The Relationship between COVID-19 and Hypothalamic–Pituitary–Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess—The CAPISCO International Expert Panel
Abstract
:1. Introduction
2. Glucocorticoids in the Management of COVID-19 Patients
3. Glucocorticoid-Induced Adrenal Insufficiency in COVID-19 Patients
4. The Impact of COVID-19 Infection on Hypothalamic–Pituitary–Adrenal Axis
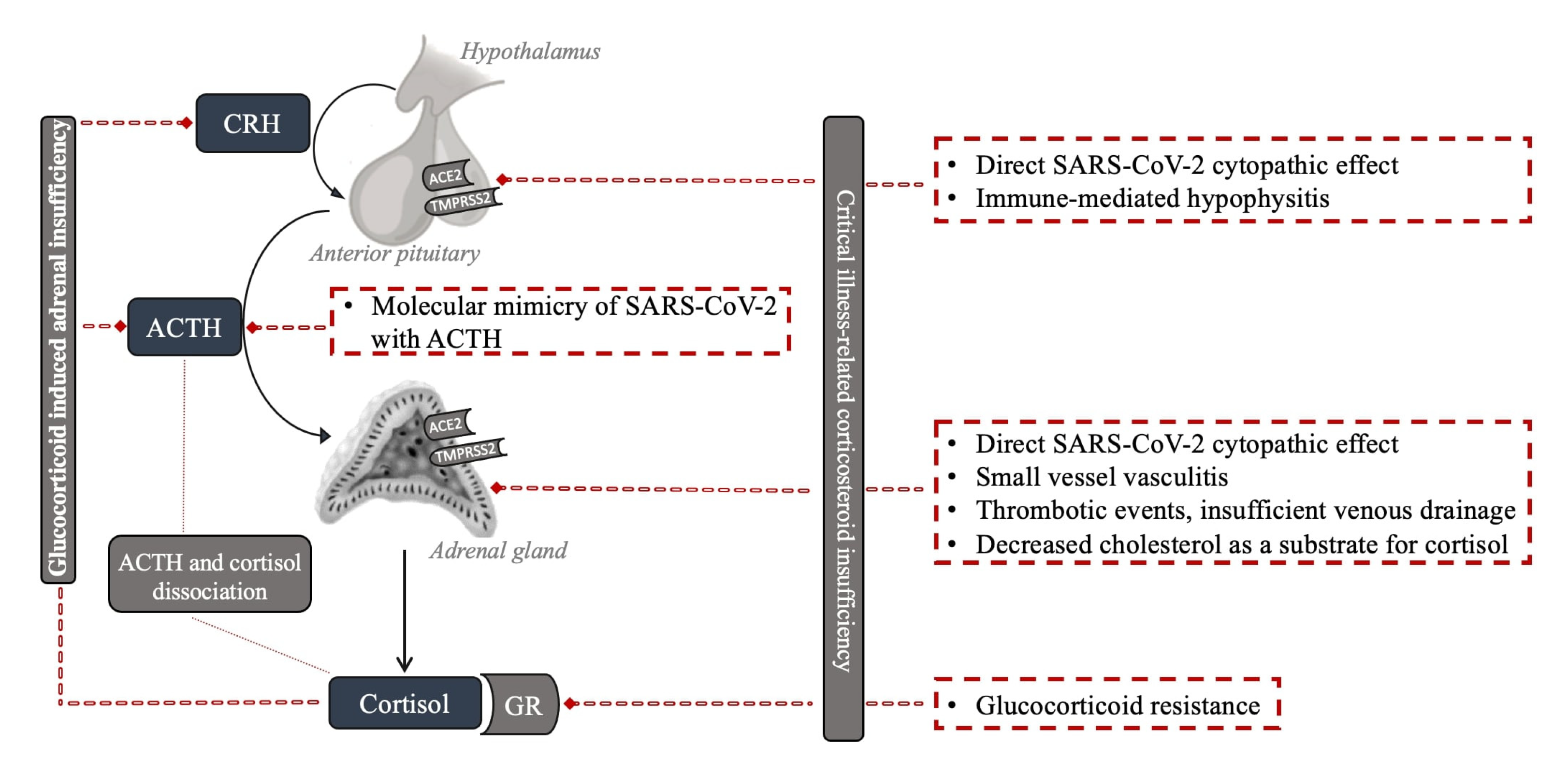
4.1. Critical Illness-Related Corticosteroid Insufficiency
4.2. Adrenal Gland
4.3. Pituitary Gland and Hypothalamus
4.4. Resistance to Cortisol Action at the Level of Glucocorticoid Receptor and Postreceptor Signaling
4.5. Dissociation between Cortisol and ACTH Regulation
4.6. Clinical Data during Acute COVID-19 Infection
4.7. Clinical Data in Patients Who Survive COVID-19 Infection
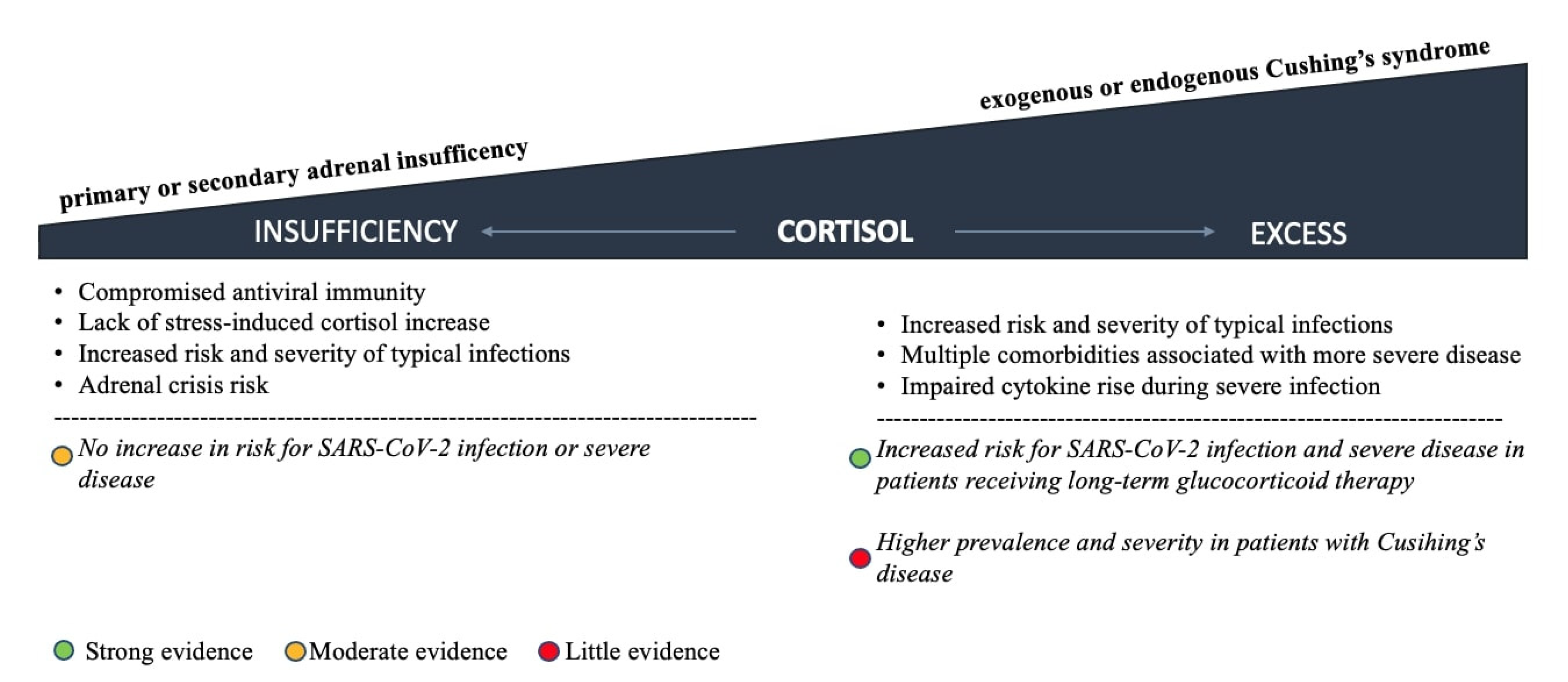
5. Pre-Existing HPA Axis Disorders and COVID-19 Outcomes
5.1. Adrenal Insufficiency
5.2. Glucocorticoid Excess
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puig-Domingo, M.; Marazuela, M.; Yildiz, B.O.; Giustina, A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine 2021, 72, 301–316. [Google Scholar] [CrossRef]
- Clarke, S.A.; Abbara, A.; Dhillo, W.S. Impact of COVID-19 on the Endocrine System: A Mini-review. Endocrinology 2021, 163, bqab203. [Google Scholar] [CrossRef]
