VARS2 Depletion Leads to Activation of the Integrated Stress Response and Disruptions in Mitochondrial Fatty Acid Oxidation
Abstract
:1. Introduction
2. Results
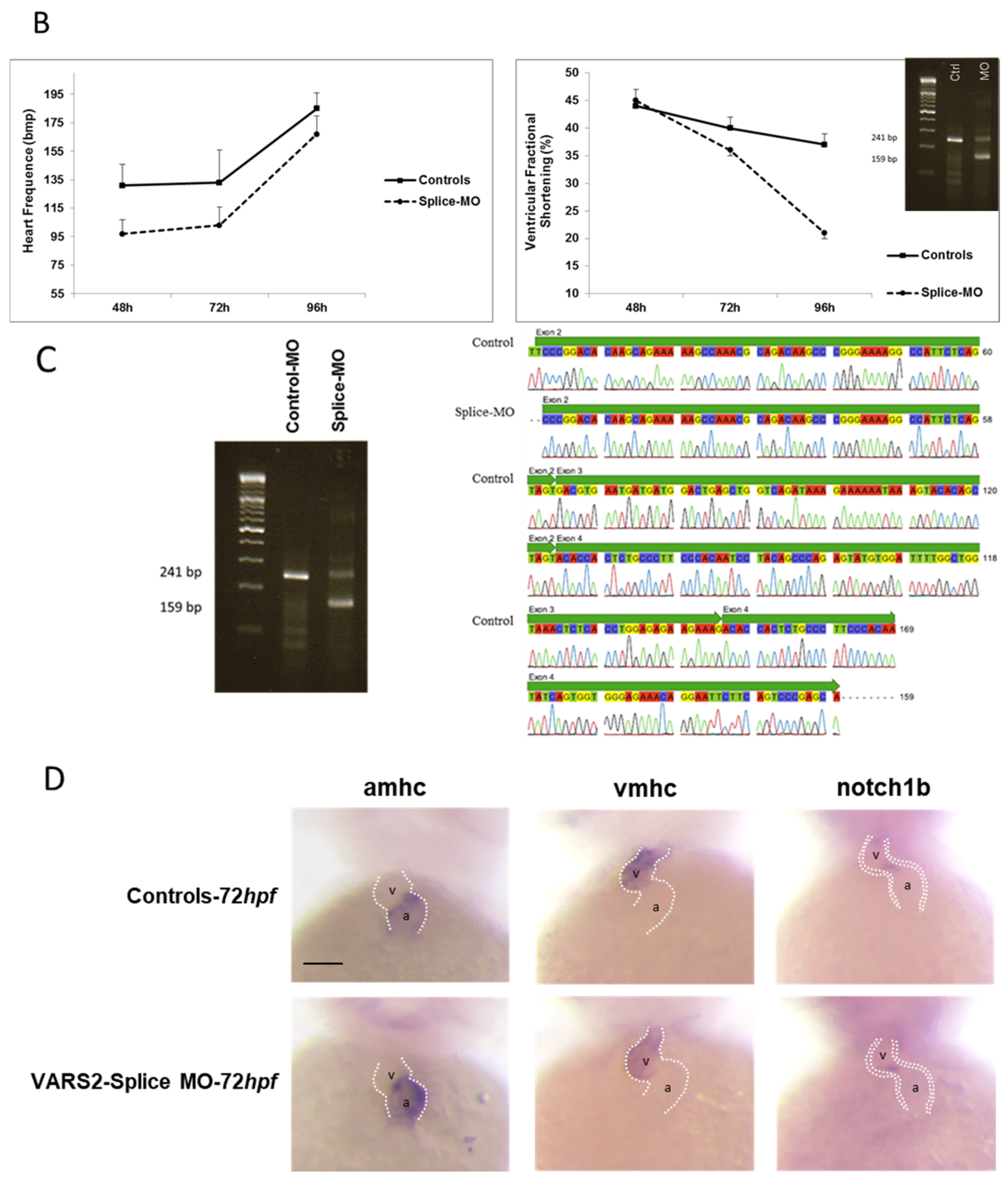
2.1. Transient VARS2 Knockdown Leads to Heart Failure in Zebrafish
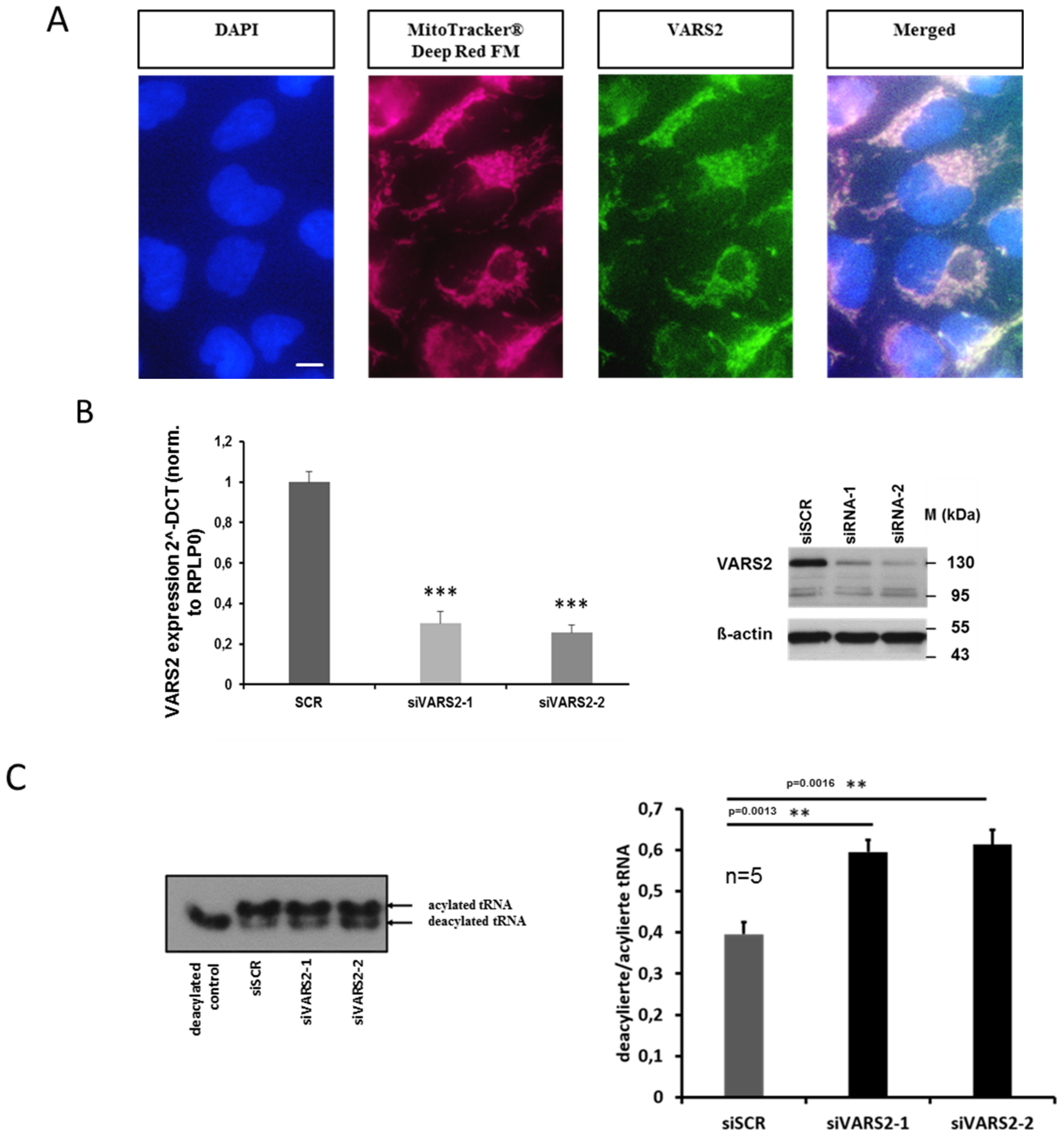
2.2. VARS2-Depleted HEK293A Cells Showed Reduced Enzymatic Activity
2.3. Heterozygous VARS2 Knockout (VARS2+/− Knockout) was Successfully Achieved in HEK293A Cells
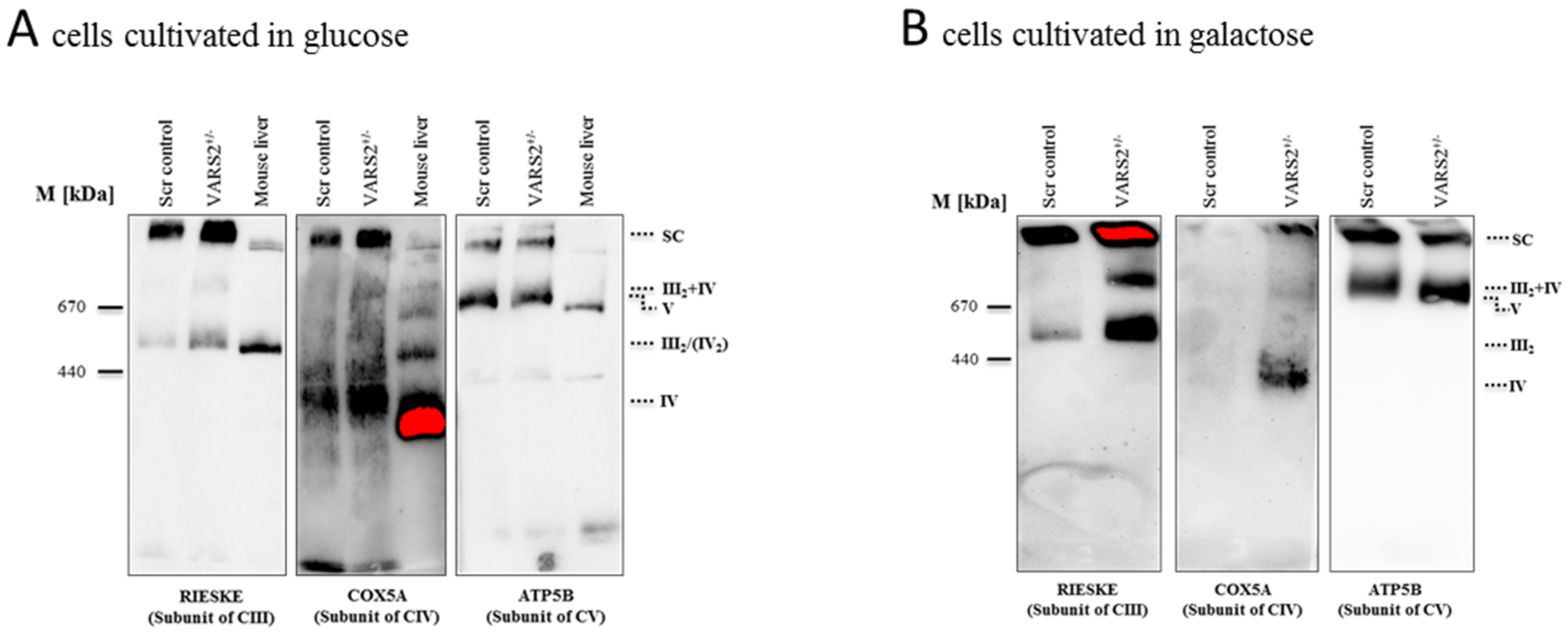
2.4. VARS2 Depletion Leads to Rearrangement of the Electron Transport Chain (ETC) Complexes without Significant Respiratory Chain Deficiencies
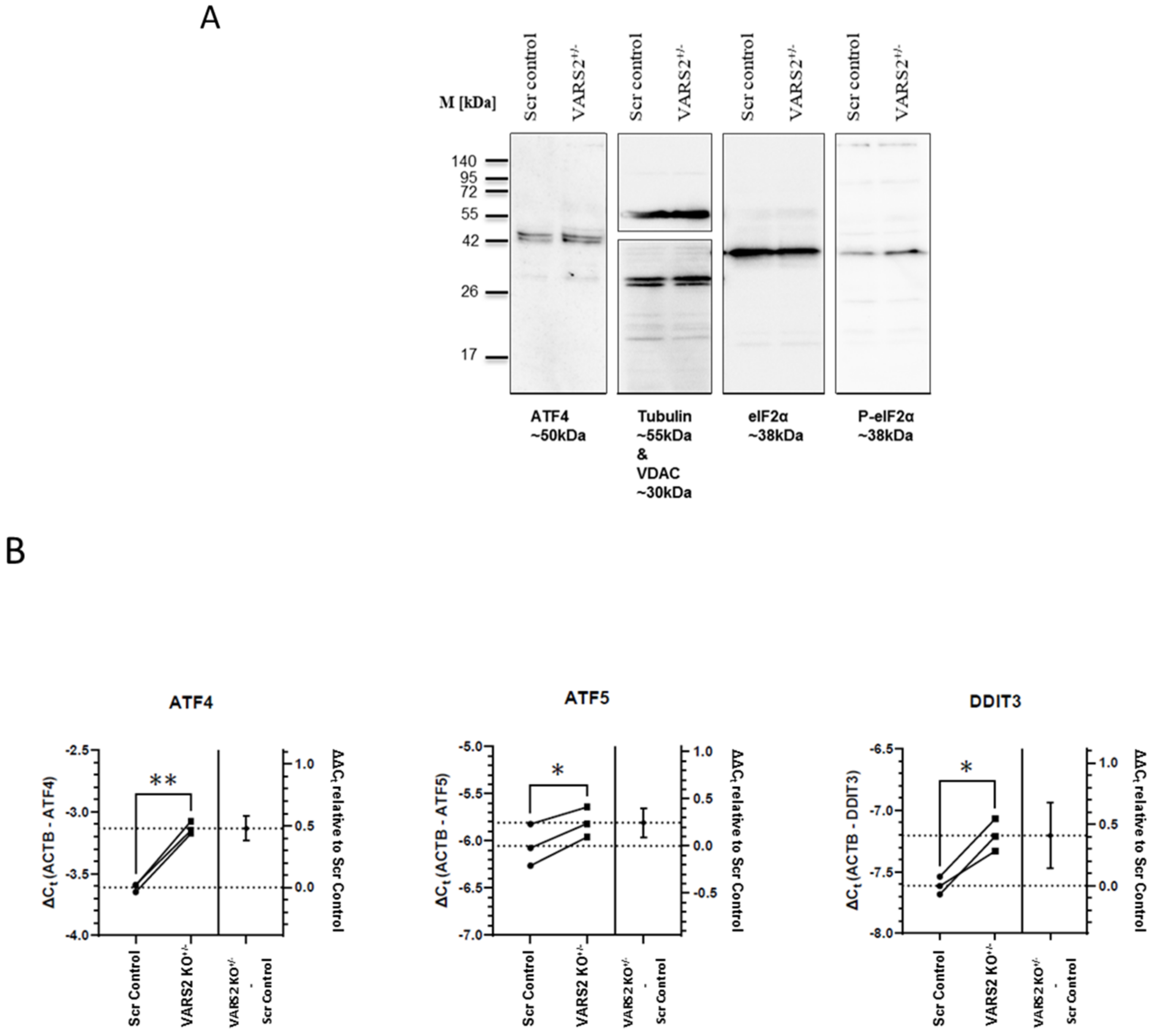
2.5. VARS2 Depletion Leads to Activation of the Integrated Stress Response (ISR)
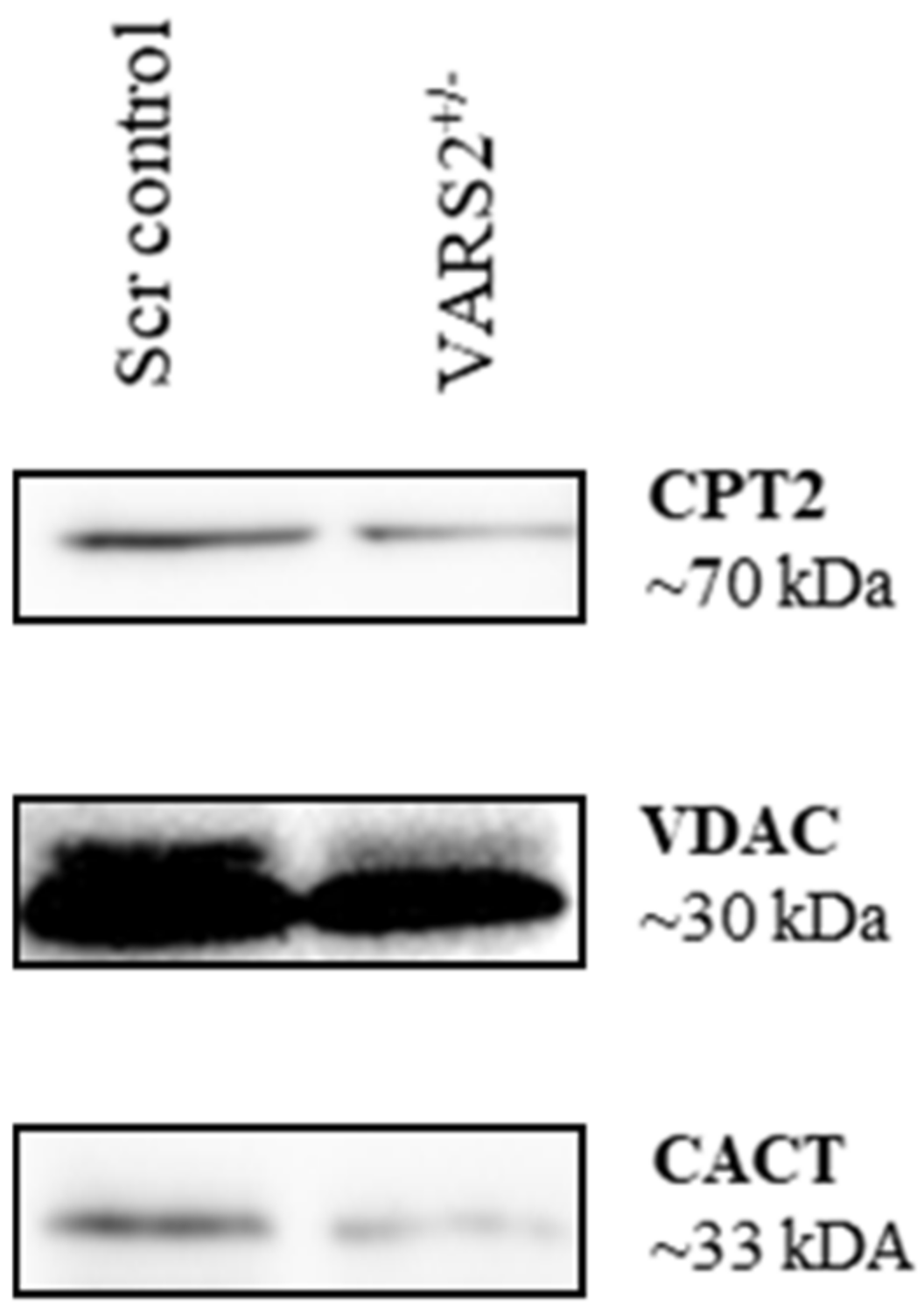
2.6. Disruptions in Mitochondrial FAO Are a Possible Pathomechanism Involved in Adaptive Changes in VARS2-Deficient Cells
3. Discussion
4. Materials and Methods
4.1. Zebrafish Strains
4.2. Morpholino Injection Procedures, Phenotyping, and RNA in Situ Hybridization
4.3. Manipulating the Expression of VARS2 Gene in HEK293A Cells
4.4. Immunohistochemical Studies in HEK293A Cells
4.5. Primers
4.6. Northern Blot
4.7. Establishing a Monoclonal VARS2-Knockout HEK293A Cell Line
4.8. Isolation of Mitochondria
4.9. Blue Native PAGE (BN-PAGE)
4.10. Seahorse
