and biochemical studies of a plant formamidopyrimidine-DNA glycosylase reveal why eukaryotic Fpg glycosylases do not excise 8-oxoguanine. DNA Repair 2012, 11, 714–725. [Google Scholar] [CrossRef]
- Prakash, A.; Cao, V.B.; Doublié, S. Phosphorylation sites identified in the NEIL1 DNA glycosylase are potential targets for the JNK1 kinase. PLoS ONE 2016, 11, e0157860. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, L.; Zhang, J.; Yue, Z.; Song, J.; Zong, S.; Liu, M.; Stovicek, O.; Gao, Y.Q.; Yi, C. Tautomerization-dependent recognition and excision of oxidation damage in base-excision DNA repair. Proc. Natl. Acad. Sci. USA 2016, 113, 7792–7797. [Google Scholar] [CrossRef]
- Kwon, K.; Cao, C.; Stivers, J.T. A novel zinc snap motif conveys structural stability to 3-methyladenine DNA glycosylase I. J. Biol. Chem. 2003, 278, 19442–19446. [Google Scholar] [CrossRef]
- Nakamura, T.; Okabe, K.; Hirayama, S.; Chirifu, M.; Ikemizu, S.; Morioka, H.; Nakabeppu, Y.; Yamagata, Y. Structure of the mammalian adenine DNA glycosylase MUTYH: Insights into the base excision repair pathway and cancer. Nucleic Acids Res. 2021, 49, 7154–7163. [Google Scholar] [CrossRef]
- Li, H.; Endutkin, A.V.; Bergonzo, C.; Campbell, A.J.; de los Santos, C.; Grollman, A.; Zharkov, D.O.; Simmerling, C. A dynamic checkpoint in oxidative lesion discrimination by formamidopyrimidine–DNA glycosylase. Nucleic Acids Res. 2016, 44, 683–694. [Google Scholar] [CrossRef]
- Endutkin, A.V.; Koptelov, S.S.; Popov, A.V.; Torgasheva, N.A.; Lomzov, A.A.; Tsygankova, A.R.; Skiba, T.V.; Afonnikov, D.A.; Zharkov, D.O. Residue coevolution reveals functionally important intramolecular interactions in formamidopyrimidine-DNA glycosylase. DNA Repair 2018, 69, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Endutkin, A.V.; Zharkov, D.O. Critical sites of DNA backbone integrity for damaged base removal by formamidopyrimidine–DNA glycosylase. Biochemistry 2019, 58, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Silvian, L.F.; Wang, J.; Steitz, T.A. Insights into editing from an Ile-tRNA synthetase structure with tRNAIle and mupirocin. Science 1999, 285, 1074–1077. [Google Scholar] [CrossRef]
- Wallace, B.D.; Berman, Z.; Mueller, G.A.; Lin, Y.; Chang, T.; Andres, S.N.; Wojtaszek, J.L.; DeRose, E.F.; Appel, C.D.; London, R.E.; et al. APE2 Zf-GRF facilitates 3′-5′ resection of DNA damage following oxidative stress. Proc. Natl Acad. Sci. USA 2017, 114, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Gamsjaeger, R.; Liew, C.K.; Loughlin, F.E.; Crossley, M.; Mackay, J.P. Sticky fingers: Zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 2007, 32, 63–70. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Wojtaszek, J.L.; Greer, B.H.; Haldar, T.; Gates, K.S.; Williams, R.S.; Eichman, B.F. An autoinhibitory role for the GRF zinc finger domain of DNA glycosylase NEIL3. J. Biol. Chem. 2020, 295, 15566–15575. [Google Scholar] [CrossRef]
- Albelazi, M.S.; Martin, P.R.; Mohammed, S.; Mutti, L.; Parsons, J.L.; Elder, R.H. The biochemical role of the human NEIL1 and NEIL3 DNA glycosylases on model DNA replication forks. Genes 2019, 10, 315. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Roldán-Arjona, T.; Ariza, R.R.; Córdoba-Cañero, D. DNA base excision repair in plants: An unfolding story with familiar and novel characters. Front. Plant Sci. 2019, 10, 1055. [Google Scholar] [CrossRef]
- Iyer, L.M.; Abhiman, S.; Aravind, L. Natural history of eukaryotic DNA methylation systems. Prog. Mol. Biol. Transl. Sci. 2011, 101, 25–104. [Google Scholar] [CrossRef]
