Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum
Abstract
:1. Introduction
2. Results
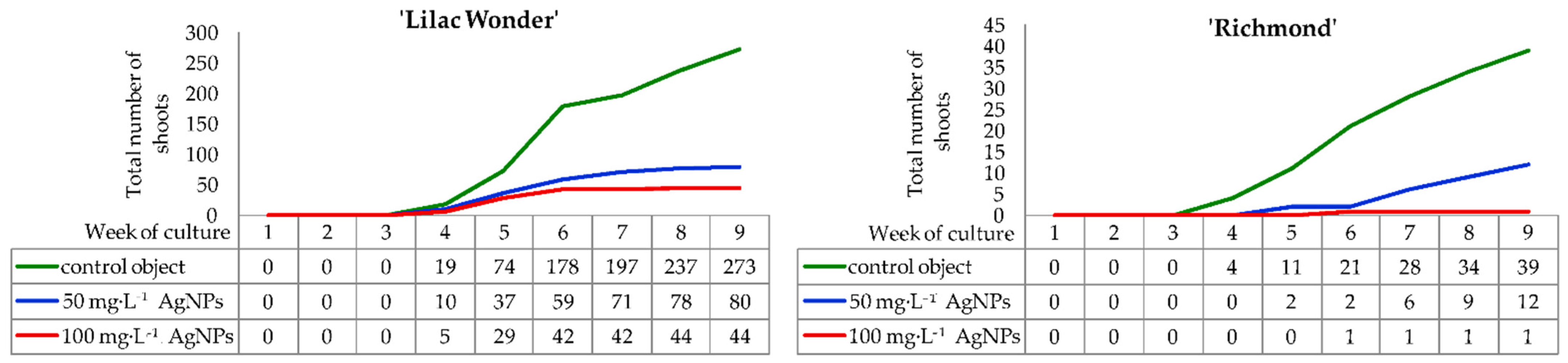
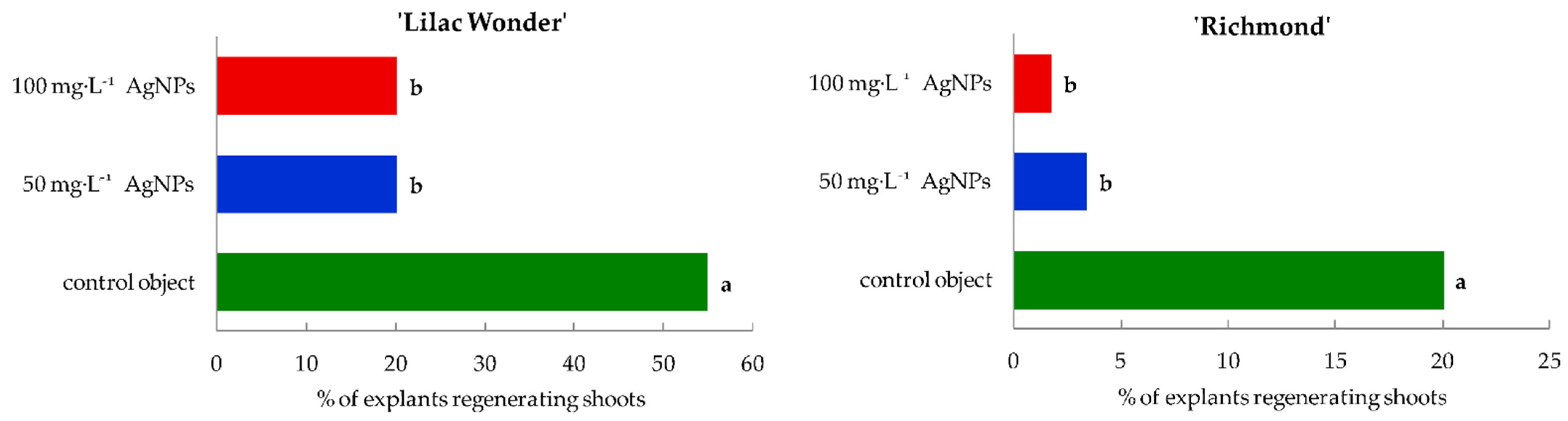
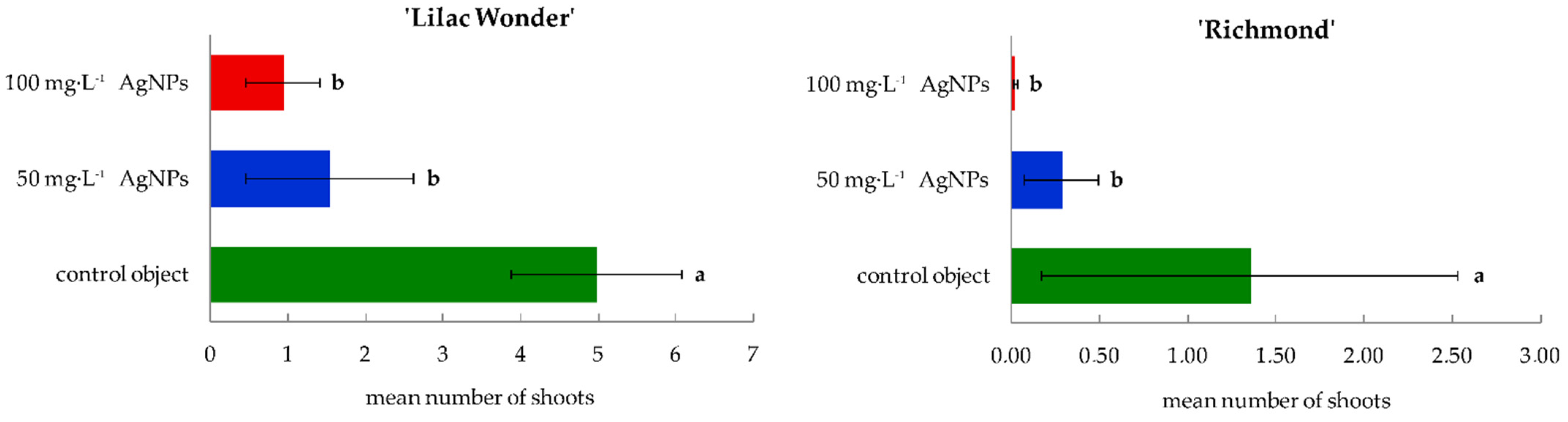
2.1. In Vitro Culture—Course and Efficiency of Adventitious Organogenesis
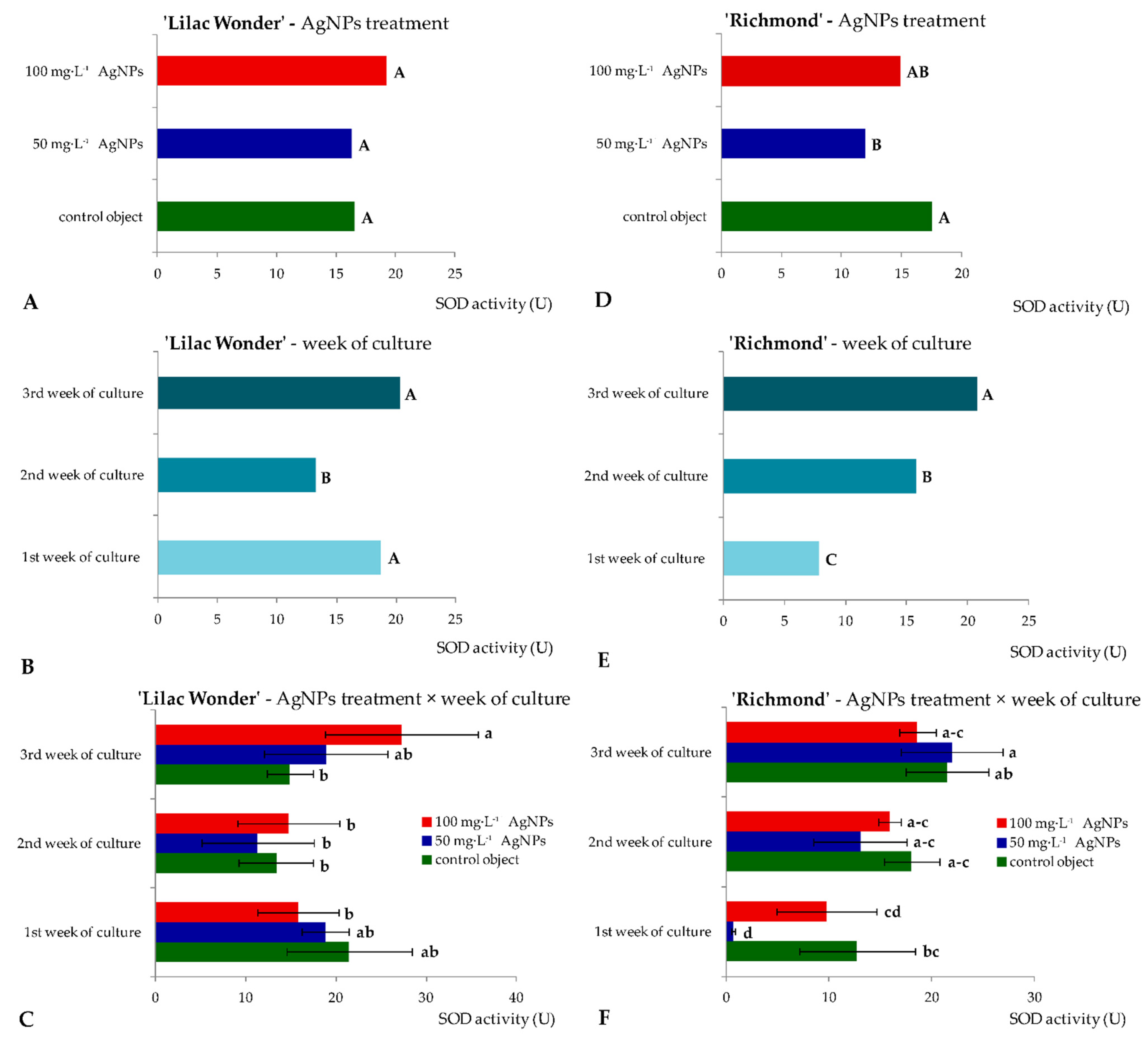
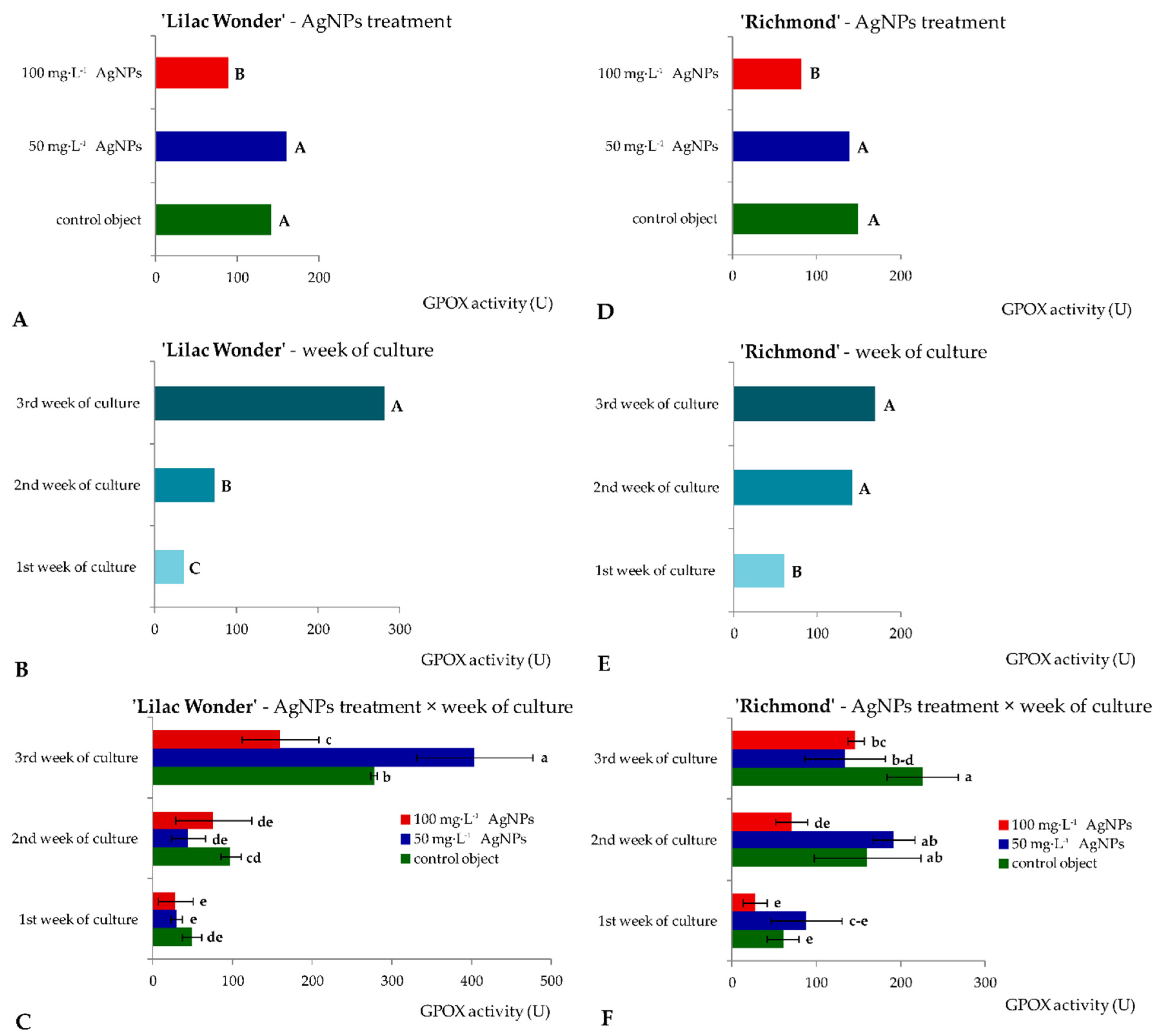
2.2. In Vitro Culture—Biosynthesis of Metabolites and Enzymatic Activity of Leaf Explants
2.3. Phenotype Analysis of Ex Vitro Grown Plants
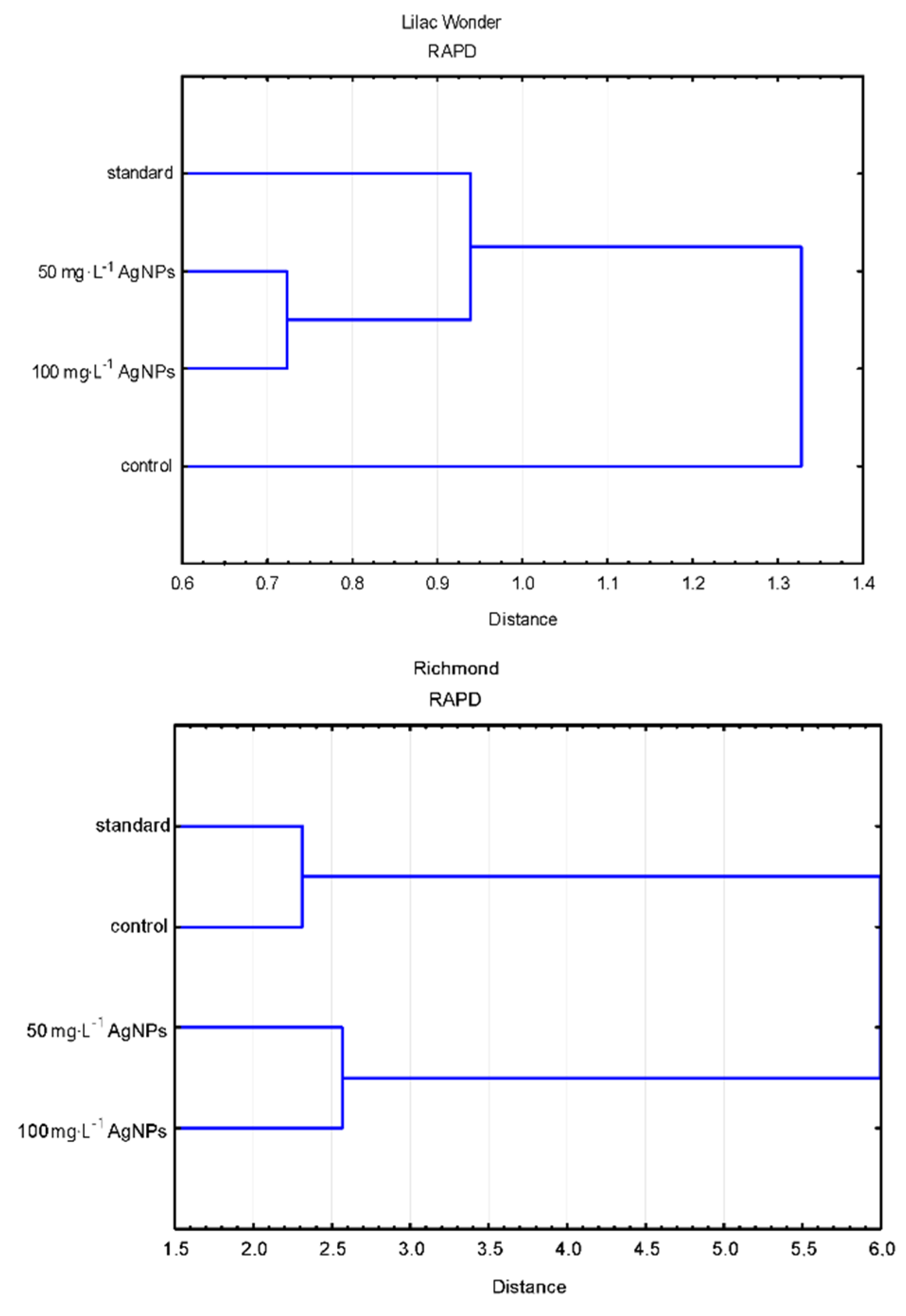
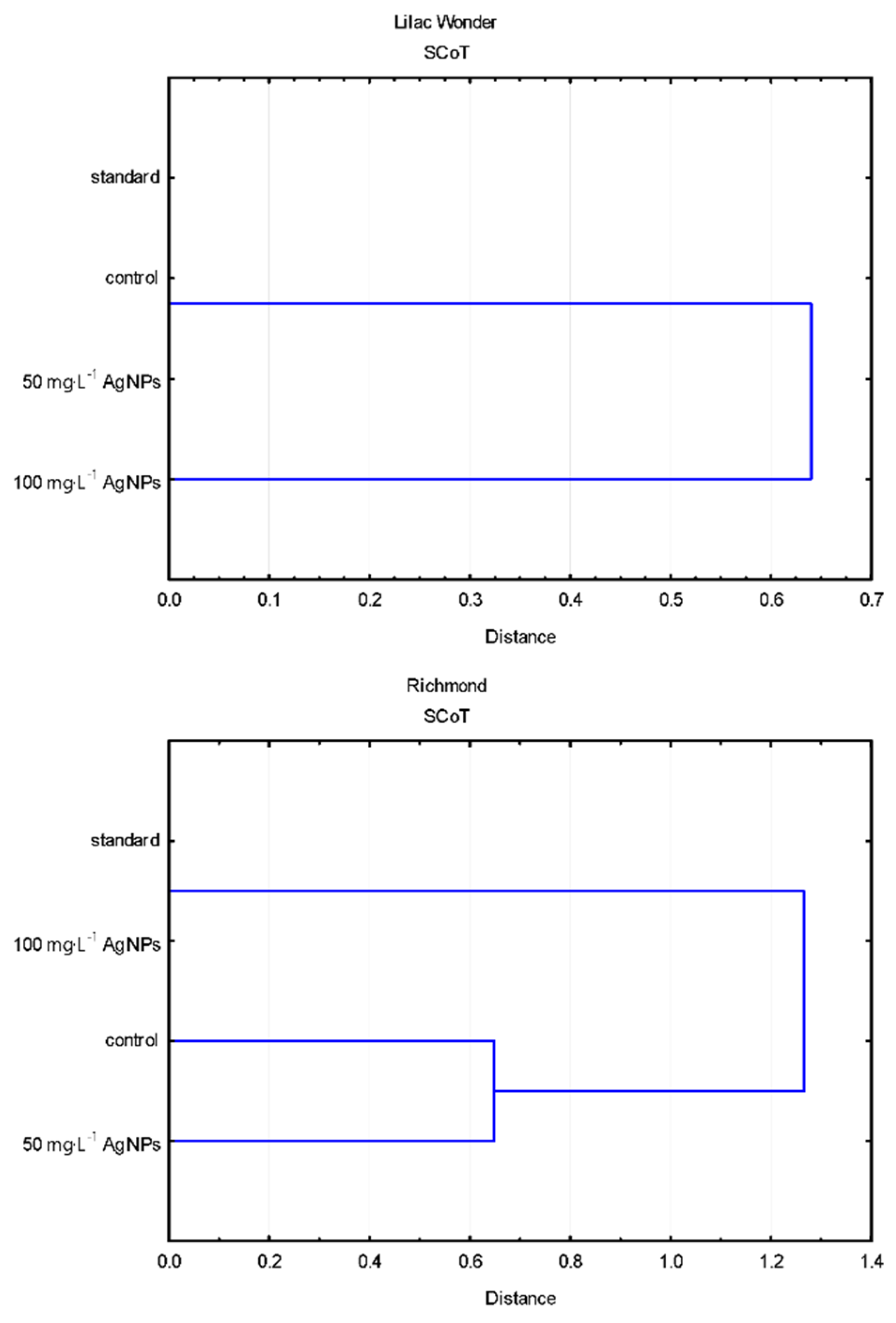
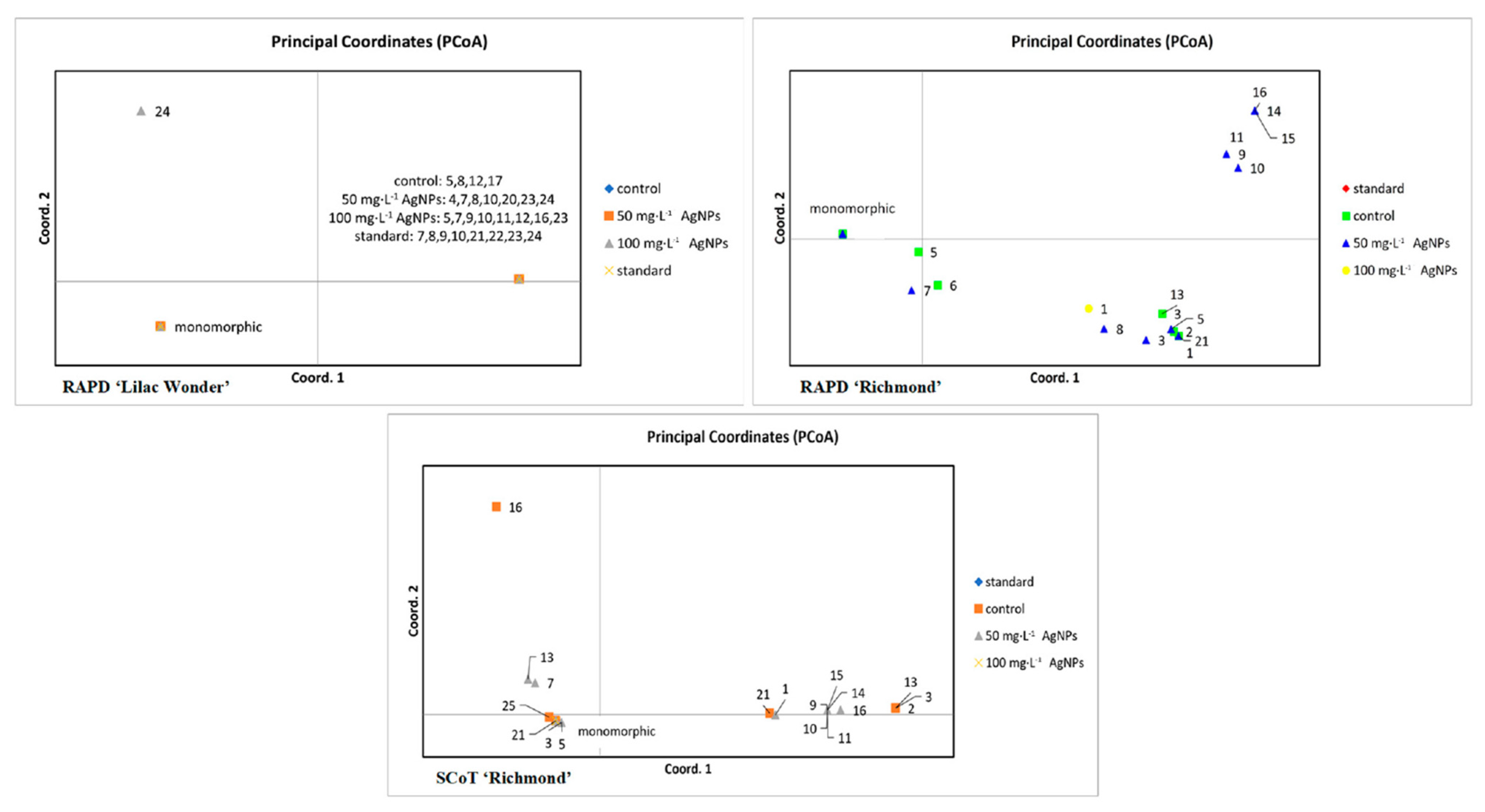
2.4. Analysis of Genetic Fidelity of Ex Vitro Grown Plants
3. Discussion
3.1. Effect of Silver Nanoparticles on the Regeneration Efficiency in Leaf Explants
3.2. Effect of Silver Nanoparticles on the Biochemical Events in Leaf Explants
3.3. Effect of Silver Nanoparticles on the Phenotype Stability
3.4. Effect of Silver Nanoparticles on the Genetic Stability
4. Materials and Methods