- Mung, S.M.; Jude, E.B. Interplay between endocrinology, metabolism and COVID-19 infection. Clin. Med. 2021, 21, e499–e504. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. In fatal COVID-19, the immune response can control the virus but kill the patient. Proc. Natl. Acad. Sci. USA 2020, 117, 30009–30011. [Google Scholar] [CrossRef]
- Ferraù, F.; Ceccato, F.; Cannavò, S.; Scaroni, C. What we have to know about corticosteroids use during Sars-Cov-2 infection. J. Endocrinol. Investig. 2021, 44, 693–701. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Henneicke, H. The Role of Glucocorticoids in the Management of COVID-19. Horm. Metab. Res. 2021, 53, 9–15. [Google Scholar] [CrossRef]
- Luo, M.-H.; Qian, Y.-Q.; Huang, D.-L.; Luo, J.-C.; Su, Y.; Wang, H.; Yu, S.-J.; Liu, K.; Tu, G.-W.; Luo, Z. Tailoring glucocorticoids in patients with severe COVID-19: A narrative review. Ann. Transl. Med. 2021, 9, 1261. [Google Scholar] [CrossRef]
- Landolf, K.M.; Lemieux, S.M.; Rose, C.; Johnston, J.P.; Adams, C.D.; Altshuler, J.; Berger, K.; Dixit, D.; Effendi, M.K.; Heavner, M.S.; et al. Corticosteroid use in ARDS and its application to evolving therapeutics for coronavirus disease 2019 (COVID-19): A systematic review. Pharmacotherapy 2022, 42, 71–90. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (2019-nCoV) Infection is Suspected: Interim Guidance, 28 January 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/330893 (accessed on 2 April 2022).
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Rochwerg, B.; Siemieniuk, R.A.; Agoritsas, T.; Lamontagne, F.; Askie, L.; Lytvyn, L.; Agarwal, A.; Leo, Y.-S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for covid-19. BMJ 2020, 370, m3379. [Google Scholar]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2022. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 2 April 2022).
- Veronese, N.; Demurtas, J.; Yang, L.; Tonelli, R.; Barbagallo, M.; Lopalco, P.; Lagolio, E.; Celotto, S.; Pizzol, D.; Zou, L.; et al. Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature. Front. Med. 2020, 7, 170. [Google Scholar] [CrossRef]
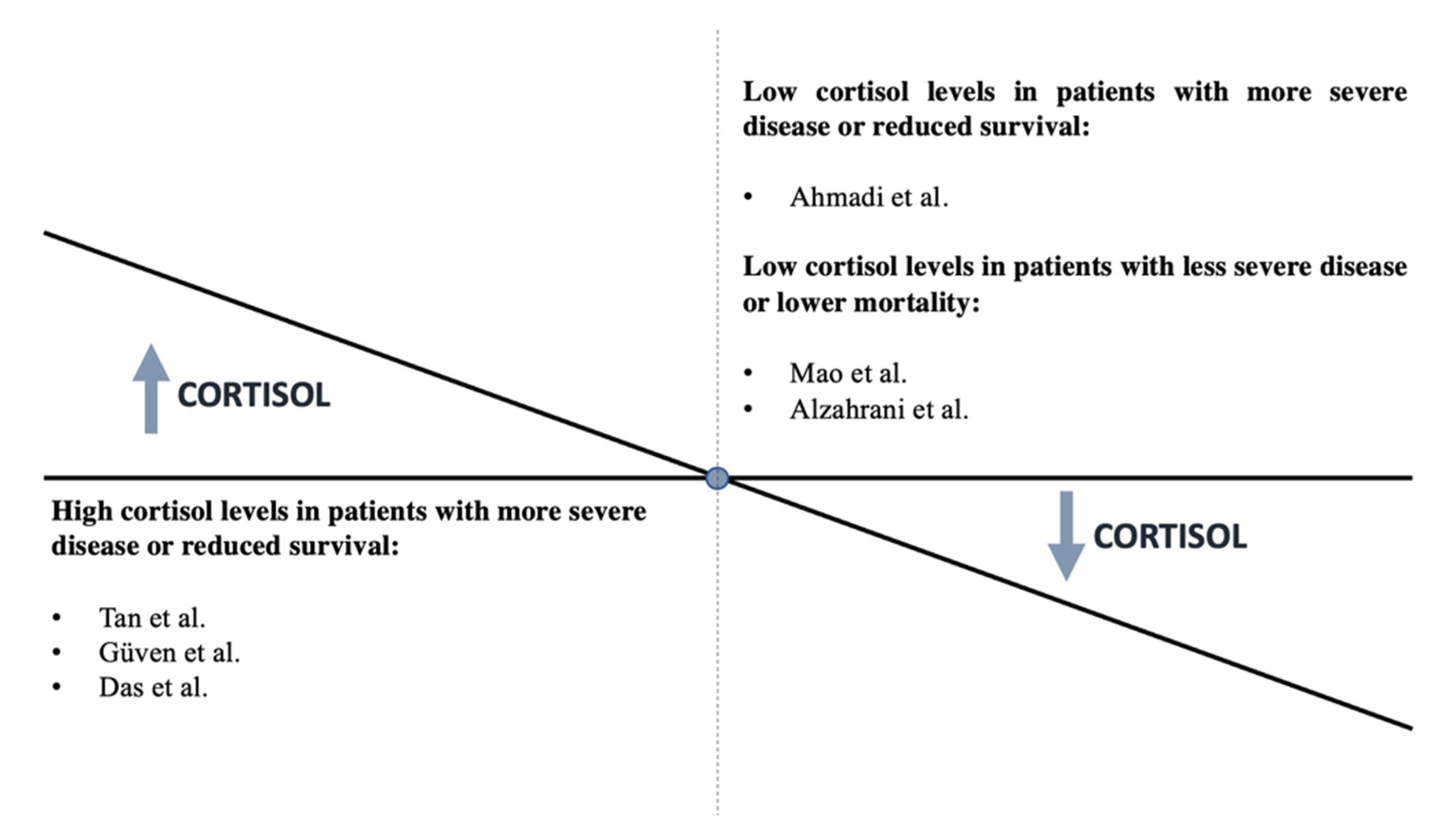
- Mao, Y.; Xu, B.; Guan, W.; Xu, D.; Li, F.; Ren, R.; Zhu, X.; Gao, Y.; Jiang, L. The Adrenal Cortex, an Underestimated Site of SARS-CoV-2 Infection. Front. Endocrinol 2020, 11, 593179. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Mukhtar, N.; Aljomaiah, A.; Aljamei, H.; Bakhsh, A.; Alsudani, N.; Elsayed, N.; Alrashidi, N.; Fadel, R.; Alsudani, N. The Impact of COVID-19 Viral Infection on the Hypothalamic-Pituitary-Adrenal Axis. Endocr Pr. Off. J. Am. Coll Endocrinol Am. Assoc. Clin. Endocrinol. 2021, 27, 83–89. [Google Scholar] [CrossRef]
- Edwards, C. New Horizons: Does Mineralocorticoid Receptor Activation by Cortisol Cause ATP Release and COVID-19 Complications? J. Clin. Endocrinol Metab. 2021, 106, 622–635. [Google Scholar] [CrossRef]
- Vogel, F.; Reincke, M. Endocrine risk factors for COVID-19: Endogenous and exogenous glucocorticoid excess. Rev. Endocr. Metab. Disord. 2022, 23, 233–250. [Google Scholar] [CrossRef]
- Pinzón, M.A.; Ortiz, S.; Holguín, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias, C.A.; Muñoz, B.; Quiceno, J.; Jaramillo, D.; et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef]
- Ranjbar, K.; Moghadami, M.; Mirahmadizadeh, A.; Fallahi, M.J.; Khaloo, V.; Shahriarirad, R.; Erfani, A.; Khodamoradi, Z.; Saadi, M. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021, 21, 337. [Google Scholar]
- Van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gogali, A.; Kyriakopoulos, C.; Kostikas, K. Corticosteroids in COVID-19: One size does not fit all. Eur. Respir. J. 2021, 57, 2100224. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, Y. Glucocorticoids are double-edged sword in the treatment of COVID-19 and cancers. Int. J. Biol. Sci. 2021, 17, 1530–1537. [Google Scholar] [CrossRef]
- Li, J.; Liao, X.; Zhou, Y.; Wang, L.; Yang, H.; Zhang, W.; Zhang, Z.; Kang, Y. Association between glucocorticoids treatment and viral clearance delay in patients with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 1063. [Google Scholar] [CrossRef] [PubMed]
- Berton, A.M.; Prencipe, N.; Giordano, R.; Ghigo, E.; Grottoli, S. Systemic steroids in patients with COVID-19: Pros and contras, an endocrinological point of view. J. Endocrinol. Investig. 2021, 44, 873–875. [Google Scholar] [CrossRef]
- Prete, A.; Bancos, I. Glucocorticoid induced adrenal insufficiency. BMJ 2021, 374, n1380. [Google Scholar] [CrossRef] [PubMed]
- Broersen, L.H.A.; Pereira, A.M.; Jorgensen, J.O.L.; Dekkers, O. Adrenal Insufficiency in Corticosteroids Use: Systematic Review and Meta-Analysis. J. Clin. Endocrinol Metab. 2015, 100, 2171–2180. [Google Scholar] [CrossRef]
- Laugesen, K.; Broersen, L.H.A.; Hansen, S.B.; Dekkers, O.M.; Sørensen, H.T.; Jorgensen, J.O.L. Management of endocrine disease: Glucocorticoid-induced adrenal insufficiency: Replace while we wait for evidence? Eur J. Endocrinol. 2021, 184, R111–R222. [Google Scholar] [CrossRef]
- Annane, D.; Sébille, V.; Troché, G.; Raphaël, J.C.; Gajdos, P.; Bellissant, E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 2000, 283, 1038–1045. [Google Scholar] [CrossRef]
- Annane, D.; Pastores, S.M.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; Marik, P.E.; et al. Critical illness-related corticosteroid insufficiency (CIRCI): A narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 2017, 43, 1781–1792. [Google Scholar] [CrossRef]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017, 43, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Téblick, A.; Gunst, J.; van den Berghe, G. Critical Illness-induced Corticosteroid Insufficiency: What It Is Not and What It Could Be. J. Clin. Endocrinol. Metab. 2022, 107, 2057–2064. Available online: https://pubmed.ncbi.nlm.nih.gov/35358303 (accessed on 2 April 2022). [CrossRef] [PubMed]
- Wong, D.W.L.; Klinkhammer, B.M.; Djudjaj, S.; Villwock, S.; Timm, M.C.; Buhl, E.M.; Wucherpfennig, S.; Cacchi, C.; Braunschweig, T.; Knuchel-Clarke, R. Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19. Patients Cells 2021, 10, 1900. [Google Scholar] [CrossRef]
- Santana, M.F.; Borba, M.G.S.; Baía-Da-Silva, D.C.; Val, F.; Alexandre, M.A.A.; Brito-Sousa, J.D.; Melo, G.C.; Queiroga, M.V.O.; Farias, M.E.L.; Camilo, C.C.; et al. Case Report: Adrenal Pathology Findings in Severe COVID-19: An Autopsy Study. Am. J. Trop Med. Hyg. 2020, 103, 1604–1607. [Google Scholar] [CrossRef]
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Iuga, A.C.; Marboe, C.C.; Yilmaz, M.; Lefkowitch, J.H.; Gauran, C.; Lagana, S.M. Adrenal Vascular Changes in COVID-19 Autopsies. Arch. Pathol. Lab. Med. 2020, 144, 1159–1160. [Google Scholar] [CrossRef]
- Salzano, C.; Saracino, G.; Cardillo, G. Possible Adrenal Involvement in Long COVID Syndrome. Medicina 2021, 57, 1087. [Google Scholar] [CrossRef]
- Piticchio, T.; le Moli, R.; Tumino, D.; Frasca, F. Relationship between betacoronaviruses and the endocrine system: A new key to understand the COVID-19 pandemic—A comprehensive review. J. Endocrinol. Investig. 2021, 44, 1553–1570. [Google Scholar] [CrossRef]
- Bellastella, G.; Maiorino, M.I.; Esposito, K. Endocrine complications of COVID-19: What happens to the thyroid and adrenal glands? J. Endocrinol. Investig. 2020, 43, 1169–1170. [Google Scholar] [CrossRef]
- Leyendecker, P.; Ritter, S.; Riou, M.; Wackenthaler, A.; Meziani, F.; Roy, C.; Ohana, M. Acute adrenal infarction as an incidental CT finding and a potential prognosis factor in severe SARS-CoV-2 infection: A retrospective cohort analysis on 219 patients. Eur. Radiol. 2021, 31, 895–900. [Google Scholar] [CrossRef]
- Machado, I.F.R.; Menezes, I.Q.; Figueiredo, S.R.; Coelho, F.M.A.; Terrabuio, D.R.B.; Ramos, D.V.; Fagundes, G.; Maciel, A.; Latronico, A.; Fragoso, M. Primary adrenal insufficiency due to bilateral adrenal infarction in COVID-19: A case report. J. Clin. Endocrinol. Metab. 2021, dgab557. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Santos, E.; Cervera, R.; Piette, J.-C.; de la Red, G.; Gil, V.; Font, J.; Couch, R.; Ingelmo, M.; Asherson, R. Adrenal involvement in the antiphospholipid syndrome: Clinical and immunologic characteristics of 86 patients. Medicine 2003, 82, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Presotto, F.; Fornasini, F.; Betterle, C.; Federspil, G.; Rossato, M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: Report of a case and review of the literature. Eur. J. Endocrinol. 2005, 153, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Ramon, I.; Mathian, A.; Bachelot, A.; Hervier, B.; Haroche, J.; Huong, D.B.-L.T.; Costedoat-Chalumeau, N.; Wechsler, B.; Karmali, R.; Velkeniers, B.; et al. Primary Adrenal Insufficiency Due to Bilateral Adrenal Hemorrhage-Adrenal Infarction in the Antiphospholipid Syndrome: Long-Term Outcome of 16 Patients. J. Clin. Endocrinol. Metab. 2013, 98, 3179–3189. [Google Scholar] [CrossRef] [Green Version]
- Vakhshoori, M.; Heidarpour, M.; Bondariyan, N.; Sadeghpour, N.; Mousavi, Z. Adrenal Insufficiency in Coronavirus Disease 2019 (COVID-19)-Infected Patients without Preexisting Adrenal Diseases: A Systematic Literature Review. Int. J. Endocrinol. 2021, 2021, 2271514. [Google Scholar] [CrossRef]
- Piccioli, A.; Chini, G.; Mannelli, M.; Serio, M. Bilateral massive adrenal hemorrhage due to sepsis: Report of two cases. J. Endocrinol. Investig. 1994, 17, 821–824. [Google Scholar] [CrossRef]
- Chien, J.-Y.; Jerng, J.-S.; Yu, C.-J.; Yang, P.-C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005, 33, 1688–1693. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M. COVID-19 and the endocrine system: Exploring the unexplored. J. Endocrinol. Investig. 2020, 43, 1027–1031. [Google Scholar] [CrossRef]
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beasley, M.B.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; AlRasheed, R. Pathophysiology of SARS-CoV-2: The Mount Sinai COVID-19 autopsy experience. Mod. Pathol Off. J. United States Can. Acad Pathol Inc. 2021, 34, 1456–1467. [Google Scholar] [CrossRef]
- Pal, R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 2020, 68, 251–252. [Google Scholar] [CrossRef]
- Siejka, A.; Barabutis, N. Adrenal insufficiency in the COVID-19 era. Am. J. Physiol. 2021, 320, E784–E785. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.K.-S.; Kwek, D.S.-K.; Ng, A.W.-K.; Ong, K.-C.; Kaw, G.J.-L.; Lee, L.S.-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin. Endocrinol. 2005, 63, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.; Raber, J.; Banks, W.; Erickson, M. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef]
- Wei, L.; Sun, S.; Zhang, J.; Zhu, H.; Xu, Y.; Ma, Q.; McNutt, M.A.M.A.; Korteweg, C.; Gu, J. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochem. Cell Biol. 2010, 88, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wheatland, R. Molecular mimicry of ACTH in SARS—Implications for corticosteroid treatment and prophylaxis. Med. Hypotheses 2004, 63, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Singh, A.; Kral, T.; Solomon, S. The immune-hypothalamic-pituitary-adrenal axis. Endocr. Rev. 1989, 10, 92–112. [Google Scholar] [CrossRef]
- Soni, A.; Pepper, G.M.; Wyrwinski, P.M.; Ramirez, N.E.; Simon, R.; Pina, T.; Gruenspan, H.; Vaca, C.E. Adrenal insufficiency occurring during septic shock: Incidence, outcome, and relationship to peripheral cytokine levels. Am. J. Med. 1995, 98, 266–271. [Google Scholar] [CrossRef]
- Natarajan, R.; Ploszaj, S.; Horton, R.; Nadler, J. Tumor necrosis factor and interleukin-1 are potent inhibitors of angiotensin-II-induced aldosterone synthesis. Endocrinology 1989, 125, 3084–3089. [Google Scholar] [CrossRef]
- Guarner, J.; Paddock, C.D.; Bartlett, J.; Zaki, S.R. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod. Pathol. 2008, 21, 1113–1120. [Google Scholar] [CrossRef] [Green Version]
- Vassiliadi, D.A.; Vassiliou, A.G.; Ilias, I.; Tsagarakis, S.; Kotanidou, A.; Dimopoulou, I. Pituitary—Adrenal Responses and Glucocorticoid Receptor Expression in Critically Ill Patients with COVID-19. Int. J. Mol. Sci. 2021, 22, 11473. [Google Scholar] [CrossRef]
- Awasthi, S.; Wagner, T.; Venkatakrishnan, A.J.; Puranik, A.; Hurchik, M.; Agarwal, V.; Conrad, I.; Kirkup, C.; Arunachalam, R.; O’Horo, J.; et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Boonen, E.; Walker, B.R. Reduced cortisol metabolism during critical illness. N. Engl. J. Med. 2013, 369, 481. [Google Scholar] [PubMed]
- Bornstein, S.R.; Engeland, W.C.; Ehrhart-Bornstein, M.; Herman, J.P. Dissociation of ACTH and glucocorticoids. Trends Endocrinol. Metab. 2008, 19, 175–180. [Google Scholar] [CrossRef]
- Ahmadi, I.; Estabraghnia Babaki, H.; Maleki, M.; Jarineshin, H.; Kaffashian, M.R.; Hassaniazad, M.; Kenarkoohi, A.; Ghanbarnejad, A.; Falahi, S.; Jahromi, M.; et al. Changes in Physiological Levels of Cortisol and Adrenocorticotropic Hormone upon Hospitalization Can Predict SARS-CoV-2 Mortality: A Cohort Study. Int. J. Endocrinol. 2022, 2022, 4280691. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 659–660. [Google Scholar] [CrossRef]
- Güven, M.; Gültekin, H. Could serum total cortisol level at admission predict mortality due to coronavirus disease 2019 in the intensive care unit? A prospective study. Sao Paulo Med. J. 2021, 139, 398–404. [Google Scholar] [CrossRef]
- Das, L.; Dutta, P.; Walia, R.; Mukherjee, S.; Suri, V.; Puri, G.D.; Mahajan, V.; Malhotra, P.; Chaudhary, S.; Gupta, R.; et al. Spectrum of Endocrine Dysfunction and Association with Disease Severity in Patients with COVID-19: Insights From a Cross-Sectional, Observational Study. Front. Endocrinol. 2021, 12, 645787. [Google Scholar] [CrossRef]
- Clarke, S.; Phylactou, M.; Patel, B.; Mills, E.G.; Muzi, B.; Izzi-Engbeaya, C.; Choudhury, S.; Khoo, B.; Meeran, K.; Comninos, A.N.; et al. Normal Adrenal and Thyroid Function in Patients Who Survive COVID-19 Infection. J. Clin. Endocrinol. Metab. 2021, 106, 2208–2220. [Google Scholar] [CrossRef]
- Catalán, I.P.; Martí, C.R.; de la Sota, D.P.; Álvarez, A.C.; Gimeno, M.J.E.; Juana, S.F.; Rodríguez, G.H.; Bajo, E.D.; Gaya, N.T.; Blasco, J.U.; et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J. Med. Virol. 2022, 94, 205–210. [Google Scholar] [CrossRef]
- Chifu, I.; Detomas, M.; Dischinger, U.; Kimpel, O.; Megerle, F.; Hahner, S.; Fassnacht, M.; Altieri, B. Management of Patients with Glucocorticoid-Related Diseases and COVID-19. Front. Endocrinol. 2021, 12, 705214. [Google Scholar] [CrossRef]
- Akbas, E.M.; Akbas, N. COVID-19, adrenal gland, glucocorticoids, and adrenal insufficiency. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czechoslov. 2021, 165, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Kim, J.H.; Moon, S.J.; Lee, W.Y. Encountering COVID-19 as Endocrinologists. Endocrinol. Metab. 2020, 35, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.; Giustina, A.; Puig-Domingo, M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020, 21, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K. Managing common endocrine disorders amid COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Tresoldi, A.S.; Sumilo, D.; Perrins, M.; Toulis, K.; Prete, A.; Reddy, N.; Wass, J.A.H.; Arlt, W.; Nirantharakumar, K. Increased Infection Risk in Addison’s Disease and Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2020, 105, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Smans, L.C.C.J.; Souverein, P.C.; Leufkens, H.G.M.; Hoepelman, A.I.M.; Zelissen, P.M.J. Increased use of antimicrobial agents and hospital admission for infections in patients with primary adrenal insufficiency: A cohort study. Eur. J. Endocrinol. 2013, 168, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Dineen, R.; Thompson, C.J.; Sherlock, M. Adrenal crisis: Prevention and management in adult patients. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819848218. [Google Scholar] [CrossRef]
- Isidori, A.M.; Venneri, M.A.; Graziadio, C.; Simeoli, C.; Fiore, D.; Hasenmajer, V.; Sbardella, E.; Gianfrilli, D.; Pozza, C.; Pasqualetti, V. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): A single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 173–185. [Google Scholar] [CrossRef]
- Sabbadin, C.; Betterle, C.; Scaroni, C.; Ceccato, F. Frequently Asked Questions in Patients with Adrenal Insufficiency in the Time of COVID-19. Front. Endocrinol. 2021, 12, 805647. [Google Scholar] [CrossRef]
- Carosi, G.; Morelli, V.; del Sindaco, G.; Serban, A.L.; Cremaschi, A.; Frigerio, S.; Rodari, G.; Profka, E.; Indirli, R.; Mungari, R.; et al. Adrenal Insufficiency at the Time of COVID-19: A Retrospective Study in Patients Referring to a Tertiary Center. J. Clin. Endocrinol. Metab. 2021, 106, e1354–e1361. [Google Scholar] [CrossRef]
- Martino, M.; Aboud, N.; Cola, M.F.; Giancola, G.; Ciarloni, A.; Salvio, G.; Arnaldi, G. Impact of COVID-19 pandemic on psychophysical stress in patients with adrenal insufficiency: The CORTI-COVID study. J. Endocrinol Invest. 2021, 44, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Marcus, H.J.; Baldeweg, S.E. The direct and indirect impact of the COVID-19 pandemic on the care of patients with pituitary disease: A cross sectional study. Pituitary 2021, 24, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Suresh, M.; Abbondanza, T.; Vaidya, A.; Bancos, I. The Impact of the COVID-19 Pandemic on Self-Reported Outcomes in Patients with Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 2021, 106, e2469–e2479. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.B.; Mirmira, R.G.; Stewart, P.M. Our Response to COVID-19 as Endocrinologists and Diabetologists. J. Clin. Endocrinol. Metab. 2020, 105, 1299–1301. [Google Scholar] [CrossRef] [Green Version]
- Katznelson, L.; Gadelha, M. Glucocorticoid use in patients with adrenal insufficiency following administration of the COVID-19 vaccine: A pituitary society statement. Pituitary 2021, 24, 143–145. [Google Scholar] [CrossRef]
- Guarnotta, V.; Ferrigno, R.; Martino, M.; Barbot, M.; Isidori, A.M.; Scaroni, C.; Ferrante, A.; Arnaldi, G.; Pivonello, R.; Giordano, C. Glucocorticoid excess and COVID-19 disease. Rev. Endocr. Metab. Disord. 2021, 22, 703–714. [Google Scholar] [CrossRef]
- Berlińska, A.; Świątkowska-Stodulska, R.; Sworczak, K. Old Problem, New Concerns: Hypercortisolemia in the Time of COVID-19. Front. Endocrinol. 2021, 12, 711612. [Google Scholar] [CrossRef]
- Pivonello, R.; Ferrigno, R.; Isidori, A.M.; Biller, B.M.K.; Grossman, A.B.; Colao, A. COVID-19 and Cushing’s syndrome: Recommendations for a special population with endogenous glucocorticoid excess. Lancet Diabetes Endocrinol. 2020, 8, 654–656. [Google Scholar] [CrossRef]
- Serban, A.L.; Ferrante, E.; Carosi, G.; Indirli, R.; Arosio, M.; Mantovani, G. COVID-19 in Cushing disease: Experience of a single tertiary centre in Lombardy. J. Endocrinol. Invest. 2021, 44, 1335–1336. [Google Scholar] [CrossRef]
- Beretta, F.; Dassie, F.; Parolin, M.; Boscari, F.; Barbot, M.; Busetto, L.; Mioni, R.; de Carlo, E.; Scaroni, C.; Fallo, F.; et al. Practical Considerations for the Management of Cushing’s Disease and COVID-19: A Case Report. Front. Endocrinol. 2020, 11, 554. [Google Scholar] [CrossRef]
- Yuno, A.; Kenmotsu, Y.; Takahashi, Y.; Nomoto, H.; Kameda, H.; Cho, K.; Nakamura, A.; Yamashita, Y.; Nakamura, J.; Nakakubo, S.; et al. Successful management of a patient with active Cushing’s disease complicated with coronavirus disease 2019 (COVID-19) pneumonia. Endocr. J. 2021, 68, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Belaya, Z.; Golounina, O.; Melnichenko, G.; Tarbaeva, N.; Pashkova, E.; Gorokhov, M.; Kalashnikov, V.; Dzeranova, L.; Fadeev, V.; Volchkov, P.; et al. Clinical course and outcome of patients with ACTH-dependent Cushing’s syndrome infected with novel coronavirus disease-19 (COVID-19): Case presentations. Endocrine 2021, 72, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.Q.; Mendonca, B.B. Adrenal Insufficiency and Glucocorticoid Use During the COVID-19 Pandemic. Clinics 2020, 75, e2022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensterle, M.; Herman, R.; Janež, A.; Mahmeed, W.A.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; et al. The Relationship between COVID-19 and Hypothalamic–Pituitary–Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess—The CAPISCO International Expert Panel. Int. J. Mol. Sci. 2022, 23, 7326. https://doi.org/10.3390/ijms23137326
Jensterle M, Herman R, Janež A, Mahmeed WA, Al-Rasadi K, Al-Alawi K, Banach M, Banerjee Y, Ceriello A, Cesur M, et al. The Relationship between COVID-19 and Hypothalamic–Pituitary–Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess—The CAPISCO International Expert Panel. International Journal of Molecular Sciences. 2022; 23(13):7326. https://doi.org/10.3390/ijms23137326
Chicago/Turabian StyleJensterle, Mojca, Rok Herman, Andrej Janež, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, and et al. 2022. "The Relationship between COVID-19 and Hypothalamic–Pituitary–Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess—The CAPISCO International Expert Panel" International Journal of Molecular Sciences 23, no. 13: 7326. https://doi.org/10.3390/ijms23137326
APA StyleJensterle, M., Herman, R., Janež, A., Mahmeed, W. A., Al-Rasadi, K., Al-Alawi, K., Banach, M., Banerjee, Y., Ceriello, A., Cesur, M., Cosentino, F., Galia, M., Goh, S.-Y., Kalra, S., Kempler, P., Lessan, N., Lotufo, P., Papanas, N., Rizvi, A. A., ... Rizzo, M. (2022). The Relationship between COVID-19 and Hypothalamic–Pituitary–Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess—The CAPISCO International Expert Panel. International Journal of Molecular Sciences, 23(13), 7326. https://doi.org/10.3390/ijms23137326