- Hong, S.; Hashimoto, H.; Kow, Y.W.; Zhang, X.; Cheng, X. The carboxy-terminal domain of ROS1 is essential for 5-methylcytosine DNA glycosylase activity. J. Mol. Biol. 2014, 426, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Doblas, J.T.; Morales-Ruiz, T.; Ariza, R.R.; Martínez-Macías, M.I.; Roldán-Arjona, T. The C-terminal domain of Arabidopsis ROS1 DNA demethylase interacts with histone H3 and is required for DNA binding and catalytic activity. DNA Repair 2022, 115, 103341. [Google Scholar] [CrossRef] [PubMed]
- Metz, A.H.; Hollis, T.; Eichman, B.F. DNA damage recognition and repair by 3-methyladenine DNA glycosylase I (TAG). EMBO J. 2007, 26, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yan, X.; Carter, L.G.; Liu, H.; Graham, S.; Coote, P.J.; Naismith, J. A model for 3-methyladenine recognition by 3-methyladenine DNA glycosylase I (TAG) from Staphylococcus aureus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Raetz, A.G.; David, S.S. When you’re strange: Unusual features of the MUTYH glycosylase and implications in cancer. DNA Repair 2019, 80, 16–25. [Google Scholar] [CrossRef]
- Yudkina, A.V.; Shilkin, E.S.; Endutkin, A.V.; Makarova, A.V.; Zharkov, D.O. Reading and misreading 8-oxoguanine, a paradigmatic ambiguous nucleobase. Crystals 2019, 9, 269. [Google Scholar] [CrossRef]
- Engstrom, L.M.; Brinkmeyer, M.K.; Ha, Y.; Raetz, A.G.; Hedman, B.; Hodgson, K.O.; Solomon, E.I.; David, S.S. A zinc linchpin motif in the MUTYH glycosylase interdomain connector is required for efficient repair of DNA damage. J. Am. Chem. Soc. 2014, 136, 7829–7832. [Google Scholar] [CrossRef]
- Nuñez, N.N.; Khuu, C.; Babu, C.S.; Bertolani, S.J.; Rajavel, A.N.; Spear, J.E.; Armas, J.A.; Wright, J.D.; Siegel, J.B.; Lim, C.; et al. The zinc linchpin motif in the DNA repair glycosylase MUTYH: Identifying the Zn2+ ligands and roles in damage recognition and repair. J. Am. Chem. Soc. 2018, 140, 13260–13271. [Google Scholar] [CrossRef]
- Luncsford, P.J.; Chang, D.-Y.; Shi, G.; Bernstein, J.; Madabushi, A.; Patterson, D.N.; Lu, A.-L.; Toth, E.A. A structural hinge in eukaryotic MutY homologues mediates catalytic activity and Rad9–Rad1–Hus1 checkpoint complex interactions. J. Mol. Biol. 2010, 403, 351–370. [Google Scholar] [CrossRef]
- Parker, A.; Gu, Y.; Mahoney, W.; Lee, S.-H.; Singh, K.K.; Lu, A.-L. Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair. J. Biol. Chem. 2001, 276, 5547–5555. [Google Scholar] [CrossRef]
- Luncsford, P.J.; Manvilla, B.A.; Patterson, D.N.; Malik, S.S.; Jin, J.; Hwang, B.-J.; Gunther, R.; Kalvakolanu, S.; Lipinski, L.J.; Yuan, W.; et al. Coordination of MYH DNA glycosylase and APE1 endonuclease activities via physical interactions. DNA Repair 2013, 12, 1043–1052. [Google Scholar] [CrossRef]
- Brinkmeyer, M.K.; David, S.S. Distinct functional consequences of MUTYH variants associated with colorectal cancer: Damaged DNA affinity, glycosylase activity and interaction with PCNA and Hus1. DNA Repair 2015, 34, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-J.; Jin, J.; Gao, Y.; Shi, G.; Madabushi, A.; Yan, A.; Guan, X.; Zalzman, M.; Nakajima, S.; Lan, L.; et al. SIRT6 protein deacetylase interacts with MYH DNA glycosylase, APE1 endonuclease, and Rad9–Rad1–Hus1 checkpoint clamp. BMC Mol. Biol. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Silva, R.M.B.; O’Brien, E. Redox chemistry in the genome: Emergence of the [4Fe4S] cofactor in repair and replication. Annu. Rev. Biochem. 2019, 88, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Khodour, Y.; Kaguni, L.S.; Stiban, J. Iron–sulfur clusters in nucleic acid metabolism: Varying roles of ancient cofactors. Enzymes 2019, 45, 225–256. [Google Scholar] [CrossRef]
- Piersen, C.E.; Prince, M.A.; Augustine, M.L.; Dodson, M.L.; Lloyd, R.S. Purification and cloning of Micrococcus luteus ultraviolet endonuclease, an N-glycosylase/abasic lyase that proceeds via an imino enzyme-DNA intermediate. J. Biol. Chem. 1995, 270, 23475–23484. [Google Scholar] [CrossRef]
- Mol, C.D.; Arvai, A.S.; Begley, T.J.; Cunningham, R.P.; Tainer, J.A. Structure and activity of a thermostable thymine-DNA glycosylase: Evidence for base twisting to remove mismatched normal DNA bases. J. Mol. Biol. 2002, 315, 373–384. [Google Scholar] [CrossRef]
- Hinks, J.A.; Evans, M.C.W.; de Miguel, Y.; Sartori, A.A.; Jiricny, J.; Pearl, L.H. An iron-sulfur cluster in the family 4 uracil-DNA glycosylases. J. Biol. Chem. 2002, 277, 16936–16940. [Google Scholar] [CrossRef]
- Kosaka, H.; Hoseki, J.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Crystal structure of family 5 uracil-DNA glycosylase bound to DNA. J. Mol. Biol. 2007, 373, 839–850. [Google Scholar] [CrossRef]
- Sang, P.B.; Srinath, T.; Patil, A.G.; Woo, E.-J.; Varshney, U. A unique uracil-DNA binding protein of the uracil DNA glycosylase superfamily. Nucleic Acids Res. 2015, 43, 8452–8463. [Google Scholar] [CrossRef]
- Cunningham, R.P.; Asahara, H.; Bank, J.F.; Scholes, C.P.; Salerno, J.C.; Surerus, K.; Münck, E.; McCracken, J.; Peisach, J.; Emptage, M.H. Endonuclease III is an iron-sulfur protein. Biochemistry 1989, 28, 4450–4455. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; O’Handley, S.; Cunningham, R.P.; Johnson, M.K. The role of the iron-sulfur cluster in Escherichia coli endonuclease III: A resonance Raman study. J. Biol. Chem. 1992, 267, 16135–16137. [Google Scholar] [CrossRef]
- Boon, E.M.; Livingston, A.L.; Chmiel, N.H.; David, S.S.; Barton, J.K. DNA-mediated charge transport for DNA repair. Proc. Natl. Acad. Sci. USA 2003, 100, 12543–12547. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.K.; Yavin, E.; Lukianova, O.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry 2005, 44, 8397–8407. [Google Scholar] [CrossRef]
- Yavin, E.; Boal, A.K.; Stemp, E.D.A.; Boon, E.M.; Livingston, A.L.; O’Shea, V.L.; David, S.S.; Barton, J.K. Protein–DNA charge transport: Redox activation of a DNA repair protein by guanine radical. Proc. Natl. Acad. Sci. USA 2005, 102, 3546–3551. [Google Scholar] [CrossRef]
- Gorodetsky, A.A.; Boal, A.K.; Barton, J.K. Direct electrochemistry of endonuclease III in the presence and absence of DNA. J. Am. Chem. Soc. 2006, 128, 12082–12083. [Google Scholar] [CrossRef][Green Version]
- Yavin, E.; Stemp, E.D.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. Electron trap for DNA-bound repair enzymes: A strategy for DNA-mediated signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 3610–3614. [Google Scholar] [CrossRef]
- Boal, A.K.; Yavin, E.; Barton, J.K. DNA repair glycosylases with a [4Fe–4S] cluster: A redox cofactor for DNA-mediated charge transport? J. Inorg. Biochem. 2007, 101, 1913–1921. [Google Scholar] [CrossRef][Green Version]
- Boal, A.K.; Genereux, J.C.; Sontz, P.A.; Gralnick, J.A.; Newman, D.K.; Barton, J.K. Redox signaling between DNA repair proteins for efficient lesion detection. Proc. Natl. Acad. Sci. USA 2009, 106, 15237–15242. [Google Scholar] [CrossRef]
- Romano, C.A.; Sontz, P.A.; Barton, J.K. Mutants of the base excision repair glycosylase, endonuclease III: DNA charge transport as a first step in lesion detection. Biochemistry 2011, 50, 6133–6145. [Google Scholar] [CrossRef]
