Low-Dose Rifabutin Increases Cytotoxicity in Antimitotic-Drug-Treated Resistant Cancer Cells by Exhibiting Strong P-gp-Inhibitory Activity
Abstract
:1. Introduction
2. Results
2.1. VIC + Rifabutin Exhibits Increased Cytotoxicity in P-gp-Overexpressing Drug-Resistant KBV20C Cancer Cells
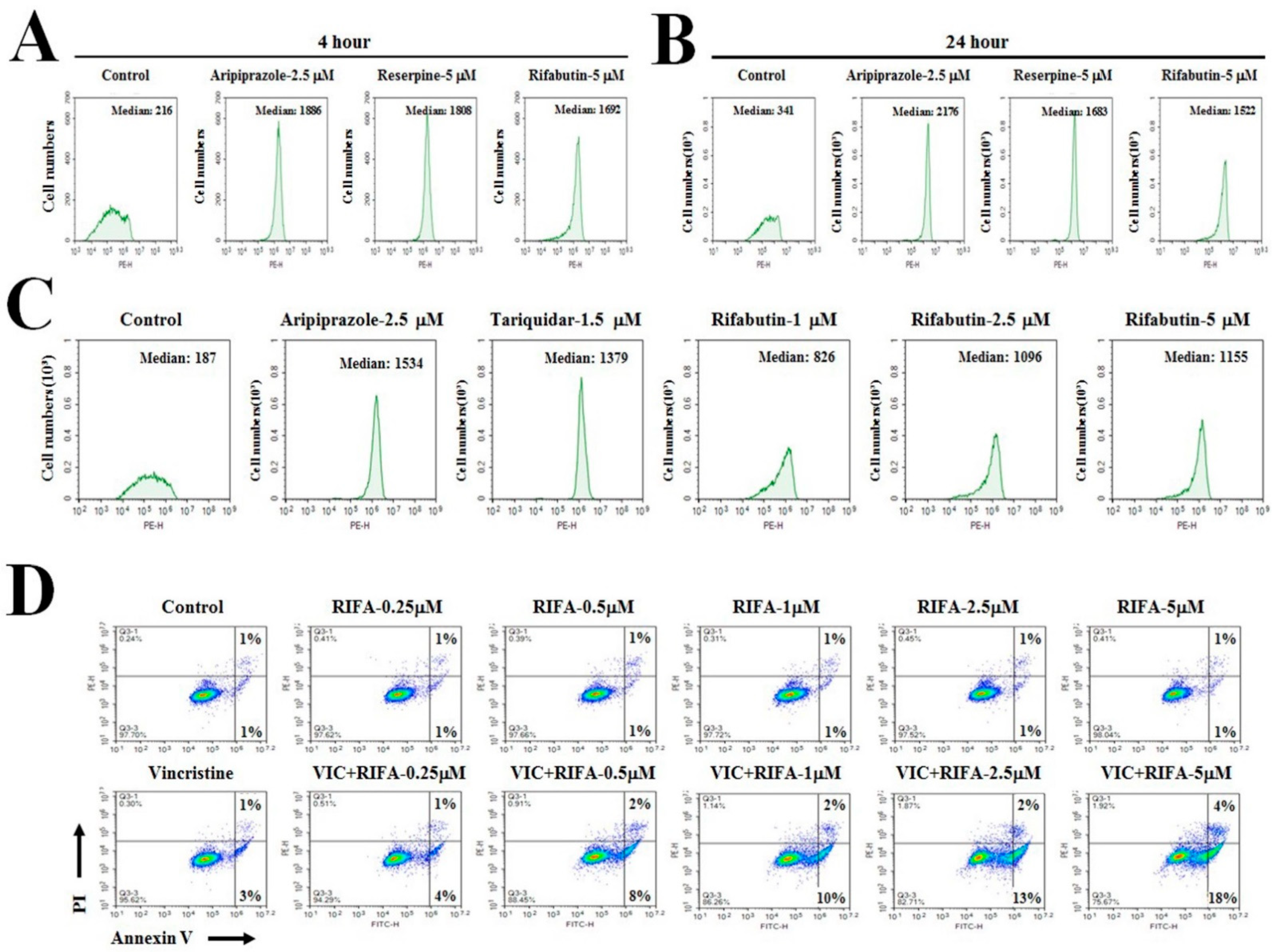
2.2. Low-Dose Rifabutin Has Strong P-gp-Inhibitory Activity after 4 h of Treatment
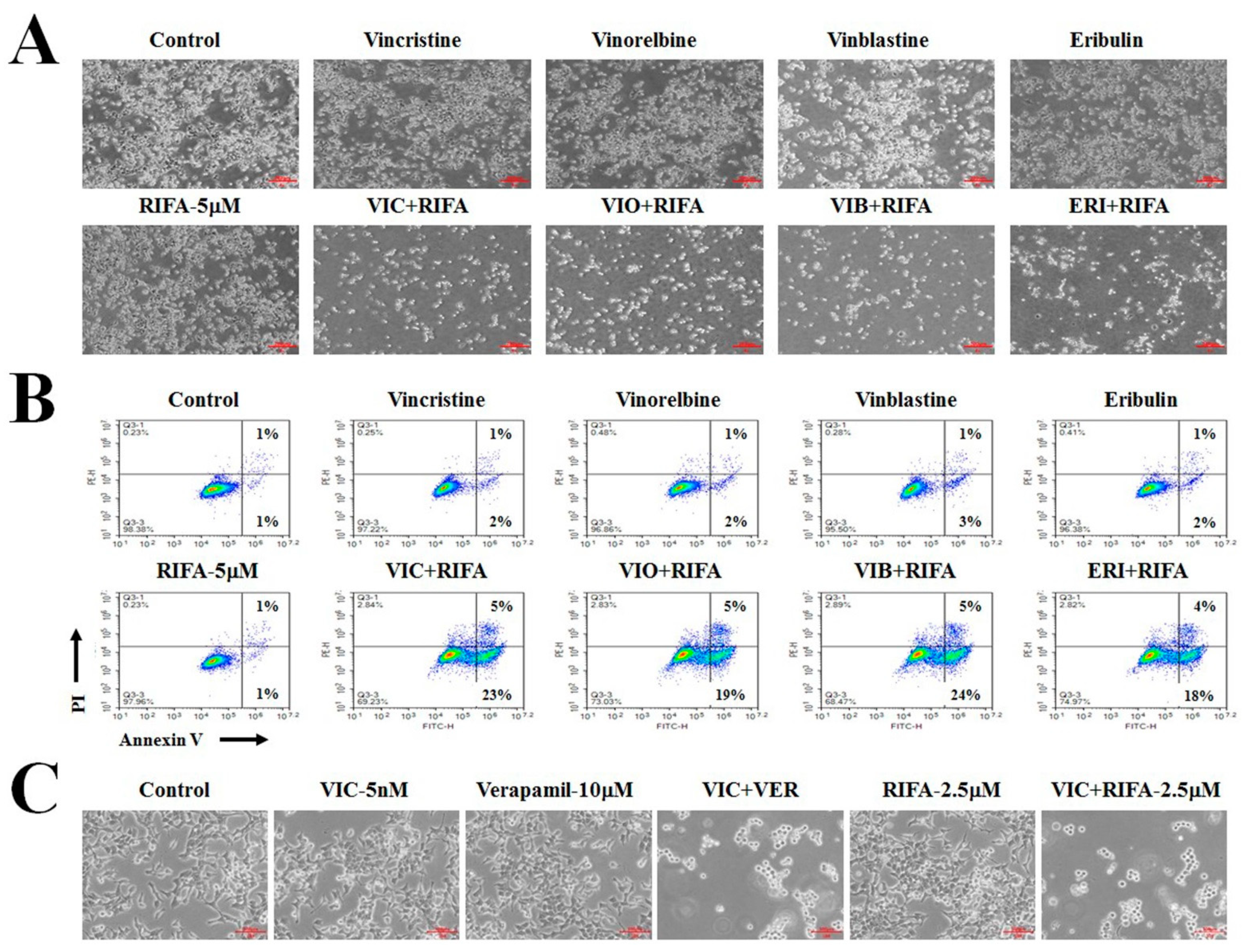
2.3. Rifabutin Causes Dose-Dependent Increases in Cytotoxicity in VIC-Treated KBV20C Cells by Inducing Early Apoptosis
2.4. Rifabutin Induces G2-Arrest and Increases DNA Damage in VIC-Treated Resistant KBV20C Cells
2.5. Co-Treatment with Rifabutin Increases the Cytotoxicity of Other Antimitotic Drugs in KBV20C Cells
2.6. Other Antibiotic Drugs (Rifampin, Rifapentine, and Rifaximin) Exhibit Minor P-gp-Inhibitory Activity
3. Discussion
4. Methods and Materials
4.1. Reagents and Cell Culture
4.2. Microscopic Observation
4.3. Cell Viability Assay
4.4. Colony Forming Assay
4.5. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.6. Annexin V Analysis
4.7. Rhodamine123 Uptake Tests
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug resistance |
| P-gp | P-glycoprotein |
| FACS | Fluorescence-activated cell sorting |
| FDA | United States Food and Drug Administration |
| DMSO | Dimethyl sulfoxide |
| C-PARP | Cleaved poly ADP ribose polymerase |
| ANOVA | One-way analysis of variance |
| SDS | Sodium dodecyl sulfate |
| PAGE | Polyacrylamide gel electrophoresis |
| FITC | Fluorescein isothiocyanate |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
References
- Rao, C.V.; Kurkjian, C.D.; Yamada, H.Y. Mitosis-targeting natural products for cancer prevention and therapy. Curr. Drug Targets 2012, 13, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Florian, S.; Mitchison, T.J. Anti-Microtubule Drugs. Methods Mol. Biol. 2016, 1413, 403–421. [Google Scholar] [PubMed]