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meder, B.; Ruhle, F.; Weis, T.; Homuth, G.; Keller, A.; Franke, J.; Peil, B.; Lorenzo Bermejo, J.; Frese, K.; Huge, A.; et al. A genome-wide association study identifies 6p21 as novel risk locus for dilated cardiomyopathy. Eur. Heart J. 2014, 35, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Sammani, A.; Kayvanpour, E.; Bosman, L.P.; Sedaghat-Hamedani, F.; Proctor, T.; Gi, W.T.; Broezel, A.; Jensen, K.; Katus, H.A.; Te Riele, A.; et al. Predicting sustained ventricular arrhythmias in dilated cardiomyopathy: A meta-analysis and systematic review. ESC Heart Fail. 2020, 7, 1430–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacher, J.M.; Schimmel, P. An editing-defective aminoacyl-trna synthetase is mutagenic in aging bacteria via the sos response. Proc. Natl. Acad. Sci. USA 2007, 104, 1907–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, Y.S.; Lee, S.J.; Moon, J.H.; Kang, B.W.; Kim, J.G.; Sohn, S.K.; Jung, J.H.; Park, H.Y.; Park, J.Y.; Kim, H.J.; et al. Vars2 v552v variant as prognostic marker in patients with early breast cancer. Med. Oncol. 2011, 28, 1273–1280. [Google Scholar] [CrossRef]
- Cheong, H.S.; Lee, J.H.; Yu, S.J.; Yoon, J.H.; Lee, H.S.; Cheong, J.Y.; Cho, S.W.; Park, N.H.; Park, B.L.; Namgoong, S.; et al. Association of vars2-sfta2 polymorphisms with the risk of chronic hepatitis b in a korean population. Liver. Int. 2015, 35, 1934–1940. [Google Scholar] [CrossRef]
- Ma, K.; Xie, M.; He, X.; Liu, G.; Lu, X.; Peng, Q.; Zhong, B.; Li, N. A novel compound heterozygous mutation in vars2 in a newborn with mitochondrial cardiomyopathy: A case report of a chinese family. BMC Med. Genet. 2018, 19, 202. [Google Scholar] [CrossRef]