- Sontz, P.A.; Mui, T.P.; Fuss, J.O.; Tainer, J.A.; Barton, J.K. DNA charge transport as a first step in coordinating the detection of lesions by repair proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Grodick, M.A.; Segal, H.M.; Zwang, T.J.; Barton, J.K. DNA-mediated signaling by proteins with 4Fe–4S clusters is necessary for genomic integrity. J. Am. Chem. Soc. 2014, 136, 6470–6478. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.C.M.; Zwang, T.J.; Barton, J.K. The oxidation state of [4Fe4S] clusters modulates the DNA-binding affinity of DNA repair proteins. J. Am. Chem. Soc. 2017, 139, 12784–12792. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.B.; Grodick, M.A.; Barton, J.K. UvrC coordinates an O2-sensitive [4Fe4S] cofactor. J. Am. Chem. Soc. 2020, 142, 10964–10977. [Google Scholar] [CrossRef]
- Sarre, A.; Ökvist, M.; Klar, T.; Hall, D.R.; Smalås, A.O.; McSweeney, S.; Timmins, J.; Moe, E. Structural and functional characterization of two unusual endonuclease III enzymes from Deinococcus radiodurans. J. Struct. Biol. 2015, 191, 87–99. [Google Scholar] [CrossRef]
- Moe, E.; Sezer, M.; Hildebrandt, P.; Todorovic, S. Surface enhanced vibrational spectroscopic evidence for an alternative DNA-independent redox activation of endonuclease III. Chem. Commun. 2015, 51, 3255–3257. [Google Scholar] [CrossRef]
- Moe, E.; Rollo, F.; Silveira, C.M.; Sezer, M.; Hildebrandt, P.; Todorovic, S. Spectroelectrochemical insights into structural and redox properties of immobilized endonuclease III and its catalytically inactive mutant. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 149–154. [Google Scholar] [CrossRef]
- Mildvan, A.S.; Xia, Z.; Azurmendi, H.F.; Saraswat, V.; Legler, P.M.; Massiah, M.A.; Gabelli, S.B.; Bianchet, M.A.; Kang, L.-W.; Amzel, L.M. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 2005, 433, 129–143. [Google Scholar] [CrossRef]
- McLennan, A.G. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 2006, 63, 123–143. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Moroz, O.V.; Wilson, K.S.; Murzin, A.G. House cleaning, a part of good housekeeping. Mol. Microbiol. 2006, 59, 5–19. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Ohta, E.; Abolhassani, N. MTH1 as a nucleotide pool sanitizing enzyme: Friend or foe? Free Radic. Biol. Med. 2017, 107, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Endutkin, A.V.; Zharkov, D.O. Substrate specificities of DNA glycosylases in vitro and in vivo. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; Royal Society of Chemistry: London, UK, 2021; Volume 1, pp. 175–203. [Google Scholar] [CrossRef]
- Noll, D.M.; Gogos, A.; Granek, J.A.; Clarke, N.D. The C-terminal domain of the adenine-DNA glycosylase MutY confers specificity for 8-oxoguanine adenine mispairs and may have evolved from MutT, an 8-oxo-dGTPase. Biochemistry 1999, 38, 6374–6379. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.E.; House, P.G.; Thiviyanathan, V.; Luxon, B.A.; Zhang, S.; Lloyd, R.S.; Gorenstein, D.G. Structural similarities between MutT and the C-terminal domain of MutY. Biochemistry 2000, 39, 7331–7336. [Google Scholar] [CrossRef]
- Fromme, J.C.; Banerjee, A.; Huang, S.J.; Verdine, G.L. Structural basis for removal of adenine mispaired with 8-oxoguanine by MutY adenine DNA glycosylase. Nature 2004, 427, 652–656. [Google Scholar] [CrossRef]
- Lee, S.; Verdine, G.L. Atomic substitution reveals the structural basis for substrate adenine recognition and removal by adenine DNA glycosylase. Proc. Natl. Acad. Sci. USA 2009, 106, 18497–18502. [Google Scholar] [CrossRef]
- Russelburg, L.P.; O’Shea Murray, V.L.; Demir, M.; Knutsen, K.R.; Sehgal, S.L.; Cao, S.; David, S.S.; Horvath, M.P. Structural basis for finding OG lesions and avoiding undamaged G by the DNA glycosylase MutY. ACS Chem. Biol. 2020, 15, 93–102. [Google Scholar] [CrossRef]
- Hickerson, R.P.; Chepanoske, C.L.; Williams, S.D.; David, S.S.; Burrows, C.J. Mechanism-based DNA-protein cross-linking of MutY via oxidation of 8-oxoguanosine. J. Am. Chem. Soc. 1999, 121, 9901–9902. [Google Scholar] [CrossRef]
- Bernards, A.S.; Miller, J.K.; Bao, K.K.; Wong, I. Flipping duplex DNA inside out: A double base-flipping reaction mechanism by Escherichia coli MutY adenine glycosylase. J. Biol. Chem. 2002, 277, 20960–20964. [Google Scholar] [CrossRef]
- Wang, L.; Chakravarthy, S.; Verdine, G.L. Structural basis for the lesion-scanning mechanism of the MutY DNA glycosylase. J. Biol. Chem. 2017, 292, 5007–5017. [Google Scholar] [CrossRef]
- Nakamura, T.; Meshitsuka, S.; Kitagawa, S.; Abe, N.; Yamada, J.; Ishino, T.; Nakano, H.; Tsuzuki, T.; Doi, T.; Kobayashi, Y.; et al. Structural and dynamic features of the MutT protein in the recognition of nucleotides with the mutagenic 8-oxoguanine base. J. Biol. Chem. 2010, 285, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.M.; Jemth, A.-S.; Desroses, M.; Loseva, O.; Helleday, T.; Högbom, M.; Stenmark, P. Crystal structure of human MTH1 and the 8-oxo-dGMP product complex. FEBS Lett. 2011, 585, 2617–2621. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Cillo, J.; Clarke, N.D.; Lu, A.-L. Specific recognition of A/G and A/7,8-dihydro-8-oxoguanine (8-oxoG) mismatches by Escherichia coli MutY: Removal of the C-terminal domain preferentially affects A/8-oxoG recognition. Biochemistry 1996, 35, 16665–16671. [Google Scholar] [CrossRef] [PubMed]
- Manuel, R.C.; Lloyd, R.S. Cloning, overexpression, and biochemical characterization of the catalytic domain of MutY. Biochemistry 1997, 36, 11140–11152. [Google Scholar] [CrossRef]
- Al-Tassan, N.; Chmiel, N.H.; Maynard, J.; Fleming, N.; Livingston, A.L.; Williams, G.T.; Hodges, A.K.; Davies, D.R.; David, S.S.; Sampson, J.R.; et al. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef]
- Livingston, A.L.; Kundu, S.; Pozzi, M.H.; Anderson, D.W.; David, S.S. Insight into the roles of tyrosine 82 and glycine 253 in the Escherichia coli adenine glycosylase MutY. Biochemistry 2005, 44, 14179–14190. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.A.; Chmiel, N.H.; David, S.S. Insight into the functional consequences of hMYH variants associated with colorectal cancer: Distinct differences in the adenine glycosylase activity and the response to AP endonucleases of Y150C and G365D murine MYH. DNA Repair 2005, 4, 315–325. [Google Scholar] [CrossRef]
- Kundu, S.; Brinkmeyer, M.K.; Eigenheer, R.A.; David, S.S. Ser 524 is a phosphorylation site in MUTYH and Ser 524 mutations alter 8-oxoguanine (OG):A mismatch recognition. DNA Repair 2010, 9, 1026–1037. [Google Scholar] [CrossRef]
- Hendrich, B.; Bird, A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 1998, 18, 6538–6547. [Google Scholar] [CrossRef]
- Bellacosa, A.; Cicchillitti, L.; Schepis, F.; Riccio, A.; Yeung, A.T.; Matsumoto, Y.; Golemis, E.A.; Genuardi, M.; Neri, G. MED1, a novel human methyl-CpG-binding endonuclease, interacts with DNA mismatch repair protein MLH1. Proc. Natl. Acad. Sci. USA 1999, 96, 3969–3974. [Google Scholar] [CrossRef]
- Baubec, T.; Ivánek, R.; Lienert, F.; Schübeler, D. Methylation-dependent and -independent genomic targeting principles of the MBD protein family. Cell 2013, 153, 480–492. [Google Scholar] [CrossRef]
- Shimbo, T.; Wade, P.A. Proteins that read DNA methylation. Adv. Exp. Med. Biol. 2016, 945, 303–320. [Google Scholar] [CrossRef]
- Ginder, G.D.; Williams, D.C., Jr. Readers of DNA methylation, the MBD family as potential therapeutic targets. Pharmacol. Ther. 2018, 184, 98–111. [Google Scholar] [CrossRef]
- Kondo, E.; Gu, Z.; Horii, A.; Fukushige, S. The thymine DNA glycosylase MBD4 represses transcription and is associated with methylated p16INK4a and hMLH1 genes. Mol. Cell. Biol. 2005, 25, 4388–4396. [Google Scholar] [CrossRef]
- Fukushige, S.; Kondo, E.; Gu, Z.; Suzuki, H.; Horii, A. RET finger protein enhances MBD2- and MBD4-dependent transcriptional repression. Biochem. Biophys. Res. Commun. 2006, 351, 85–92. [Google Scholar] [CrossRef]
- Meng, H.; Harrison, D.J.; Meehan, R.R. MBD4 interacts with and recruits USP7 to heterochromatic foci. J. Cell. Biochem. 2015, 116, 476–485. [Google Scholar] [CrossRef]
- Petronzelli, F.; Riccio, A.; Markham, G.D.; Seeholzer, S.H.; Stoerker, J.; Genuardi, M.; Yeung, A.T.; Matsumoto, Y.; Bellacosa, A. Biphasic kinetics of the human DNA repair protein MED1 (MBD4), a mismatch-specific DNA N-glycosylase. J. Biol. Chem. 2000, 275, 32422–32429. [Google Scholar] [CrossRef]
- Petronzelli, F.; Riccio, A.; Markham, G.D.; Seeholzer, S.H.; Genuardi, M.; Karbowski, M.; Yeung, A.T.; Matsumoto, Y.; Bellacosa, A. Investigation of the substrate spectrum of the human mismatch-specific DNA N-glycosylase MED1 (MBD4): Fundamental role of the catalytic domain. J. Cell. Physiol. 2000, 185, 473–480. [Google Scholar] [CrossRef]
- Owen, R.M.; Baker, R.D.; Bader, S.; Dunlop, M.G.; Nicholl, I.D. The identification of a novel alternatively spliced form of the MBD4 DNA glycosylase. Oncol. Rep. 2007, 17, 111–116. [Google Scholar] [CrossRef][Green Version]
- Ramiro-Merina, Á.; Ariza, R.R.; Roldán-Arjona, T. Molecular characterization of a putative plant homolog of MBD4 DNA glycosylase. DNA Repair 2013, 12, 890–898. [Google Scholar] [CrossRef]
- Cecchini, N.M.; Torres, J.R.; López, I.L.; Cobo, S.; Nota, F.; Alvarez, M.E. Alternative splicing of an exitron determines the subnuclear localization of the Arabidopsis DNA glycosylase MBD4L under heat stress. Plant J. 2022, 110, 377–388. [Google Scholar] [CrossRef]
- Otani, J.; Arita, K.; Kato, T.; Kinoshita, M.; Kimura, H.; Suetake, I.; Tajima, S.; Ariyoshi, M.; Shirakawa, M. Structural basis of the versatile DNA recognition ability of the methyl-CpG binding domain of methyl-CpG binding domain protein 4. J. Biol. Chem. 2013, 288, 6351–6362. [Google Scholar] [CrossRef]
- Walavalkar, N.M.; Cramer, J.M.; Buchwald, W.A.; Scarsdale, J.N.; Williams, D.C., Jr. Solution structure and intramolecular exchange of methyl-cytosine binding domain protein 4 (MBD4) on DNA suggests a mechanism to scan for mCpG/TpG mismatches. Nucleic Acids Res. 2014, 42, 11218–11232. [Google Scholar] [CrossRef]
- Aziz, M.A.; Schupp, J.E.; Kinsella, T.J. Modulation of the activity of methyl binding domain protein 4 (MBD4/MED1) while processing iododeoxyuridine generated DNA mispairs. Cancer Biol. Ther. 2009, 8, 1156–1163. [Google Scholar] [CrossRef]
- Ishibashi, T.; So, K.; Cupples, C.G.; Ausió, J. MBD4-mediated glycosylase activity on a chromatin template is enhanced by acetylation. Mol. Cell. Biol. 2008, 28, 4734–4744. [Google Scholar] [CrossRef]
- Screaton, R.A.; Kiessling, S.; Sansom, O.J.; Millar, C.B.; Maddison, K.; Bird, A.; Clarke, A.R.; Frisch, S.M. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: A potential link between genome surveillance and apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 5211–5216. [Google Scholar] [CrossRef]
- Sansom, O.J.; Zabkiewicz, J.; Bishop, S.M.; Guy, J.; Bird, A.; Clarke, A.R. MBD4 deficiency reduces the apoptotic response to DNA-damaging agents in the murine small intestine. Oncogene 2003, 22, 7130–7136. [Google Scholar] [CrossRef]
- Sannai, M.; Doneddu, V.; Giri, V.; Seeholzer, S.; Nicolas, E.; Yip, S.-C.; Bassi, M.R.; Mancuso, P.; Cortellino, S.; Cigliano, A.; et al. Modification of the base excision repair enzyme MBD4 by the small ubiquitin-like molecule SUMO1. DNA Repair 2019, 82, 102687. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kondo, T.; Takada, I.; Youn, M.-Y.; Yamamoto, Y.; Takahashi, S.; Matsumoto, T.; Fujiyama, S.; Shirode, Y.; Yamaoka, I.; et al. DNA demethylation in hormone-induced transcriptional derepression. Nature 2009, 461, 1007–1012. [Google Scholar] [CrossRef]
- Antoniali, G.; Malfatti, M.C.; Tell, G. Unveiling the non-repair face of the Base Excision Repair pathway in RNA processing: A missing link between DNA repair and gene expression? DNA Repair 2017, 56, 65–74. [Google Scholar] [CrossRef]
- Malfatti, M.C.; Antoniali, G.; Codrich, M.; Burra, S.; Mangiapane, G.; Dalla, E.; Tell, G. New perspectives in cancer biology from a study of canonical and non-canonical functions of base excision repair proteins with a focus on early steps. Mutagenesis 2020, 35, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, L.; Demir, Ö.; You, P.; Sarno, A.; Amaro, R.E.; Nilsen, H. RNA metabolism guided by RNA modifications: The role of SMUG1 in rRNA quality control. Biomolecules 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Gehring, M.; Johnson, L.; Hannon, M.; Harada, J.J.; Goldberg, R.B.; Jacobsen, S.E.; Fischer, R.L. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 2002, 110, 33–42. [Google Scholar] [CrossRef]
- Gong, Z.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T.; David, L.; Zhu, J.-K. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 2002, 111, 803–814. [Google Scholar] [CrossRef]
- Cléry, A.; Blatter, M.; Allain, F.H.-T. RNA recognition motifs: Boring? Not quite. Curr. Opin. Struct. Biol. 2008, 18, 290–298. [Google Scholar] [CrossRef]
- Tang, K.; Lang, Z.; Zhang, H.; Zhu, J.-K. The DNA demethylase ROS1 targets genomic regions with distinct chromatin modifications. Nat. Plants 2016, 2, 16169. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Jost, J.-P.; Siegmann, M.; Sun, L.; Leung, R. Mechanisms of DNA demethylation in chicken embryos. Purification and properties of a 5-methylcytosine-DNA glycosylase. J. Biol. Chem. 1995, 270, 9734–9739. [Google Scholar] [CrossRef]
- Vairapandi, M.; Duker, N.J. Partial purification and characterization of human 5-methylcytosine-DNA glycosylase. Oncogene 1996, 13, 933–938. [Google Scholar]
- Cortellino, S.; Xu, J.; Sannai, M.; Moore, R.; Caretti, E.; Cigliano, A.; Le Coz, M.; Devarajan, K.; Wessels, A.; Soprano, D.; et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 2011, 146, 67–79. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y. Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef]
- Weber, A.R.; Krawczyk, C.; Robertson, A.B.; Kuśnierczyk, A.; Vågbø, C.B.; Schuermann, D.; Klungland, A.; Schär, P. Biochemical reconstitution of TET1–TDG–BER-dependent active DNA demethylation reveals a highly coordinated mechanism. Nat. Commun. 2016, 7, 10806. [Google Scholar] [CrossRef]
- Frémont, M.; Siegmann, M.; Gaulis, S.; Matthies, R.; Hess, D.; Jost, J.-P. Demethylation of DNA by purified chick embryo 5-methylcytosine-DNA glycosylase requires both protein and RNA. Nucleic Acids Res. 1997, 25, 2375–2380. [Google Scholar] [CrossRef]
- Jost, J.-P.; Frémont, M.; Siegmann, M.; Hofsteenge, J. The RNA moiety of chick embryo 5-methylcytosine-DNA glycosylase targets DNA demethylation. Nucleic Acids Res. 1997, 25, 4545–4550. [Google Scholar] [CrossRef]
- Jost, J.-P.; Schwarz, S.; Hess, D.; Angliker, H.; Fuller-Pace, F.V.; Stahl, H.; Thiry, S.; Siegmann, M. A chicken embryo protein related to the mammalian DEAD box protein p68 is tightly associated with the highly purified protein–RNA complex of 5-MeC-DNA glycosylase. Nucleic Acids Res. 1999, 27, 3245–3252. [Google Scholar] [CrossRef][Green Version]
- Vairapandi, M.; Liebermann, D.A.; Hoffman, B.; Duker, N.J. Human DNA-demethylating activity: A glycosylase associated with RNA and PCNA. J. Cell. Biochem. 2000, 79, 249–260. [Google Scholar] [CrossRef]
- Boland, M.J.; Christman, J.K. Characterization of Dnmt3b:thymine-DNA glycosylase interaction and stimulation of thymine glycosylase-mediated repair by DNA methyltransferase(s) and RNA. J. Mol. Biol. 2008, 379, 492–504. [Google Scholar] [CrossRef]
- Zhou, L.; Ren, M.; Zeng, T.; Wang, W.; Wang, X.; Hu, M.; Su, S.; Sun, K.; Wang, C.; Liu, J.; et al. TET2-interacting long noncoding RNA promotes active DNA demethylation of the MMP-9 promoter in diabetic wound healing. Cell Death Dis. 2019, 10, 813. [Google Scholar] [CrossRef]
- Gallinari, P.; Jiricny, J. A new class of uracil-DNA glycosylases related to human thymine-DNA glycosylase. Nature 1996, 383, 735–738. [Google Scholar] [CrossRef]
| Enzyme | Substrate Specificity | Structural Superfamily |
|---|---|---|
| Human | ||
| UNG | U in single- and double-stranded DNA, any context | α/β-fold |
| TDG | T, U, 3,N4-ethenoC and oxidized/deaminated derivatives of 5-methylC opposite to G in XpG dinucleotides | α/β-fold |
| SMUG1 | U in single- and double-stranded DNA, any context | α/β-fold |
| MBD4 | T and U opposite to G in XpG dinucleotides | HhH |
| NTHL1 | Oxidized pyrimidines | HhH |
| MUTYH | A and 2-OH-A opposite to G or 8-oxoguanine | HhH |
| OGG1 | 8-oxoguanine and FapyG opposite to C | HhH |
| MPG | Ring-alkylated purines, hypoxanthine, 1,N6-ethenoA | FMT_C |
| NEIL1 | Oxidized pyrimidines and purines, ring-open N7-alkylated G modifications, psoralen cross-links | H2TH |
| NEIL2 | Oxidized pyrimidines and purines in bubble DNA | H2TH |
| NEIL3 | Oxidized pyrimidines and purines in single-stranded DNA | H2TH |
| E. coli | ||
| Ung | U in single- and double-stranded DNA, any context | α/β-fold |
| Mug | U and 3,N4-ethenoC opposite to G | α/β-fold |
| Nth | Oxidized pyrimidines | HhH |
| MutY | A opposite to G or 8-oxoguanine | HhH |
| AlkA | Ring-alkylated purines, hypoxanthine, 1,N6-ethenoA | HhH |
| Tag | Ring-alkylated purines | HhH 1 |
| Fpg | 8-oxoguanine opposite to C | H2TH |
| Nei | Oxidized pyrimidines | H2TH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torgasheva, N.A.; Diatlova, E.A.; Grin, I.R.; Endutkin, A.V.; Mechetin, G.V.; Vokhtantsev, I.P.; Yudkina, A.V.; Zharkov, D.O. Noncatalytic Domains in DNA Glycosylases. Int. J. Mol. Sci. 2022, 23, 7286. https://doi.org/10.3390/ijms23137286
Torgasheva NA, Diatlova EA, Grin IR, Endutkin AV, Mechetin GV, Vokhtantsev IP, Yudkina AV, Zharkov DO. Noncatalytic Domains in DNA Glycosylases. International Journal of Molecular Sciences. 2022; 23(13):7286. https://doi.org/10.3390/ijms23137286
Chicago/Turabian StyleTorgasheva, Natalia A., Evgeniia A. Diatlova, Inga R. Grin, Anton V. Endutkin, Grigory V. Mechetin, Ivan P. Vokhtantsev, Anna V. Yudkina, and Dmitry O. Zharkov. 2022. "Noncatalytic Domains in DNA Glycosylases" International Journal of Molecular Sciences 23, no. 13: 7286. https://doi.org/10.3390/ijms23137286
APA StyleTorgasheva, N. A., Diatlova, E. A., Grin, I. R., Endutkin, A. V., Mechetin, G. V., Vokhtantsev, I. P., Yudkina, A. V., & Zharkov, D. O. (2022). Noncatalytic Domains in DNA Glycosylases. International Journal of Molecular Sciences, 23(13), 7286. https://doi.org/10.3390/ijms23137286