- Inoue, K.; Saito, T.; Okubo, K.; Kimizuka, K.; Yamada, H.; Sakurai, T.; Ishizuna, K.; Hata, S.; Kai, T.; Kurosumi, M. Phase II clinical study of eribulin monotherapy in Japanese patients with metastatic breast cancer who had well-defined taxane resistance. Breast Cancer Res. Treat. 2016, 157, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, S.H.; Guo, X.L. New insights into Vinca alkaloids resistance mechanism and circumvention in lung cancer. Biomed. Pharmacother. 2017, 96, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updates 2021, 55, 100754. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.K. Cancer Stem Cells as a Potential Target to Overcome Multidrug Resistance. Front. Oncol. 2020, 10, 764. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar]
- Zhang, Y.; Zeng, Z.; Zhao, J.; Li, D.; Liu, M.; Wang, X. Measurement of Rhodamine 123 in Three-Dimensional Organoids: A Novel Model for P-Glycoprotein Inhibitor Screening. Basic Clin. Pharmacol. Toxicol. 2016, 119, 349–352. [Google Scholar] [CrossRef]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Wang, X.; Vongpunsawad, S.; Tromp, G.; Kuivaniemi, H. Editorial: FDA-Approved drug repositioning for P-glycoprotein overexpressing resistant cancer. Front. Oncol. 2021, 11, 632657. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, H.S. Drug Repositioning With an Anticancer Effect: Contributions to Reduced Cancer Incidence in Susceptible Individuals. In Vivo 2021, 35, 3039–3044. [Google Scholar] [CrossRef] [PubMed]
- Sivik, J.M.; Davidson, J.; Hale, C.M.; Drabick, J.J.; Talamo, G. Addition of doxycycline to ciprofloxacin for infection prophylaxis during autologous stem cell transplants for multiple myeloma. Support. Care Cancer 2018, 26, 3055–3061. [Google Scholar] [CrossRef]
- Mitrovic, A.; Kos, J. Nitroxoline: Repurposing its antimicrobial to antitumor application. Acta Biochim. Pol. 2019, 66, 521–531. [Google Scholar]
- Pfab, C.; Schnobrich, L.; Eldnasoury, S.; Gessner, A.; El-Najjar, N. Repurposing of Antimicrobial Agents for Cancer Therapy: What Do We Know? Cancers 2021, 13, 3193. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Gao, Y.; Wu, H.; Dong, W.; Liu, L. Antibiotic drug rifabutin is effective against lung cancer cells by targeting the eIF4E-beta-catenin axis. Biochem. Biophys. Res. Commun. 2016, 472, 299–305. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, A.; Kadhiravan, T.; Tharyan, P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst. Rev. 2013, 2013, CD007545. [Google Scholar] [CrossRef]
- Regazzi, M.; Carvalho, A.C.; Villani, P.; Matteelli, A. Treatment optimization in patients co-infected with HIV and Mycobacterium tuberculosis infections: Focus on drug-drug interactions with rifamycins. Clin. Pharmacokinet. 2014, 53, 489–507. [Google Scholar] [CrossRef]
- Jiang, C.; Lee, S.H.; Park, J.H.; Lee, J.S.; Park, J.W.; Kim, J.R.; Lee, S.H.; Kim, H.S.; Yoon, S. A Low Dose of Aripiprazole Has the Strongest Sensitization Effect Among 19 Repositioned Bipolar Drugs in P-gp-overexpressing Drug-resistant Cancer Cells. Anticancer Res. 2021, 41, 687–697. [Google Scholar] [CrossRef]
- Jiang, C.; Zheng, T.; Park, J.H.; Lee, J.S.; Oh, Y.; Kundu, A.; Kim, H.S.; Yoon, S. Sensitization Effects of Repurposed Blood Pressure-regulating Drugs on Drug-resistant Cancer Cells. Anticancer Res. 2021, 41, 6179–6190. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lee, J.S.; Lee, J.S.; Park, J.H.; Kim, H.S.; Yoon, S. Co-treatment of Low Dose Pacritinib, a Phase III Jak2 Inhibitor, Greatly Increases Apoptosis of P-gp Over-expressing Cancer Cells With Multidrug Resistance. Anticancer Res. 2022, 42, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lee, J.S.; Lee, J.S.; Park, J.H.; Kim, H.S.; Yoon, S. JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells. Int. J. Mol. Sci. 2022, 23, 4597. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, D.M. Rifamycins, Alone and in Combination. Cold Spring Harb. Perspect. Med. 2016, 6, a027011. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.S.; Kim, I.S.; Yoon, S. Histamine Receptor Antagonists, Loratadine and Azelastine, Sensitize P-gp-overexpressing Antimitotic Drug-resistant KBV20C Cells Through Different Molecular Mechanisms. Anticancer Res. 2019, 39, 3767–3775. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.J.; Lee, B.M.; Yoon, S. Co-treatment With HIV Protease Inhibitor Nelfinavir Greatly Increases Late-phase Apoptosis of Drug-resistant KBV20C Cancer Cells Independently of P-Glycoprotein Inhibition. Anticancer Res. 2019, 39, 3757–3765. [Google Scholar] [CrossRef]
- Kim, K.S.; Jiang, C.; Kim, J.Y.; Park, J.H.; Kim, H.R.; Lee, S.H.; Kim, H.S.; Yoon, S. Low-Dose Crizotinib, a Tyrosine Kinase Inhibitor, Highly and Specifically Sensitizes P-Glycoprotein-Overexpressing Chemoresistant Cancer Cells Through Induction of Late Apoptosis in vivo and in vitro. Front. Oncol. 2020, 10, 696. [Google Scholar] [CrossRef]
- Cheon, J.H.; Kim, K.S.; Yadav, D.K.; Kim, M.; Kim, H.S.; Yoon, S. The JAK2 inhibitors CEP-33779 and NVP-BSK805 have high P-gp inhibitory activity and sensitize drug-resistant cancer cells to vincristine. Biochem. Biophys. Res. Commun. 2017, 490, 1176–1182. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.; Lee, B.M.; Kim, H.S.; Yoon, S. P-gp Inhibition by the Anti-psychotic Drug Pimozide Increases Apoptosis, as well as Expression of pRb and pH2AX in Highly Drug-resistant KBV20C Cells. Anticancer Res. 2018, 38, 5685–5692. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Oh, Y.; Kim, H.S.; Yoon, S. Low-Dose Rifabutin Increases Cytotoxicity in Antimitotic-Drug-Treated Resistant Cancer Cells by Exhibiting Strong P-gp-Inhibitory Activity. Int. J. Mol. Sci. 2022, 23, 7383. https://doi.org/10.3390/ijms23137383
Lee JS, Oh Y, Kim HS, Yoon S. Low-Dose Rifabutin Increases Cytotoxicity in Antimitotic-Drug-Treated Resistant Cancer Cells by Exhibiting Strong P-gp-Inhibitory Activity. International Journal of Molecular Sciences. 2022; 23(13):7383. https://doi.org/10.3390/ijms23137383
Chicago/Turabian StyleLee, Ji Sun, Yunmoon Oh, Hyung Sik Kim, and Sungpil Yoon. 2022. "Low-Dose Rifabutin Increases Cytotoxicity in Antimitotic-Drug-Treated Resistant Cancer Cells by Exhibiting Strong P-gp-Inhibitory Activity" International Journal of Molecular Sciences 23, no. 13: 7383. https://doi.org/10.3390/ijms23137383