- Diodato, D.; Melchionda, L.; Haack, T.B.; Dallabona, C.; Baruffini, E.; Donnini, C.; Granata, T.; Ragona, F.; Balestri, P.; Margollicci, M.; et al. Vars2 and tars2 mutations in patients with mitochondrial encephalomyopathies. Hum. Mutat. 2014, 35, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Alsemari, A.; Al-Younes, B.; Goljan, E.; Jaroudi, D.; BinHumaid, F.; Meyer, B.F.; Arold, S.T.; Monies, D. Recessive vars2 mutation underlies a novel syndrome with epilepsy, mental retardation, short stature, growth hormone deficiency, and hypogonadism. Hum. Genomics 2017, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Baertling, F.; Alhaddad, B.; Seibt, A.; Budaeus, S.; Meitinger, T.; Strom, T.M.; Mayatepek, E.; Schaper, J.; Prokisch, H.; Haack, T.B.; et al. Neonatal encephalocardiomyopathy caused by mutations in vars2. Metab. Brain Dis. 2017, 32, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Adriao, M.; Sampaio, M.; Basto, M.A.; Rodrigues, E.; Vilarinho, L.; Teles, E.L.; Alonso, I.; Leao, M. Mitochondrial encephalopathy: First portuguese report of a vars2 causative variant. JIMD Rep. 2018, 42, 113–119. [Google Scholar]
- Begliuomini, C.; Magli, G.; Di Rocco, M.; Santorelli, F.M.; Cassandrini, D.; Nesti, C.; Deodato, F.; Diodato, D.; Casellato, S.; Simula, D.M.; et al. Vars2-linked mitochondrial encephalopathy: Two case reports enlarging the clinical phenotype. BMC Med. Genet. 2019, 20, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzman, L.; Kolic, I.; Radic Nisevic, J.; Ruzic Barsic, A.; Skarpa Prpic, I.; Prpic, I. A novel vars2 gene variant in a patient with epileptic encephalopathy. Ups J. Med. Sci. 2019, 124, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusikova, K.; Feichtinger, R.G.; Csillag, B.; Kalev, O.K.; Weis, S.; Duba, H.C.; Mayr, J.A.; Weis, D. Case report and review of the literature: A new and a recurrent variant in the vars2 gene are associated with isolated lethal hypertrophic cardiomyopathy, hyperlactatemia, and pulmonary hypertension in early infancy. Front. Pediatr. 2021, 9, 660076. [Google Scholar] [CrossRef]
- Chin, H.L.; Goh, D.L.; Wang, F.S.; Tay, S.K.H.; Heng, C.K.; Donnini, C.; Baruffini, E.; Pines, O. A combination of two novel vars2 variants causes a mitochondrial disorder associated with failure to thrive and pulmonary hypertension. J. Mol. Med. 2019, 97, 1557–1566. [Google Scholar] [CrossRef]
- Searfoss, G.H.; Paisley, B.M.; Goldstein, K.M.; Baker, T.K.; Willy, J.A. The integrated stress response regulates cell health of cardiac progenitors. Toxicol. Sci. 2019, 167, 202–210. [Google Scholar] [CrossRef]
- Taylor, R.W.; Pyle, A.; Griffin, H.; Blakely, E.L.; Duff, J.; He, L.; Smertenko, T.; Alston, C.L.; Neeve, V.C.; Best, A.; et al. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA 2014, 312, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Olahova, M. Mitochondrial oxphos biogenesis: Co-regulation of protein synthesis, import, and assembly pathways. Int. J. Mol. Sci. 2020, 21, 3820. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol 2019, 20, 267–284. [Google Scholar] [CrossRef]
- Figuccia, S.; Degiorgi, A.; Ceccatelli Berti, C.; Baruffini, E.; Dallabona, C.; Goffrini, P. Mitochondrial aminoacyl-trna synthetase and disease: The yeast contribution for functional analysis of novel variants. Int. J. Mol. Sci. 2021, 22, 4524. [Google Scholar] [CrossRef]
- Ling, J.; Reynolds, N.; Ibba, M. Aminoacyl-trna synthesis and translational quality control. Annu. Rev. Microbiol. 2009, 63, 61–78. [Google Scholar] [CrossRef]
- Sissler, M.; Gonzalez-Serrano, L.E.; Westhof, E. Recent advances in mitochondrial aminoacyl-trna synthetases and disease. Trends Mol. Med. 2017, 23, 693–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, F.; Di Meo, I.; Bellacchio, E.; Webb, B.D.; McFarland, R.; Chrzanowska-Lightowlers, Z.M.A.; He, L.; Skorupa, E.; Moroni, I.; Ardissone, A.; et al. Clinical, biochemical, and genetic features associated with vars2-related mitochondrial disease. Hum. Mutat. 2018, 39, 563–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronicka, E.; Piekutowska-Abramczuk, D.; Ciara, E.; Trubicka, J.; Rokicki, D.; Karkucinska-Wieckowska, A.; Pajdowska, M.; Jurkiewicz, E.; Halat, P.; Kosinska, J.; et al. New perspective in diagnostics of mitochondrial disorders: Two years’ experience with whole-exome sequencing at a national paediatric centre. J. Transl. Med. 2016, 14, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenaz, G.; Genova, M.L. Structure and organization of mitochondrial respiratory complexes: A new understanding of an old subject. Antioxid. Redox Signal. 2010, 12, 961–1008. [Google Scholar] [CrossRef]
- Jha, P.; Wang, X.; Auwerx, J. Analysis of mitochondrial respiratory chain supercomplexes using blue native polyacrylamide gel electrophoresis (bn-page). Curr. Protoc. Mouse Biol. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Agnew, T.; Goldsworthy, M.; Aguilar, C.; Morgan, A.; Simon, M.; Hilton, H.; Esapa, C.; Wu, Y.; Cater, H.; Bentley, L.; et al. A wars2 mutant mouse model displays oxphos deficiencies and activation of tissue-specific stress response pathways. Cell Rep. 2018, 25, 3315–3328.e3316. [Google Scholar] [CrossRef] [Green Version]
- Schonberger, J.; Seidman, C.E. Many roads lead to a broken heart: The genetics of dilated cardiomyopathy. Am. J. Hum. Genet. 2001, 69, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Brackett, J.C.; Sims, H.F.; Rinaldo, P.; Shapiro, S.; Powell, C.K.; Bennett, M.J.; Strauss, A.W. Two alpha subunit donor splice site mutations cause human trifunctional protein deficiency. J. Clin. Investig. 1995, 95, 2076–2082. [Google Scholar] [CrossRef]
- Marin-Garcia, J.; Goldenthal, M.J. Fatty acid metabolism in cardiac failure: Biochemical, genetic and cellular analysis. Cardiovasc. Res. 2002, 54, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, D.; Martin, D.; Pascale De, L.; Villain, E.; Jouvet, P.; Rabier, D.; Brivet, M.; Saudubray, J.M. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 1999, 100, 2248–2253. [Google Scholar] [CrossRef] [Green Version]
- Bonnefont, J.P.; Demaugre, F.; Prip-Buus, C.; Saudubray, J.M.; Brivet, M.; Abadi, N.; Thuillier, L. Carnitine palmitoyltransferase deficiencies. Mol. Genet Metab. 1999, 68, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Huttner, I.G.; Sedaghat-Hamedani, F.; Just, S.; Dahme, T.; Frese, K.S.; Vogel, B.; Kohler, D.; Kloos, W.; Rudloff, J.; et al. Pinch proteins regulate cardiac contractility by modulating integrin-linked kinase-protein kinase b signaling. Mol. Cell Biol. 2011, 31, 3424–3435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Braun, H.P.; Schagger, H. Blue native page. Nat. Protoc. 2006, 1, 418–428. [Google Scholar] [CrossRef]
- Rutledge, R.G.; Côté, C. Mathematics of quantitative kinetic pcr and the application of standard curves. Nucleic Acids Res. 2003, 31, e93. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−δδct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Biasin, V.; Wygrecka, M.; Marsh, L.M.; Becker-Pauly, C.; Brcic, L.; Ghanim, B.; Klepetko, W.; Olschewski, A.; Kwapiszewska, G. Meprin β contributes to collagen deposition in lung fibrosis. Sci. Rep. 2017, 7, 39969. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayvanpour, E.; Wisdom, M.; Lackner, M.K.; Sedaghat-Hamedani, F.; Boeckel, J.-N.; Müller, M.; Eghbalian, R.; Dudek, J.; Doroudgar, S.; Maack, C.; et al. VARS2 Depletion Leads to Activation of the Integrated Stress Response and Disruptions in Mitochondrial Fatty Acid Oxidation. Int. J. Mol. Sci. 2022, 23, 7327. https://doi.org/10.3390/ijms23137327
Kayvanpour E, Wisdom M, Lackner MK, Sedaghat-Hamedani F, Boeckel J-N, Müller M, Eghbalian R, Dudek J, Doroudgar S, Maack C, et al. VARS2 Depletion Leads to Activation of the Integrated Stress Response and Disruptions in Mitochondrial Fatty Acid Oxidation. International Journal of Molecular Sciences. 2022; 23(13):7327. https://doi.org/10.3390/ijms23137327
Chicago/Turabian StyleKayvanpour, Elham, Michael Wisdom, Maximilian K. Lackner, Farbod Sedaghat-Hamedani, Jes-Niels Boeckel, Marion Müller, Rose Eghbalian, Jan Dudek, Shirin Doroudgar, Christoph Maack, and et al. 2022. "VARS2 Depletion Leads to Activation of the Integrated Stress Response and Disruptions in Mitochondrial Fatty Acid Oxidation" International Journal of Molecular Sciences 23, no. 13: 7327. https://doi.org/10.3390/ijms23137327
APA StyleKayvanpour, E., Wisdom, M., Lackner, M. K., Sedaghat-Hamedani, F., Boeckel, J.-N., Müller, M., Eghbalian, R., Dudek, J., Doroudgar, S., Maack, C., Frey, N., & Meder, B. (2022). VARS2 Depletion Leads to Activation of the Integrated Stress Response and Disruptions in Mitochondrial Fatty Acid Oxidation. International Journal of Molecular Sciences, 23(13), 7327. https://doi.org/10.3390/ijms23137327