4.1. Plant Material—In Vitro Culture Conditions and Nanoparticles Treatment
4.2. Plant Material—Biochemical Array during In Vitro Culture
4.3. Plant Material—Acclimatization, Ex Vitro Growth, and Phenotype Analysis
4.4. Plant Material—Analysis of Genetic Stability of Ex Vitro Grown Plants
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadollahi, A.; Arzani, K.; Khoshghalb, H. The role of nanotechnology in horticultural crops postharvest management. Acta Hortic. 2010, 875, 49–56. [Google Scholar] [CrossRef]
- Milewska-Hendel, A.; Gawecki, R.; Zubko, M.; Stróż, D.; Kurczyńska, E. Diverse influence of nanoparticles on plant growth with a particular emphasis on crop plants. Acta Agrobot. 2016, 69, 1694. [Google Scholar] [CrossRef] [Green Version]
- Fayez, K.A.; El-Deeb, B.A.; Mostafa, N.Y. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017, 39, 155. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Tymoszuk, A.; Kulus, D. Silver nanoparticles induce genetic, biochemical, and phenotype variation in chrysanthemum. Plant Cell Tissue Org. Cult. 2020, 143, 331–344. [Google Scholar] [CrossRef]
- Tymoszuk, A. Silver nanoparticles effects on in vitro germination, growth, and biochemical activity of tomato, radish, and kale seedlings. Materials 2021, 14, 5340. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxixity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Tarafdar, J.C. Nanoparticle production, characterization ant its application to horticultural crops. In Compendium of Lectures of Winter School on Utilization of Degraded Land and Soil through Horticultural Crops for Improving Agricultural Productivity and Environmental Quality; Aishwath, O.P., Balra, S., Dubey, P.N., Mishra, B.K., Eds.; NRCSS: Ajmer, India, 2015; pp. 222–229. [Google Scholar]
- Mandal, D.; Lalrinchhani. Nanofertilizer and its application in horticulture. J. Appl. Hortic. 2021, 23, 70–77. [Google Scholar] [CrossRef]
- Rana, R.A.; Siddiqui, M.N.; Skalicky, M.; Brestic, M.; Hossain, A.; Kayesh, E.; Popov, M.; Hejnak, V.; Gupta, D.R.; Mahmud, N.U.; et al. Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae 2021, 7, 332. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Miler, N. Silver and gold nanoparticles impact on in vitro adventitious organogenesis in chrysanthemum, gerbera and Cape Primrose. Sci. Hortic. 2019, 257, 108766. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Gold nanoparticles affect the cryopreservation efficiency of in vitro-derived shoot tips of bleeding heart. Plant Cell Tissue Org. Cult. 2021, 146, 297–311. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health. 2012, 9, 1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.R.; Iacobucci, M.; Bhattacharya, P.; Ke, P.C. In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2016, 110, 2–12. [Google Scholar] [CrossRef]
- Čapek, J.; Roušar, T. Detection of oxidative stress induced by nanomaterials in cells–the roles of reactive oxygen species and glutathione. Molecules 2021, 26, 4710. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Su, J.; Jiang, J.; Zhang, F.; Liu, Y.; Ding, L.; Chen, S.; Chen, F. Current achivements and future prospects in the genetic breeding of chrysanthemum: A review. Hortic. Res. 2019, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Miler, N.; Jedrzejczyk, I.; Jakubowski, S.; Winiecki, J. Ovaries of chrysanthemum irradiated with high-energy photons and high-energy electrons can regenerate plants with novel traits. Agronomy 2021, 11, 1111. [Google Scholar] [CrossRef]
- Zalewska, M.; Tymoszuk, A.; Miler, N. New chrysanthemum culivars as a result of in vitro mutagenesis with the application of different explants types. Acta Sci. Pol. Hortorum Cultus 2011, 10, 109–123. [Google Scholar]
- Holme, I.B.; Gregersen, P.L.; Brinch-Pedersen, H. Induced genetic variation in crop plants by random or targeted mutagenesis: Convergence and differences. Front. Plant Sci. 2019, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.S.; Maria, M.; Muhammad, S.; Ali, S.M. Potential of mutation breeding to sustain food security. In Genetic Variation; Maia, R.T., de Araujo Campos, M., Eds.; Intech Open: London, UK, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Zalewska, M.; Miler, N.; Tymoszuk, A.; Drzewiecka, B.; Winiecki, J. Results of mutation breeding activity on Chrysanthemum × grandiflorum (Ramat.) Kitam. in Poland. EJPAU 2010, 13, 27. [Google Scholar]
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J. Exp. Bot. 2020, 71, 63–72. [Google Scholar] [CrossRef]
- RHSCC. The Royal Horticultural Society Colour Chart; RHSCC: London, UK, 1966. [Google Scholar]
- Miler, N.; Kulus, D. Microwave treatment can induce chrysanthemum phenotypic and genetic changes. Sci. Hortic. 2018, 227, 223–233. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Oćwieja, M. Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3). Acta Physiol. Plant. 2016, 38, 76. [Google Scholar] [CrossRef] [Green Version]
- Cvjetko, P.; Zovko, M.; Peharec Štefanić, P.; Biba, R.; Tkalec, M.; Domijan, A.M.; Vinković Vrček, I.; Letofsky-Papst, I.; Šikić, S.; Balen, B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ. Sci. Pollut. Res. 2018, 25, 5590–5602. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Ma, X.; Qalekhani, F. Effects of engineered aluminium and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L. Sci. Rep. 2020, 10, 3847. [Google Scholar] [CrossRef] [Green Version]
- Zalewska, M. Somatic Mutagenesis in Chrysanthemum (Dendranthema Grandiflora Tzvelev) Induced In Vivo and In Vitro by X and Gamma Radiation (In Polish); Wydawnictwo Uczelniane Akademii Techniczo Rolniczej w Bydgoszczy: Bydgoszcz, Poland, 1995; pp. 1–83. [Google Scholar]
- Broertjes, C.; Roest, S.; Bokelmann, G.S. Mutation breeding of Chrysanthemum morifolium Ram. using in vivo and in vitro adventitious bud techniques. Euphytica 1976, 25, 11–19. [Google Scholar] [CrossRef]
- Broertjes, C.; Keen, A. Adventitious shoots: Do they develop from one cell? Euphytica 1980, 29, 73–87. [Google Scholar] [CrossRef]
- Nabeshima, T.; Yang, S.-J.; Ohno, S.; Honda, K.; Deguchi, A.; Doi, M.; Tatsuzawa, F.; Hosokawa, M. Histogen layers contribiuting to adventitious bud formation are determined by their cell division activities. Front. Plant Sci. 2017, 8, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lema-Rumińska, J.; Zalewska, M. Studies on flower pigment of chrysanthemum mutants: Nero and Wonder Groups. Acta Sci. Pol. Hortorum Cultus 2004, 3, 125–135. [Google Scholar] [CrossRef]
- Lema-Rumińska, J.; Zalewska, M. Changes in flower colour among Lady Group of Chrysanthemum × grandiflorum (Ramat.) Kitam. as a result of mutation breeding. Fol. Hortic. Ann. 2005, 17, 61–72. [Google Scholar]
- Lema-Rumińska, J.; Kulus, D.; Tymoszuk, A.; Varejão, J.M.T.B.; Bahcevandziev, K. Profile of secondary metabolites and genetic stability analysis in new lines of Echinacea purpurea (L.) Moench micropropagated via somatic embryogenesis. Industr. Crops Prod. 2019, 142, 111851. [Google Scholar] [CrossRef]
- Kulus, D.; Rewers, M.; Serocka, M.; Mikuła, A. Cryopreservation by encapsulation-dehydration affects the vegetative growth of chrysanthemum but does not disturb its chimeric structure. Plant Cell Tissue Org. Cult. 2019, 138, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic potential of nanoparticles: Structural and functional modifications in DNA. Front. