The Calcium-Dependent Protein Kinase TaCDPK27 Positively Regulates Salt Tolerance in Wheat
Abstract
:1. Introduction
2. Results
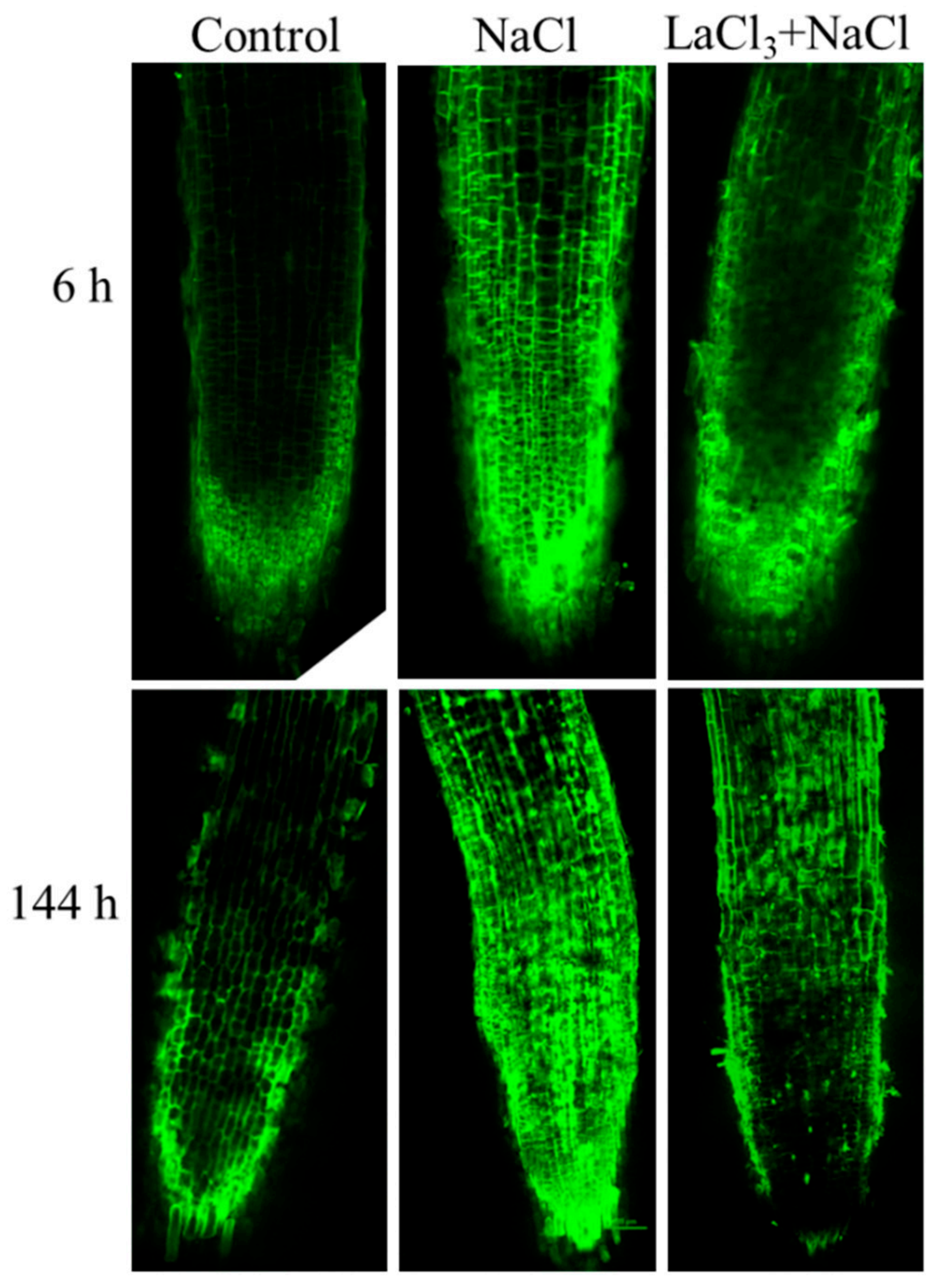
2.1. NaCl Treatment Increases Ca2+ Levels in the Roots of Wheat Seedlings
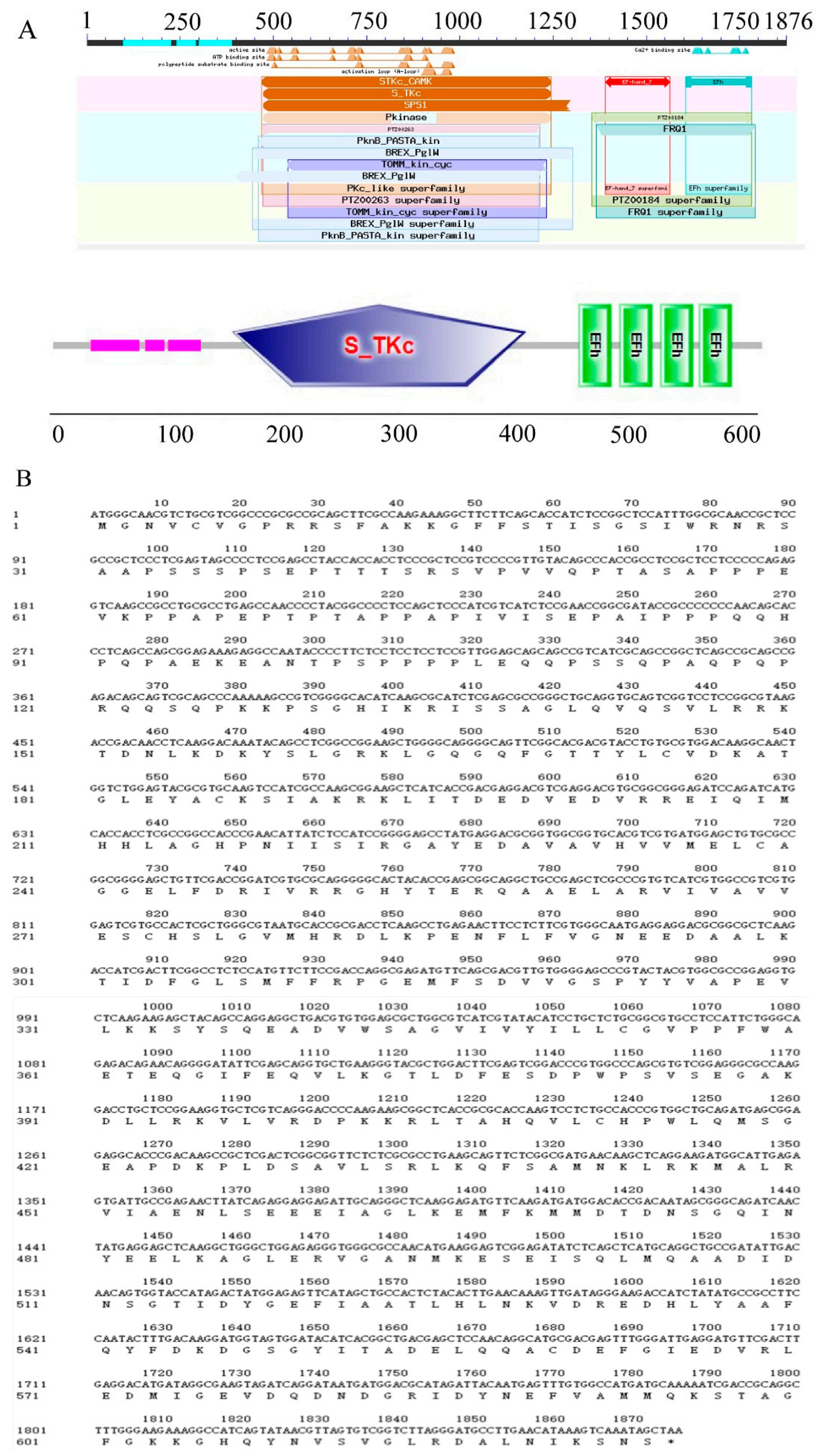
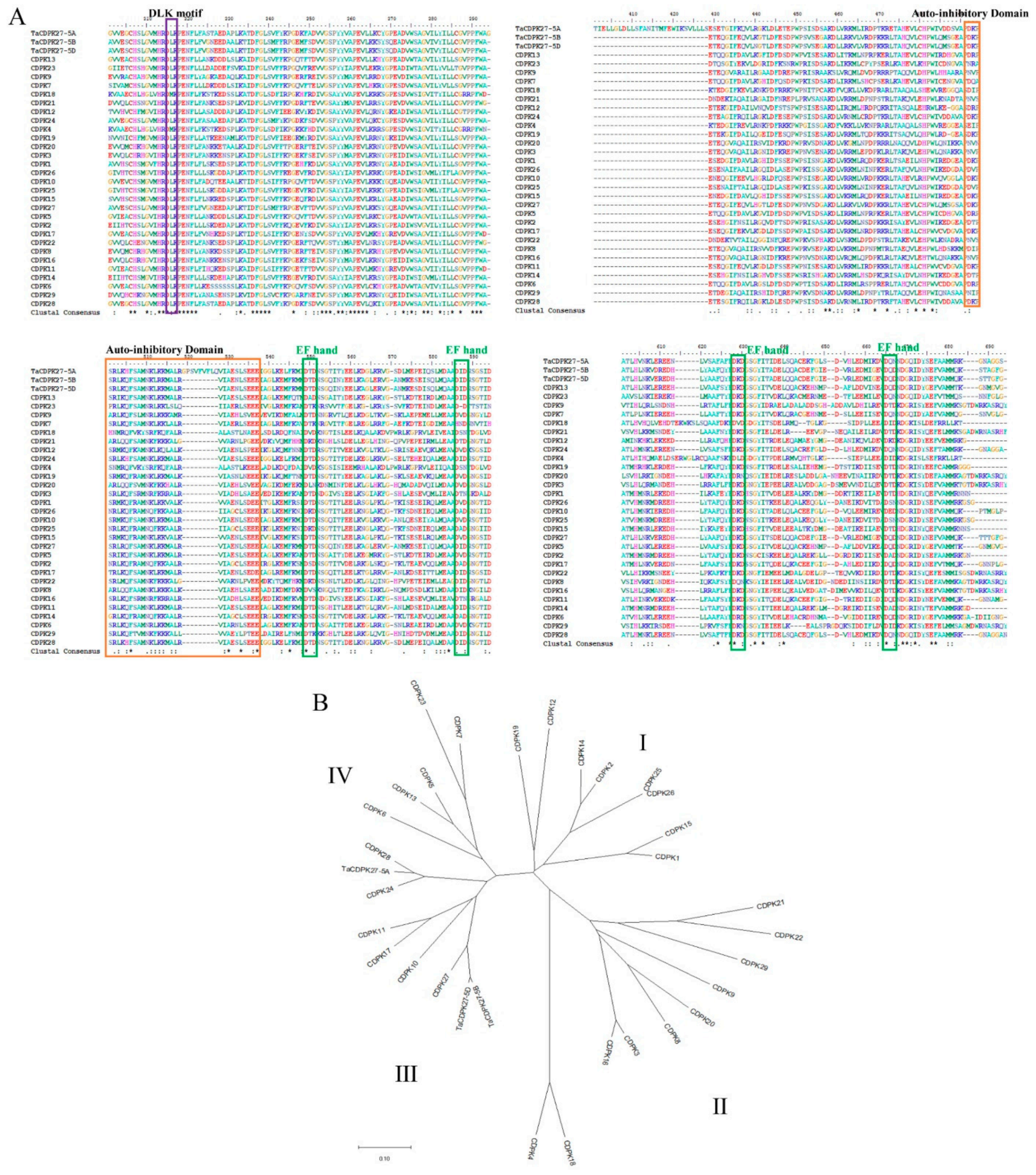
2.2. Cloning and Characterization of the Wheat CDPK Gene TaCDPK27B
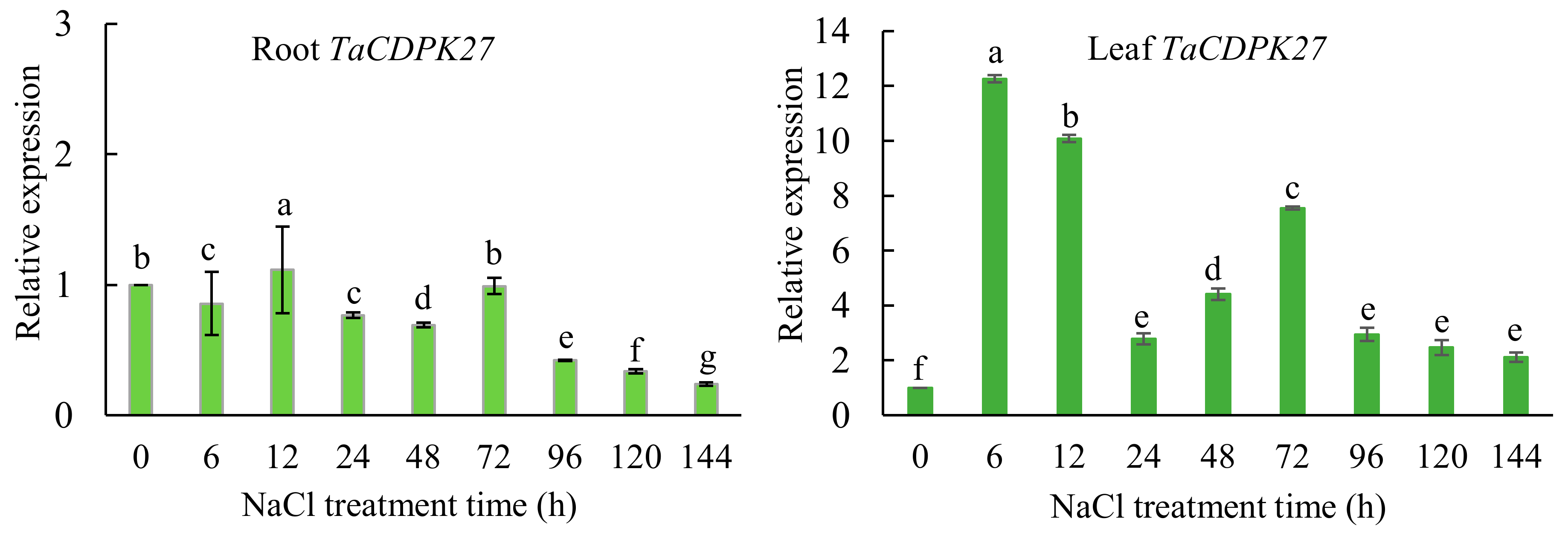
2.3. TaCDPK27 Responds to NaCl Treatment
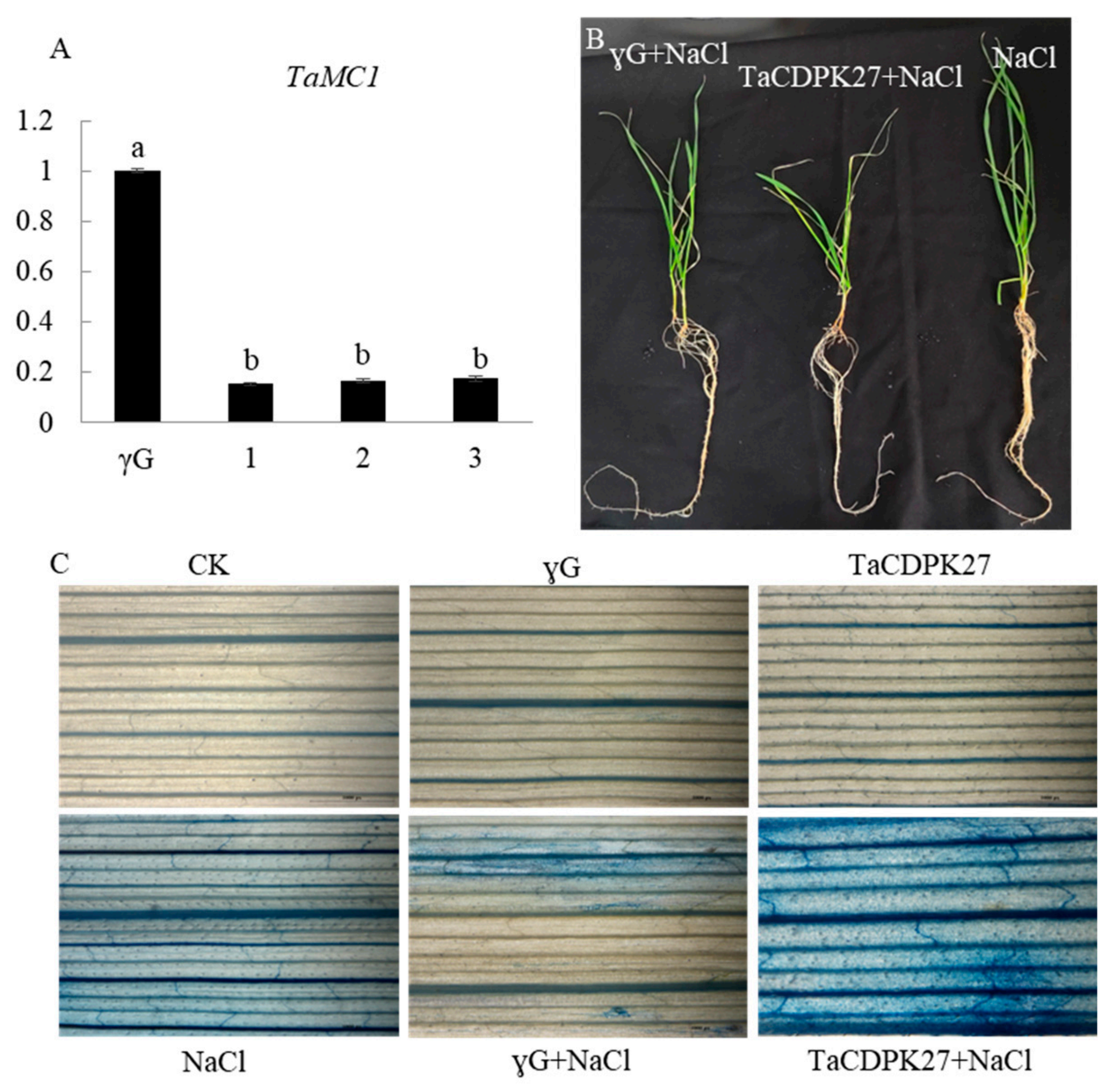
2.4. Silencing of TaCDPK27 Increases the Salt Sensitivity of Wheat Seedlings
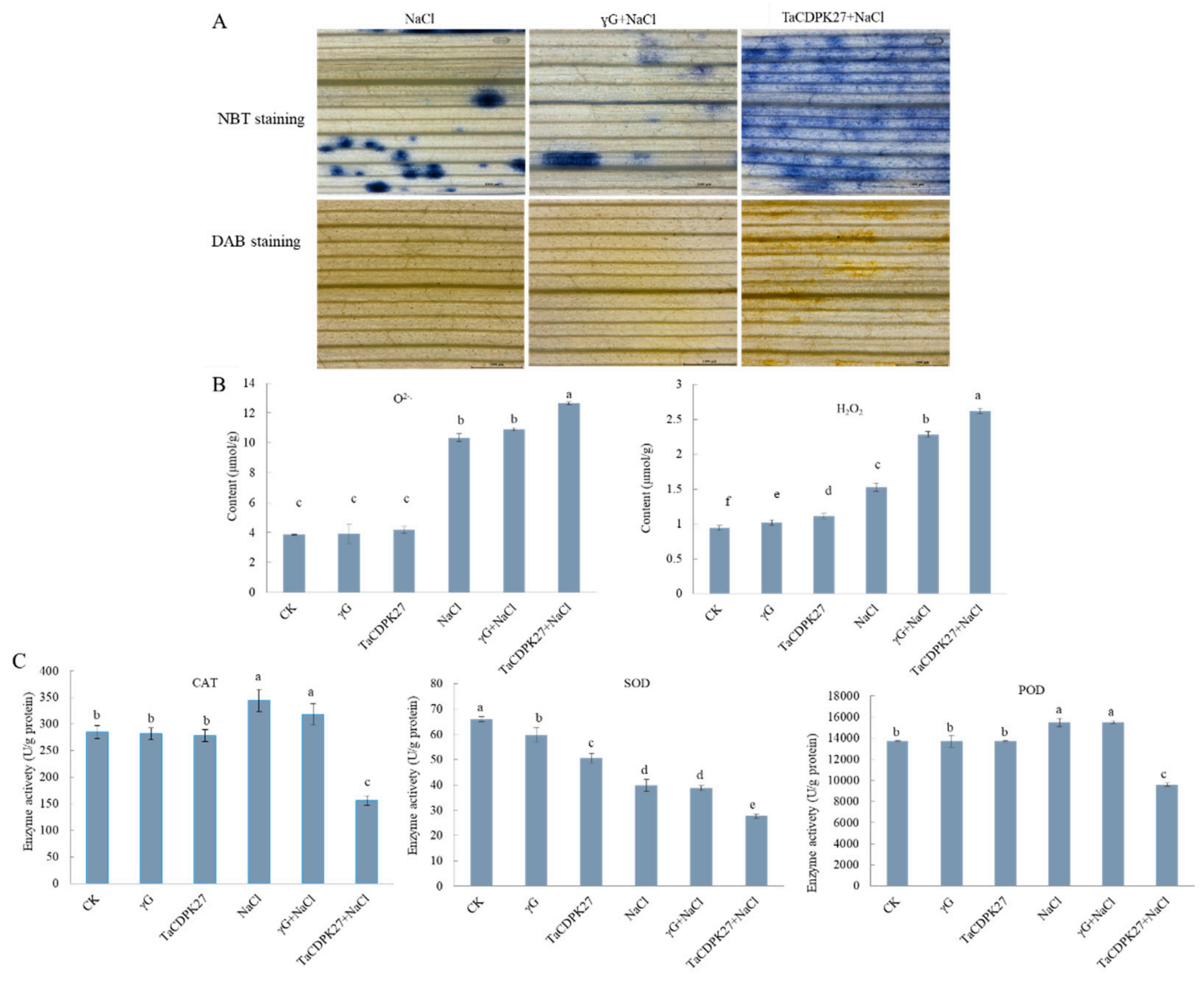
2.5. Silencing of TaCDPK27 Promoted NaCl Stress-Triggered Programmed Cell Death (PCD) in Leaves of Wheat Seedlings
2.6. Silencing TaCDPK27 Aggravates the NaCl Stress-Induced Injury of Photosystem II (PSII)
2.7. Silencing TaCDPK27 Reduces the Antioxidant Capacity of Wheat Roots and Leaves under NaCl Treatment
3. Discussion
4. Materials and Methods
4.1. Wheat Seedling Growth and Stress Conditions
4.2. Cloning, Sequencing, and Phylogenetic Analysis of TaCDPK27
4.3. Subcellular Localization of TaCDPK27
4.4. BSMV-VIGS Assay
4.5. Physiological Measurements and Histochemical Staining
4.6. qRT-PCR Assay
4.7. Cytosolic Ca2+ Fluorescence Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manghwar, H.; Hussain, A. Mechanism of tobacco osmotin gene in plant responses to biotic and abiotic stress tolerance: A brief history. BIOCELL 2022, 46, 623–632. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; El-Sharkawy, I.; Sherif, S. Salt stress signals on demand: Cellular events in the right context. Int. J. Mol. Sci. 2020, 21, 3918. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular cloning and functional analysis of GmLACS2-3 reveals its involvement in cutin and suberin biosynthesis along with abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant. Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Du, H.; Wang, Y.; Wang, H.; Yang, S.; Li, C.; Chen, N.; Yang, H.; Zhang, Y.; Zhu, Y.; et al. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J. Integr. Plant Biol. 2021, 63, 510–527. [Google Scholar] [CrossRef]
- Kolbert, Z.; Ördög, A. Involvement of nitric oxide (NO) in plant responses to metalloids. J. Hazard. Mater. 2021, 420, 126606. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kavi Kishor, P.B. Engineering salinity tolerance in plants: Progress and prospects. Planta 2020, 251, 76. [Google Scholar] [CrossRef]
- Ayaz, A.; Saqib, S.; Huang, H.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant. Physiol. Biochem. 2021, 161, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hao, L.; Zhu, B.; Jiang, Z. Plant calcium signaling in response to potassium deficiency. Int. J. Mol. Sci. 2018, 19, 3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atif, R.M.; Shahid, L.; Waqas, M.; Ali, B.; Rashid, M.A.R.; Azeem, F.; Nawaz, M.A.; Wani, S.H.; Chung, G. Insights on calcium-dependent protein kinases (cpks) signaling for abiotic stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 5298. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Cao, A.; Wang, F.; Chen, X.; Xie, S.; Shen, H.; Jin, X.; Li, H. Calcium-dependent protein kinase genes in Glycyrrhiza Uralensis appear to be involved in promoting the biosynthesis of glycyrrhizic acid and flavonoids under salt stress. Molecules 2019, 24, 1837. [Google Scholar] [CrossRef] [Green Version]
- Cieśla, A.; Mituła, F.; Misztal, L.; Fedorowicz-Stronska, O.; Janicka, S.; Tajdel-Zielinska, M.; Marczak, M.; Janicki, M.; Ludwików, A.; Sadowski, J. A role for barley calcium-dependent protein kinase CPK2a in the response to drought. Front. Plant Sci. 2016, 7, 1550. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Z.; Li, H.X.; Xu, M.J.; Wang, P.F.; Xiao, X.H.; Kang, G.Z. Functional characterization and regulatory mechanism of wheat CPK34 kinase in response to drought stress. BMC Genomics 2020, 21, 577. [Google Scholar] [CrossRef]
- Bundó, M.; Coca, M. Calcium-dependent protein kinase OsCPK10 mediates both drought tolerance and blast disease resistance in rice plants. J. Exp. Bot. 2017, 68, 2963–2975. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, X.; Chang, S.; Chu, Z.; Wang, H.; Han, S.; Wang, Y. Calcium-dependent protein kinase 21 phosphorylates 14-3-3 proteins in response to ABA signaling and salt stress in rice. Biochem. Biophys Res. Commun. 2017, 493, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Borkiewicz, L.; Polkowska-Kowalczyk, L.; Cieśla, J.; Sowiński, P.; Jończyk, M.; Rymaszewski, W.; Szymańska, K.P.; Jaźwiec, R.; Muszyńska, G.; Szczegielniak, J. Expression of maize calcium-dependent protein kinase (ZmCPK11) improves salt tolerance in transgenic Arabidopsis plants by regulating sodium and potassium homeostasis and stabilizing photosystem II. Physiol. Plant 2020, 168, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wu, C.; Luo, C.; Wei, M.; Qu, S.; Wang, S. Overexpression of MdCPK1a gene, a calcium dependent protein kinase in apple, increase tobacco cold tolerance via scavenging ROS accumulation. PLoS ONE 2020, 15, e0242139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Bi, Z.; Liu, Q.; Xu, T.; Yu, N.; Cao, Y.; Zhu, A.; Wu, W.; Zhan, X.; et al. Impaired function of the calcium-dependent protein kinase, OsCPK12, leads to early senescence in rice (Oryza sativa L.). Front. Plant Sci. 2019, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaghan, J.; Matschi, S.; Shorinola, O.; Rovenich, H.; Matei, A.; Segonzac, C.; Malinovsky, F.G.; Rathjen, J.P.; MacLean, D.; Romeis, T.; et al. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 2014, 16, 605–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Shen, F.; Hong, Y.; Rong, W.; Du, L.; Liu, X.; Xu, H.; Ma, L.; Zhang, Z. The wheat calcium-dependent protein kinase TaCPK7-D positively regulates host resistance to sharp eyespot disease. Mol. Plant Pathol. 2016, 17, 1252–1264. [Google Scholar] [CrossRef]
- Gong, J.; Shi, T.; Li, Y.; Wang, H.; Li, F. Genome-wide identification and characterization of calcium metabolism related gene families in Arabidopsis thaliana and their regulation by Bacillus amyloliquefaciens under high calcium stress. Front. Plant Sci. 2021, 12, 707496. [Google Scholar] [CrossRef]
- Zuo, R.; Hu, R.; Chai, G.; Xu, M.; Qi, G.; Kong, Y.; Zhou, G. Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populus trichocarpa). Mol. Biol. Rep. 2013, 40, 2645–2662. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; He, Q.; Daud, M.K.; Chen, J.; Zhu, S. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS ONE 2014, 9, e98189. [Google Scholar]
- Zou, B.; Ding, Y.; Liu, H.; Hua, J. Silencing of copine genes confers common wheat enhanced resistance to powdery mildew. Mol. Plant Pathol. 2018, 19, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, W.; Xie, X.; Wang, Z.; Guan, P.; Peng, H.; Jiao, Y.; Ni, Z.; Sun, Q.; Guo, W. A collinearity-incorporating homology inference strategy for connecting emerging assemblies in the Triticeae Tribe as a Pilot Practice in the Plant Pangenomic Era. Mol. Plant 2020, 13, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Zhou, J.; Zhang, X.; Feng, Z.; Wei, F.; Zhao, L.; Zhang, Y.; Feng, H.; Zhu, H. Calcium-dependent protein kinase GhCDPK28 was dentified and involved in verticillium wilt resistance in cotton. Front. Plant Sci. 2021, 12, 772649. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Q.; Yang, J.; Xie, G.; Liu, J.H. PtrCDPK10 of Poncirus trifoliata functions in dehydration and drought tolerance by reducing ROS accumulation via phosphorylating PtrAPX. Plant Sci. 2020, 291, 110320. [Google Scholar] [CrossRef]
- İbrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M.; et al. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotechnol. 2021, 329, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Lamers, J.; van der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis calcium-dependent protein kinases (CDPKs) and their roles in plant growth regulation and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Sun, H.; Zhao, N.; Jing, X.; Shen, X.; Chen, S. The Arabidopsis Ca2+-dependent protein kinase CPK27 is required for plant response to salt-stress. Gene 2015, 563, 203–214. [Google Scholar] [CrossRef]
- Hunter, K.; Kimura, S.; Rokka, A.; Tran, H.C.; Toyota, M.; Kukkonen, J.P.; Wrzaczek, M. CRK2 enhances salt tolerance by regulating callose deposition in connection with PLDα1. Plant Physiol. 2019, 180, 2004–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance. J. Plant Physiol. 2015, 185, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, H.T.; El-Helely, A.A. Programmed cell death in plants: Insights into developmental and stress-induced cell death. Curr. Protein Pept. Sci. 2021, 22, 873–889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hong, Q.; Li, Y.; Li, Q.; Wang, M. Autophagy contributes to regulate the ROS levels and PCD progress in TMV-infected tomatoes. Plant Sci. 2018, 269, 12–19. [Google Scholar] [CrossRef]
- Yue, J.Y.; Wang, Y.J.; Jiao, J.L.; Wang, H.Z. Silencing of ATG2 and ATG7 promotes programmed cell death in wheat via inhibition of autophagy under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112761. [Google Scholar] [CrossRef]
- Yue, J.Y.; Wang, Y.J.; Jiao, J.L.; Wang, H.Z. Comparative transcriptomic and metabolic profiling provides insight into the mechanism by which the autophagy inhibitor 3-MA enhances salt stress sensitivity in wheat seedlings. BMC Plant Biol. 2021, 21, 577. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Yu, B.; Yang, W.; Yue, J.; Wang, H. Monitoring autophagy in wheat living cells by visualization of fluorescence protein-tagged ATG8. Plant Cell Tiss Organ. Cult. 2018, 134, 481–489. [Google Scholar] [CrossRef]
