Different Metabolism and Toxicity of TRANS Fatty Acids, Elaidate and Vaccenate Compared to Cis-Oleate in HepG2 Cells
Abstract
:1. Introduction
2. Results
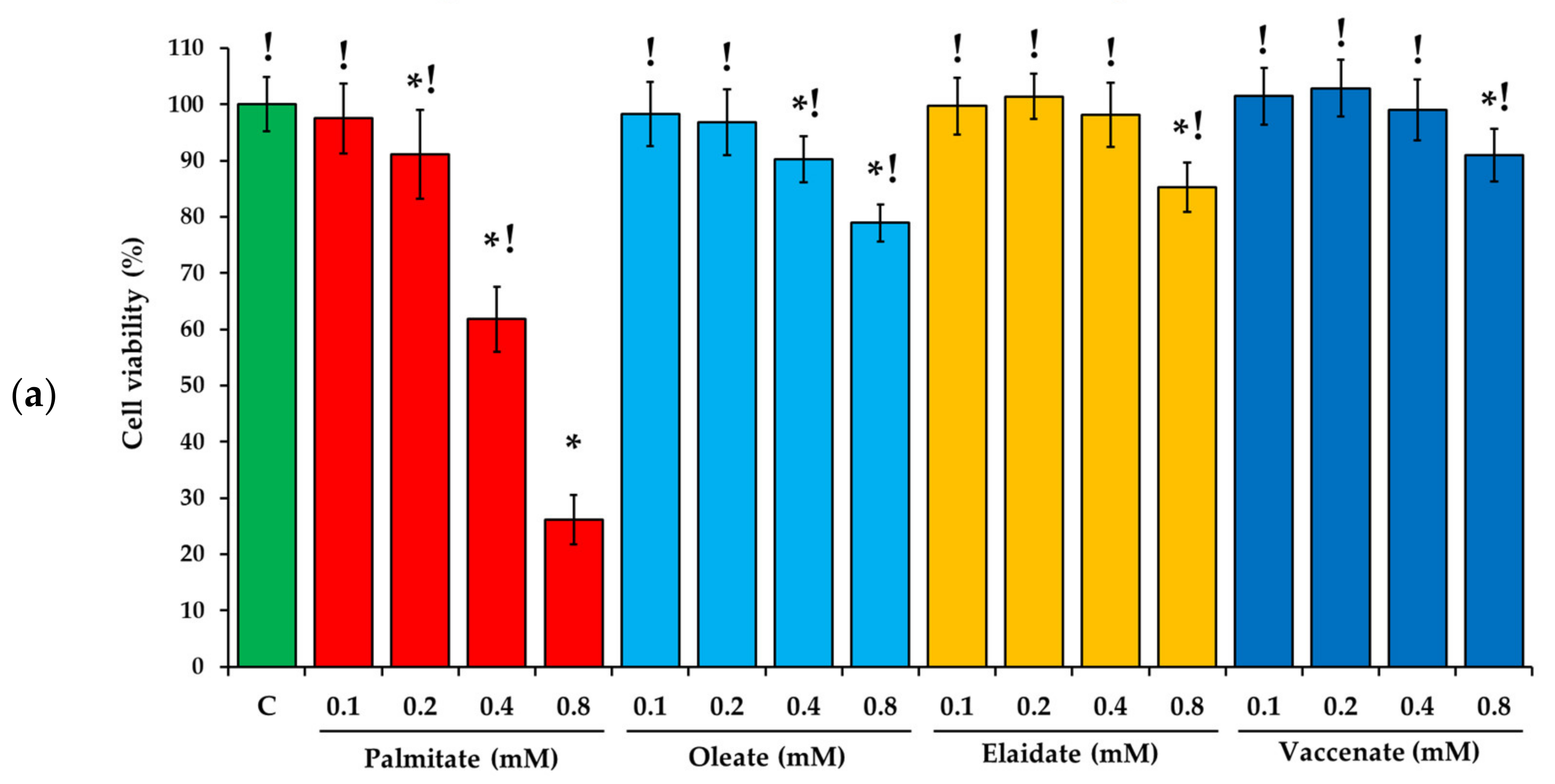
2.1. Fatty Acid Treatments and Viability of HepG2 Cells
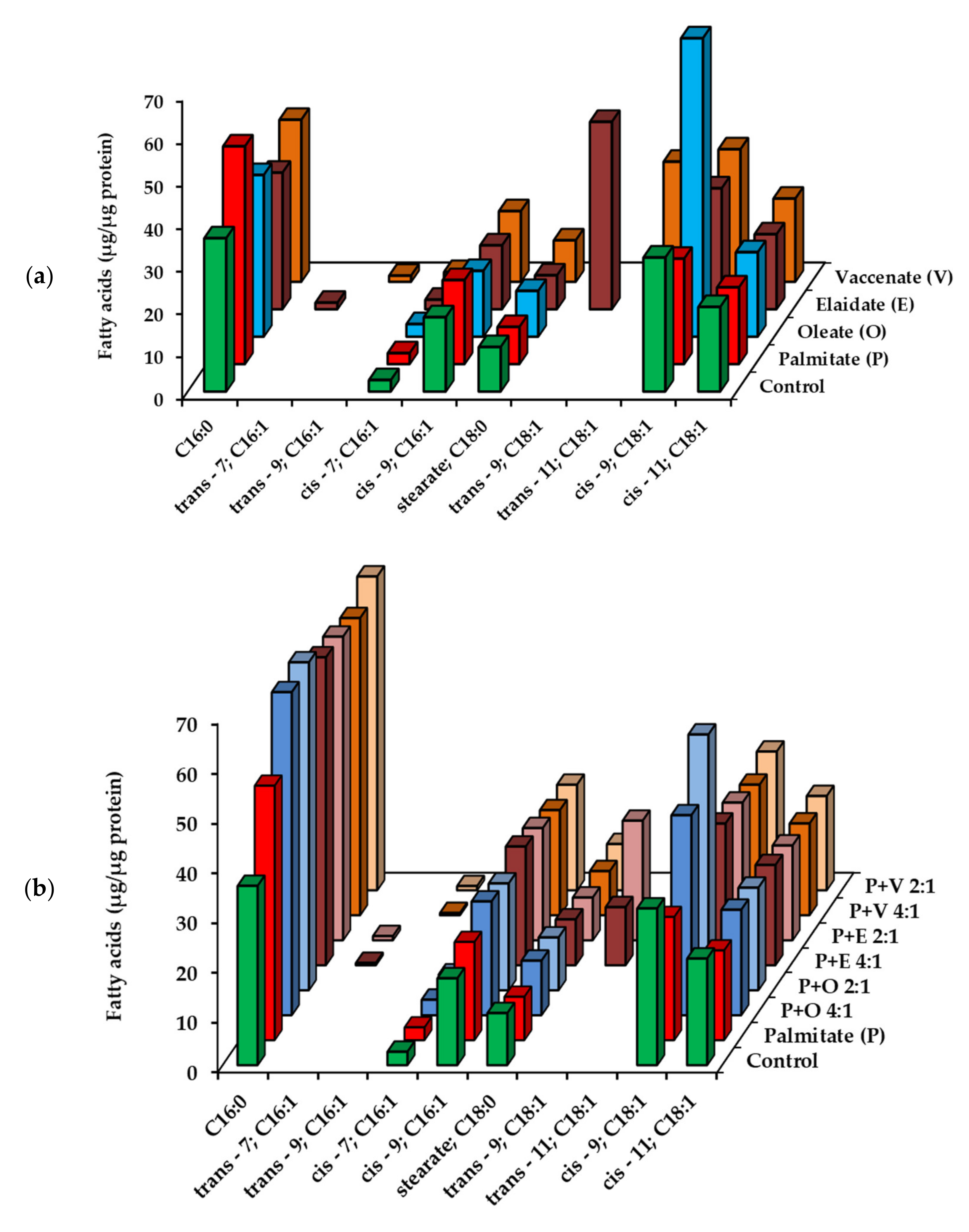
2.2. Utilization of Fatty Acids by HepG2 Cells at Low, Non-Toxic Concentration
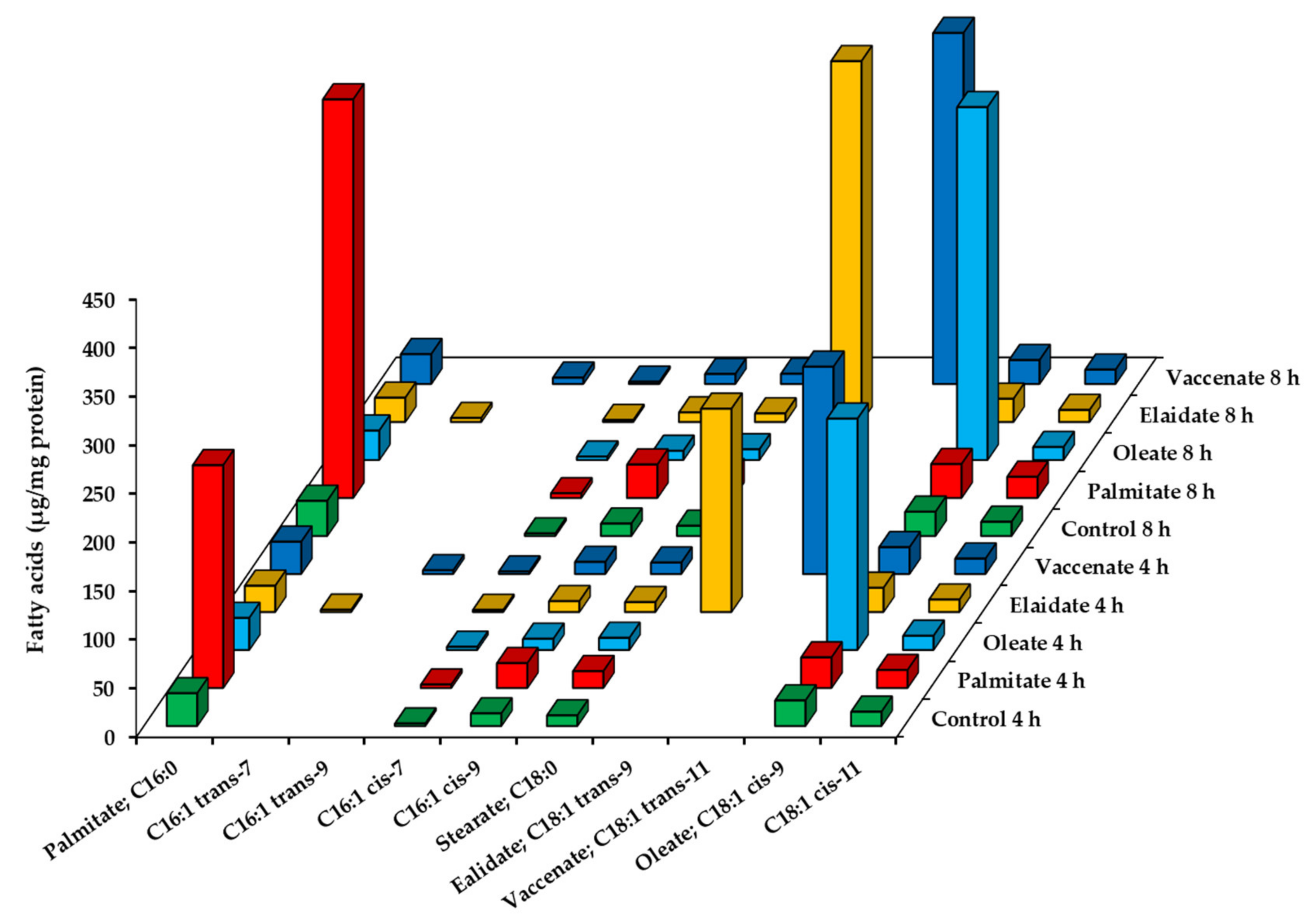
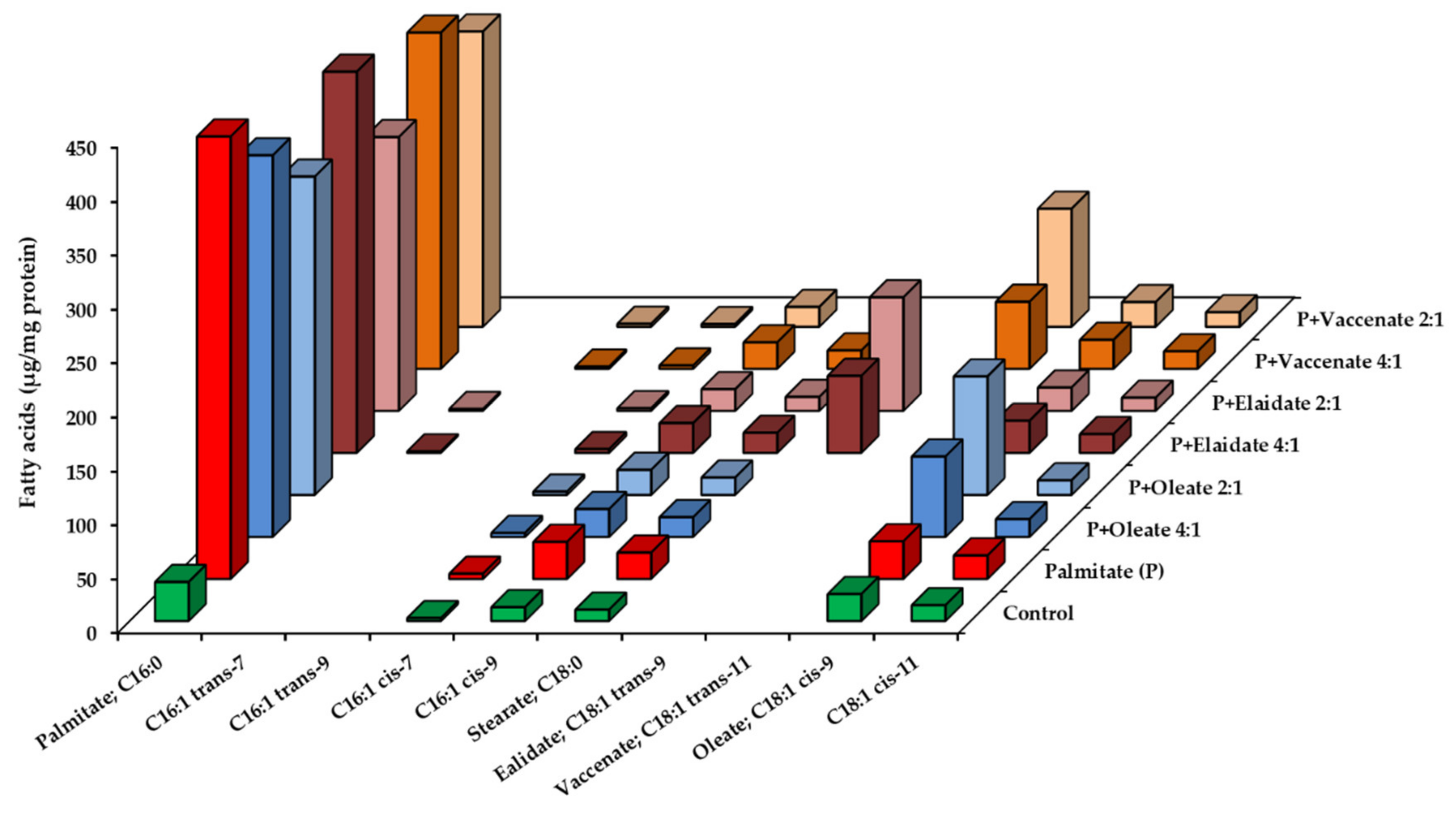
2.3. Utilization of Fatty Acids by HepG2 Cells at High, Toxic Concentration
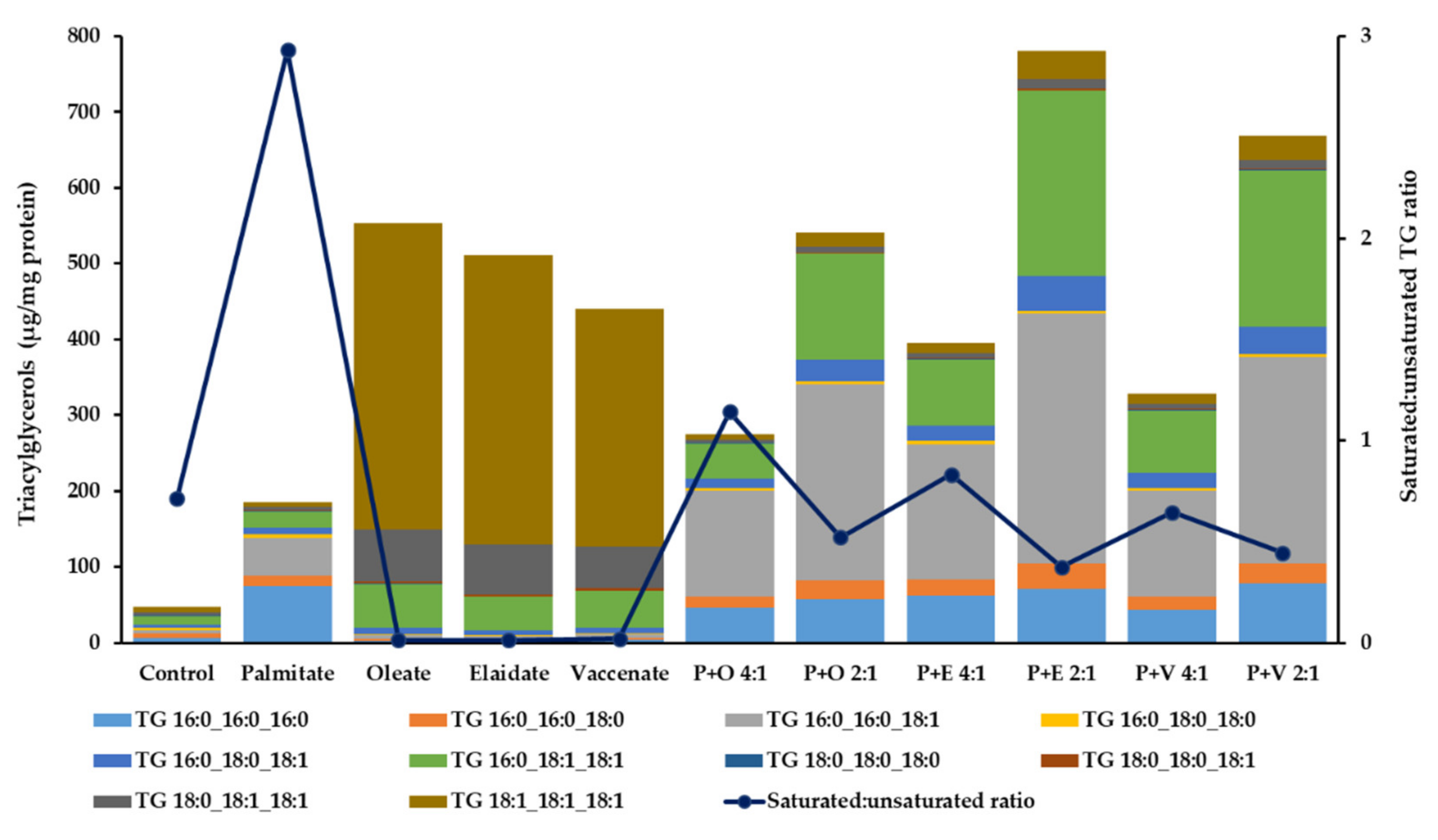
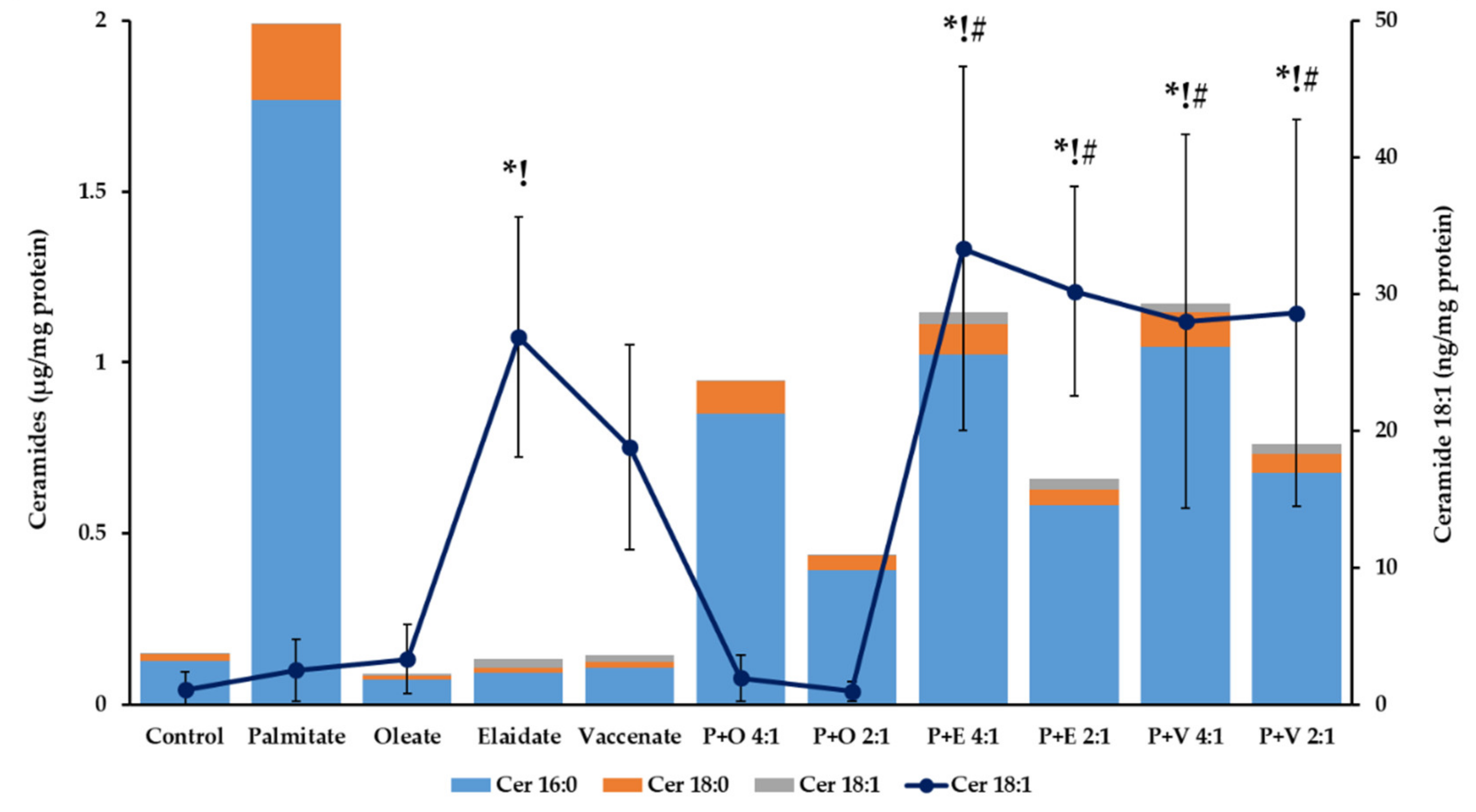
2.4. Cellular Triacylglycerol, Diacylglycerol and Ceramide Contents in Lipotoxicity
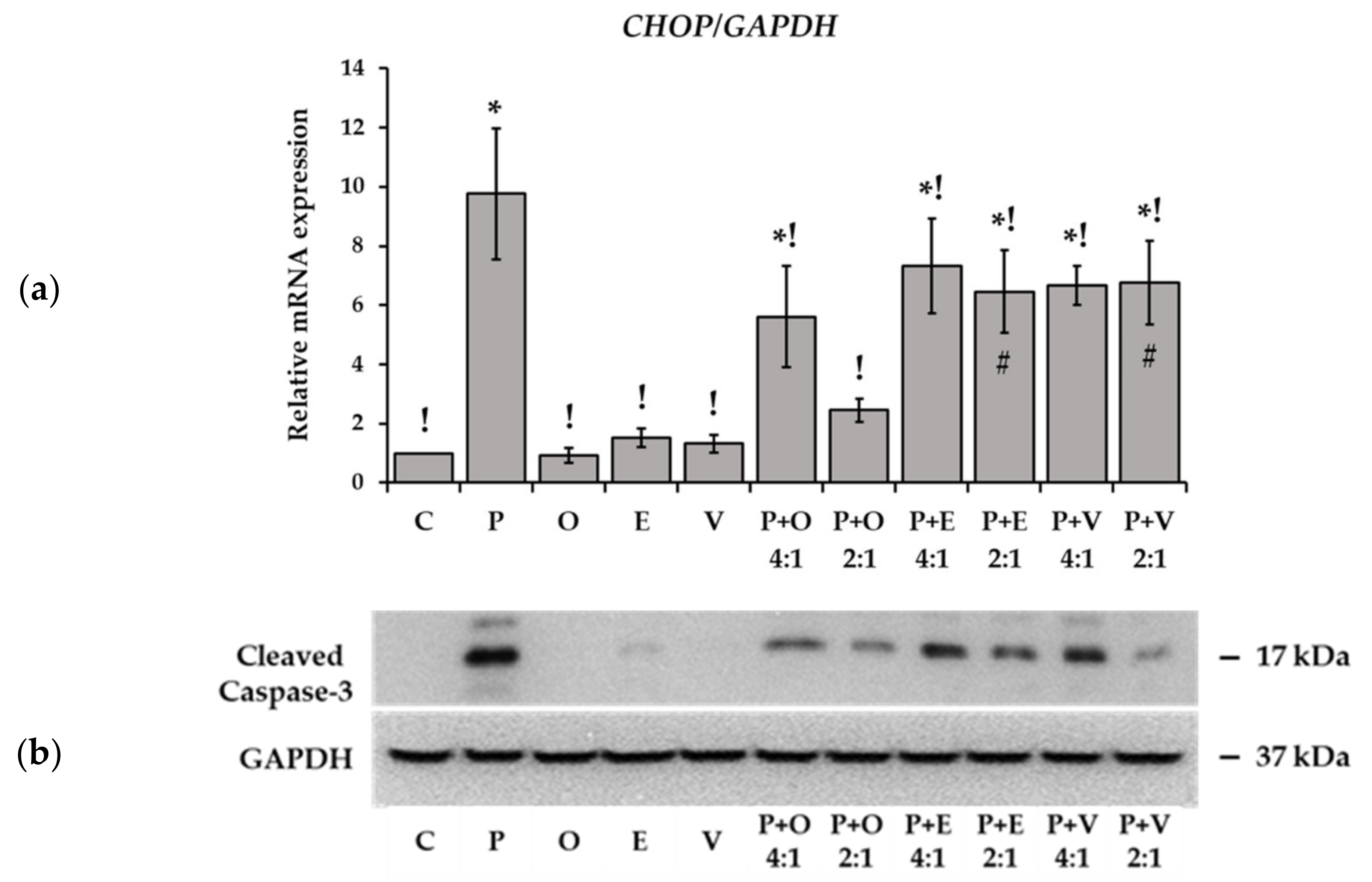
2.5. Markers of Stress and Apoptosis in Fatty Acid Treated Cells
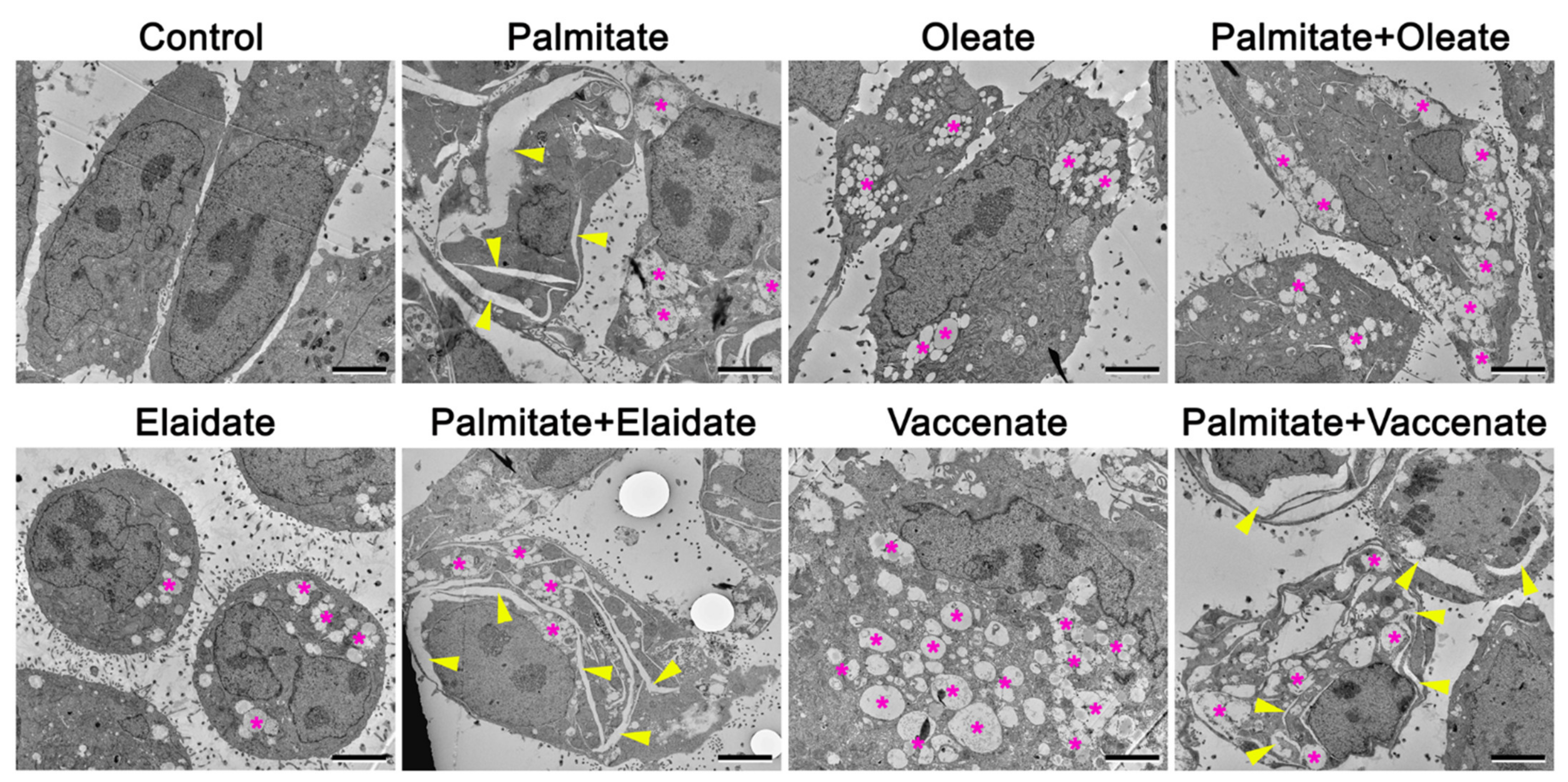
2.6. Changes in Cellular Ultrastructure upon High Concentration Fatty Acid Supplementations
3. Discussion
4. Materials and Methods
4.1. Materials Used
4.2. Cell Culture
4.3. Cell Treatment with BSA-Conjugated Fatty Acids
4.4. Analysis of Lipid Contents
4.4.1. GC-FID Analysis of Fatty Acid Profiles
4.4.2. HPLC-MS/MS Analysis of Triacylglycerols, Diacylglycerols and Ceramides
4.5. Electron Microscopy
4.6. Cell Viability
4.7. Western Blot Analysis
4.8. qPCR Analysis
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Kuhnt, K.; Baehr, M.; Rohrer, C.; Jahreis, G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur. J. Lipid Sci. Technol. 2011, 113, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, C.M.; Edel, A.L.; Patenaude, A.F.; McCullough, R.S.; Blackwood, D.P.; Chouinard, P.Y.; Paquin, P.; Lamarche, B.; Pierce, G.N. Dietary vaccenic acid has antiatherogenic effects in LDLr−/− mice. J. Nutr. 2010, 140, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D. Natural trans fat, dairy fat, partially hydrogenated oils, and cardiometabolic health: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. Heart J. 2016, 37, 1079–1081. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Salmeron, J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Willett, W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001, 73, 1019–1026. [Google Scholar] [CrossRef]
- Wang, X.; Gupta, J.; Kerslake, M.; Rayat, G.; Proctor, S.D.; Chan, C.B. Trans-11 vaccenic acid improves insulin secretion in models of type 2 diabetes in vivo and in vitro. Mol. Nutr. Food Res. 2016, 60, 846–857. [Google Scholar] [CrossRef]
- Prada, M.; Wittenbecher, C.; Eichelmann, F.; Wernitz, A.; Kuxhaus, O.; Kroger, J.; Weikert, C.; Schulze, M.B. Plasma Industrial and Ruminant Trans Fatty Acids and Incident Type 2 Diabetes in the EPIC-Potsdam Cohort. Diabetes Care 2022, 45, 845–853. [Google Scholar] [CrossRef]
- Sarnyai, F.; Donko, M.B.; Matyasi, J.; Gor-Nagy, Z.; Marczi, I.; Simon-Szabo, L.; Zambo, V.; Somogyi, A.; Csizmadia, T.; Low, P.; et al. Cellular toxicity of dietary trans fatty acids and its correlation with ceramide and diglyceride accumulation. Food Chem. Toxicol. 2019, 124, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Sarnyai, F.; Somogyi, A.; Gor-Nagy, Z.; Zambo, V.; Szelenyi, P.; Matyasi, J.; Simon-Szabo, L.; Kereszturi, E.; Toth, B.; Csala, M. Effect of cis- and trans-Monounsaturated Fatty Acids on Palmitate Toxicity and on Palmitate-induced Accumulation of Ceramides and Diglycerides. Int. J. Mol. Sci. 2020, 21, 2626. [Google Scholar] [CrossRef] [Green Version]
- Summers, S.A. Editorial: The Role of Ceramides in Diabetes and Cardiovascular Disease. Front. Endocrinol. 2021, 12, 667885. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. De novo sphingolipid biosynthesis: A necessary, but dangerous, pathway. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef] [Green Version]
- Tippetts, T.S.; Holland, W.L.; Summers, S.A. Cholesterol—The devil you know; ceramide—The devil you don’t. Trends Pharmacol. Sci. 2021, 42, 1082–1095. [Google Scholar] [CrossRef]
- Hirsch, J.; Farquhar, J.W.; Ahrens, E.H., Jr.; Peterson, M.L.; Stoffel, W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am. J. Clin. Nutr. 1960, 8, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, R.N.; King, I.B.; Patterson, R.E.; Psaty, B.M.; Kestin, M.; Heckbert, S.R. Assessment of trans-fatty acid intake with a food frequency questionnaire and validation with adipose tissue levels of trans-fatty acids. Am. J. Epidemiol. 1998, 148, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.P.; Li, D.; Sinclair, A.J. The occurrence of trans-18:1 isomers in plasma lipids classes in humans. Eur. J. Clin. Nutr. 2001, 55, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef]
- Abraham, R.A.; Bahl, V.K.; Parshad, R.; Seenu, V.; Roy, A.; Golandaz, S.; Dorairaj, P.; Ramakrishnan, L. Content of trans fatty acids in human cheek epithelium: Comparison with serum and adipose tissue. Biomed Res. Int. 2013, 2013, 276174. [Google Scholar] [CrossRef] [Green Version]
- Johnston, P.V.; Johnson, O.C.; Kummerow, F.A. Occurrence of trans fatty acids in human tissue. Science 1957, 126, 698–699. [Google Scholar] [CrossRef]
- Zambo, V.; Simon-Szabo, L.; Szelenyi, P.; Kereszturi, E.; Banhegyi, G.; Csala, M. Lipotoxicity in the liver. World J. Hepatol. 2013, 5, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Sommerweiss, D.; Gorski, T.; Richter, S.; Garten, A.; Kiess, W. Oleate rescues INS-1E beta-cells from palmitate-induced apoptosis by preventing activation of the unfolded protein response. Biochem. Biophys. Res. Commun. 2013, 441, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef] [Green Version]
- Scholz, A.; Navarrete-Munoz, E.M.; Garcia-de-la-Hera, M.; Fernandez-Somoano, A.; Tardon, A.; Santa-Marina, L.; Pereda-Pereda, E.; Romaguera, D.; Guxens, M.; Beneito, A.; et al. Association between trans fatty acid intake and overweight including obesity in 4 to 5-year-old children from the INMA study. Pediatr. Obes. 2019, 14, e12528. [Google Scholar] [CrossRef]
- Wigger, D.; Gulbins, E.; Kleuser, B.; Schumacher, F. Monitoring the Sphingolipid de novo Synthesis by Stable-Isotope Labeling and Liquid Chromatography-Mass Spectrometry. Front. Cell Dev. Biol. 2019, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Vendel Nielsen, L.; Krogager, T.P.; Young, C.; Ferreri, C.; Chatgilialoglu, C.; Norregaard Jensen, O.; Enghild, J.J. Effects of elaidic acid on lipid metabolism in HepG2 cells, investigated by an integrated approach of lipidomics, transcriptomics and proteomics. PLoS ONE 2013, 8, e74283. [Google Scholar] [CrossRef] [Green Version]
- Krogager, T.P.; Nielsen, L.V.; Kahveci, D.; Dyrlund, T.F.; Scavenius, C.; Sanggaard, K.W.; Enghild, J.J. Hepatocytes respond differently to major dietary trans fatty acid isomers, elaidic acid and trans-vaccenic acid. Proteome Sci. 2015, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.Y.; Degrace, P.; Gresti, J.; Loreau, O.; Clouet, P. Vaccenic and elaidic acid equally esterify into triacylglycerols, but differently into phospholipids of fed rat liver cells. Lipids 2011, 46, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Woldseth, B.; Retterstol, K.; Christophersen, B.O. Monounsaturated trans fatty acids, elaidic acid and trans-vaccenic acid, metabolism and incorporation in phospholipid molecular species in hepatocytes. Scand. J. Clin. Lab. Investig. 1998, 58, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.B.; Zou, Q.; Lv, X.; Zhou, R.L.; Niu, X.; Weng, C.; Chen, F.; Fan, Y.W.; Deng, Z.Y.; Li, J. 9t18:1 and 11t18:1 activate the MAPK pathway to regulate the expression of PLA2 and cause inflammation in HUVECs. Food Funct. 2020, 11, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, S.B.; He, Y.M.; Zhuo, C.F.; Zhou, R.L.; Chen, F.; Li, H.Y.; Deng, Z.Y. 9c11tCLA modulates 11t18:1 and 9t18:1 induced inflammations differently in human umbilical vein endothelial cells. Sci. Rep. 2018, 8, 1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, C.A.; Baker, E.J.; De Souza, C.O.; Miles, E.A.; Calder, P.C. Differential Effects of Ruminant and Industrial 18-Carbon trans-Monounsaturated Fatty Acids (trans Vaccenic and Elaidic) on the Inflammatory Responses of an Endothelial Cell Line. Molecules 2021, 26, 5834. [Google Scholar] [CrossRef] [PubMed]
- Van Greevenbroek, M.M.; Robertus-Teunissen, M.G.; Erkelens, D.W.; de Bruin, T.W. Lipoprotein secretion by intestinal Caco-2 cells is affected differently by trans and cis unsaturated fatty acids: Effect of carbon chain length and position of the double bond. Am. J. Clin. Nutr. 1998, 68, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haj Ahmed, S.; Kharroubi, W.; Zarrouk, A.; Brahmi, F.; Nury, T.; Lizard, G.; Hammami, M. Protective effects of bezafibrate against elaidic acid-induced accumulation of lipid droplets in monocytic cells. Curr. Res. Transl. Med. 2017, 65, 20–30. [Google Scholar] [CrossRef]
- Oteng, A.B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Ma, W.W.; Zhao, L.; Yuan, L.H.; Yu, H.L.; Wang, H.; Gong, X.Y.; Wei, F.; Xiao, R. Elaidic acid induces cell apoptosis through induction of ROS accumulation and endoplasmic reticulum stress in SHSY5Y cells. Mol. Med. Rep. 2017, 16, 9337–9346. [Google Scholar] [CrossRef] [Green Version]
- Sauvat, A.; Chen, G.; Muller, K.; Tong, M.; Aprahamian, F.; Durand, S.; Cerrato, G.; Bezu, L.; Leduc, M.; Franz, J.; et al. Trans-Fats Inhibit Autophagy Induced by Saturated Fatty Acids. EBioMedicine 2018, 30, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Somogyi, A.; Mátyási, J.; Gór-Nagy, Z.; Sarnyai, F.; Csala, M.; Tóth, B. Application of Gas Chromatography—Flame Ionization Detection to Study Cellular Incorporation of Dietary Trans Fatty Acids of Medical Importance. Period. Polytech. Chem. Eng. 2021, 65, 149–157. [Google Scholar] [CrossRef]
- Somogyi, A.; Berinkeiné Donkó, M.; Sarnyai, F.; Becskereki, G.; Csala, M.; Tóth, B. Simultaneous Quantitative Determination of Different Ceramide and Diacylglycerol Species in Cultured Cells by Using Liquid Chromatography–Electrospray Tandem Mass Spectrometry. Period. Polytech. Chem. Eng. 2020, 64, 421–429. [Google Scholar] [CrossRef]



| Treatment (8 h) | TGs 1 (μg/mg Protein) | DGs 2 (ng/mg Protein) | Ceramides 3 (ng/mg Protein) |
|---|---|---|---|
| Control | 31.4 ± 11.4 ! | 333.9 ± 39.1 ! | 159.6 ± 20.2 ! |
| Palmitate | 64.4 ± 24.0 * | 560.2 ± 100.3 * | 237.3 ± 12.3 * |
| cis-Oleate | 52.7 ± 17.2 | 388.9 ± 81.9 ! | 98.3 ± 16.4 *,! |
| trans-Elaidate | 45.8 ± 13.6 | 443.0 ± 68.0 ! | 91.9 ± 16.4 *,! |
| trans-Vaccenate | 48.4 ± 8.6 | 365.1 ± 67.1 ! | 120.7 ± 25.4 *,! |
| Palmitate + Oleate (4:1) | 59.0 ± 15.3 | 506.4 ± 72.0 * | 208.4 ± 37.3 * |
| Palmitate + Oleate (2:1) | 97.7 ± 37.7 *,! | 514.4 ± 94.7 * | 210.6 ± 29.5 * |
| Palmitate + Elaidate (4:1) | 56.1 ± 16.9 | 514.4 ± 45.0 * | 202.5 ± 35.6 * |
| Palmitate + Elaidate (2:1) | 96.3 ± 32.0 *,! | 586.1 ± 114.8 * | 207.5 ± 33.5 * |
| Palmitate + Vaccenate (4:1) | 61.1 ± 12.9 * | 538.8 ± 113.7 * | 214.9 ± 38.4 * |
| Palmitate + Vaccenate (2:1) | 98.2 ± 29.4 *,! | 502.9 ± 107.1 * | 212.4 ± 32.9 * |
| Overall FA Content 1 (μg/mg Protein) | ||
|---|---|---|
| Treatment | at 4 h | at 8 h |
| Control | 101.0 ± 21.1 ! | 101.5 ± 30.7 ! |
| Palmitate | 325.3 ± 43.8 * | 507.0 ± 101.6 * |
| cis-Oleate | 312.4 ± 36.3 * | 429.9 ± 47.2 * |
| trans-Elaidate | 299.2 ± 78.6 * | 456.7 ± 114.6 * |
| trans-Vaccenate | 320.0 ± 46.7 * | 460.3 ± 80.1 * |
| Palmitate + Oleate (4:1) | 326.5 ± 58.0 * | 492.0 ± 100.5 * |
| Palmitate + Oleate (2:1) | 312.1 ± 25.3 * | 460.2 ± 97.8 * |
| Palmitate + Elaidate (4:1) | 336.8 ± 56.5 * | 522.3 ± 120.9 * |
| Palmitate + Elaidate (2:1) | 331.0 ± 34.2 * | 429.4 ± 93.4 * |
| Palmitate + Vaccenate (4:1) | 323.7 ± 70.8 * | 463.0 ± 83.2 * |
| Palmitate + Vaccenate (2:1) | 348.6 ± 67.1 * | 457.1 ± 77.6 * |
| Overall DG Content 1 (μg/mg Protein) | ||
|---|---|---|
| Treatment | at 4 h | at 8 h |
| Control | 0.3 ± 0.1 ! | 0.3 ± 0.1 ! |
| Palmitate | 8.1 ± 2.3 * | 18.0 ± 2.3 * |
| cis-Oleate | 1.1 ± 0.2 ! | 1.5 ± 0.4 ! |
| trans-Elaidate | 1.7 ± 0.3 ! | 2.9 ± 0.4 ! |
| trans-Vaccenate | 1.4 ± 0.3 ! | 2.4 ± 0.4 ! |
| Palmitate + Oleate (4:1) | 3.7 ± 1.1 *,! | 8.9 ± 3.0 *,! |
| Palmitate + Oleate (2:1) | 2.1 ± 0.6 ! | 3.3 ± 1.1 ! |
| Palmitate + Elaidate (4:1) | 4.3 ± 1.6 *,! | 10.3 ± 3.1 *,! |
| Palmitate + Elaidate (2:1) | 2.8 ± 0.9 ! | 5.7 ± 1.6 ! |
| Palmitate + Vaccenate (4:1) | 5.1 ± 1.8 *,! | 11.2 ± 2.7 * |
| Palmitate + Vaccenate (2:1) | 3.8 ± 1.4 *,! | 7.3 ± 2.9 *,! |
| Overall Ceramide Content 1 (ng/mg Protein) | ||
|---|---|---|
| Treatment | at 4 h | at 8 h |
| Control | 171.1 ± 12.3 ! | 149.3 ± 52.4 ! |
| Palmitate | 765.3 ± 197.4 * | 1 993.9 ± 100.5 * |
| cis-Oleate | 134.8 ± 24.8 ! | 88.7 ± 25.7 ! |
| trans-Elaidate | 195.5 ± 18.0 ! | 133.8 ± 38.7 ! |
| trans-Vaccenate | 175.7 ± 23.0 ! | 144.5 ± 51.1 ! |
| Palmitate + Oleate (4:1) | 502.9 ± 125.1 *,! | 946.4 ± 593.6 *,! |
| Palmitate + Oleate (2:1) | 393.1 ± 90.9 ! | 435.5 ± 85.3 ! |
| Palmitate + Elaidate (4:1) | 591.4 ± 130.7 * | 1 146.4 ± 204.7 *,! |
| Palmitate + Elaidate (2:1) | 421.7 ± 95.0 ! | 659.6 ± 59.4 ! |
| Palmitate + Vaccenate (4:1) | 539.5 ± 137.8 * | 1 173.4 ± 466.2 *,! |
| Palmitate + Vaccenate (2:1) | 450.0 ± 113.9 *,! | 760.0 ± 281.4 ! |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarnyai, F.; Kereszturi, É.; Szirmai, K.; Mátyási, J.; Al-Hag, J.I.; Csizmadia, T.; Lőw, P.; Szelényi, P.; Tamási, V.; Tibori, K.; et al. Different Metabolism and Toxicity of TRANS Fatty Acids, Elaidate and Vaccenate Compared to Cis-Oleate in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 7298. https://doi.org/10.3390/ijms23137298
Sarnyai F, Kereszturi É, Szirmai K, Mátyási J, Al-Hag JI, Csizmadia T, Lőw P, Szelényi P, Tamási V, Tibori K, et al. Different Metabolism and Toxicity of TRANS Fatty Acids, Elaidate and Vaccenate Compared to Cis-Oleate in HepG2 Cells. International Journal of Molecular Sciences. 2022; 23(13):7298. https://doi.org/10.3390/ijms23137298
Chicago/Turabian StyleSarnyai, Farkas, Éva Kereszturi, Kitti Szirmai, Judit Mátyási, Johanna Iman Al-Hag, Tamás Csizmadia, Péter Lőw, Péter Szelényi, Viola Tamási, Kinga Tibori, and et al. 2022. "Different Metabolism and Toxicity of TRANS Fatty Acids, Elaidate and Vaccenate Compared to Cis-Oleate in HepG2 Cells" International Journal of Molecular Sciences 23, no. 13: 7298. https://doi.org/10.3390/ijms23137298