- Yue, J.; Sun, H.; Zhang, W.; Pei, D.; He, Y.; Wang, H.Z. Wheat homologs of yeast ATG6 function in autophagy and are implicated in powdery mildew immunity. BMC Plant Biol. 2015, 15, 95. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cao, C.; Guo, Z.; Zhang, Q.; Li, S.; Zhang, X.; Gong, J.; Shen, Y. Herbivore exposure alters ion fluxes and improves salt tolerance in a desert shrub. Plant Cell Environ. 2020, 43, 400–419. [Google Scholar] [CrossRef]


| Treatments | Root Length | Leaf Length |
|---|---|---|
| CK | 36.50 ± 0.860 a | 31.10 ± 0.455 a |
| γG | 32.07 ± 0.262 b | 29.23 ± 0.249 b |
| TaCDPK27 | 30.80 ± 0.170 c | 27.27 ± 0.287 c |
| NaCl | 29.00 ± 0.125 d | 25.97 ± 0.262 d |
| γG+NaCl | 25.77 ± 0.655 e | 24.33 ± 0.368 e |
| TaCDPK27+NaCl | 22.07 ± 0.478 f | 22.37 ± 0.340 f |
| Treatments | Fv/Fm | Y(II) | qP | F0 | NPQ | ETR |
|---|---|---|---|---|---|---|
| CK | 0.795 ± 0.002 a | 0.610 ± 0.002 a | 0.840 ± 0.003 a | 0.072 ± 0.000 a | 0.436 ± 0.101 f | 84.2 ± 0.309 a |
| γG | 0.788 ± 0.001 ab | 0.567 ± 0.001 b | 0.803 ± 0.002 b | 0.063 ± 0.004 b | 0.582 ± 0.016 e | 78.6 ± 1.717 b |
| TaCDPK27 | 0.779 ± 0.001 b | 0.525 ± 0.002 c | 0.759 ± 0.006 c | 0.057 ± 0.002 c | 0.762 ± 0.004 d | 70.6 ± 0.818 c |
| NaCl | 0.757 ± 0.001 c | 0.464 ± 0.001 d | 0.736 ± 0.003 d | 0.048 ± 0.001 d | 0.976 ± 0.003 c | 65.2 ± 3.626 d |
| γG+NaCl | 0.737 ± 0.002 d | 0.364 ± 0.014 e | 0.605 ± 0.002 e | 0.040 ± 0.002 e | 1.137 ± 0.107 b | 50.4 ± 1.879 e |
| TaCDPK27+NaCl | 0.713 ± 0.016 e | 0.280 ± 0.043 f | 0.464 ± 0.004 f | 0.032 ± 0.001 f | 1.602 ± 0.052 a | 38.1 ± 1.306 f |
| Term | Gene | Primer Sequences (Forward/Reverse Primer) |
|---|---|---|
| ORF amplification | Calcium-dependent protein kinase 27 (TaCDPK27) | F:ATGGGCAACGTCTGCGT |
| R:TTAGCTATTTGACTTTATGTTCAAGGCATCCCT | ||
| qRT-PCR | TaCDPK27 | F:GCCGCCTTCCAATACTTT |
| R:TTATCCTGATCTACTTCGCCTA | ||
| Subcellular localization | TaCDPK27 | F: AAGTCCGGAGCTAGCTCTAGAATGGGCAACGTCTGCGT |
| R: GCCCTTGCTCACCATGGATCCGCTATTTGACTTTATGTTCAAG | ||
| BMSV-VIGS | TaCDPK27 | F: CAAACATTTTTTTTTTTTTTTAGCTAGCGCCGCCTTCCAATACTTT |
| R:GATTCTTCTTCCGTTGCTAGCTTATCCTGATCTACTTCGCCTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.-Y.; Jiao, J.-L.; Wang, W.-W.; Wang, H.-Z. The Calcium-Dependent Protein Kinase TaCDPK27 Positively Regulates Salt Tolerance in Wheat. Int. J. Mol. Sci. 2022, 23, 7341. https://doi.org/10.3390/ijms23137341
Yue J-Y, Jiao J-L, Wang W-W, Wang H-Z. The Calcium-Dependent Protein Kinase TaCDPK27 Positively Regulates Salt Tolerance in Wheat. International Journal of Molecular Sciences. 2022; 23(13):7341. https://doi.org/10.3390/ijms23137341
Chicago/Turabian StyleYue, Jie-Yu, Jin-Lan Jiao, Wen-Wen Wang, and Hua-Zhong Wang. 2022. "The Calcium-Dependent Protein Kinase TaCDPK27 Positively Regulates Salt Tolerance in Wheat" International Journal of Molecular Sciences 23, no. 13: 7341. https://doi.org/10.3390/ijms23137341
APA StyleYue, J.-Y., Jiao, J.-L., Wang, W.-W., & Wang, H.-Z. (2022). The Calcium-Dependent Protein Kinase TaCDPK27 Positively Regulates Salt Tolerance in Wheat. International Journal of Molecular Sciences, 23(13), 7341. https://doi.org/10.3390/ijms23137341





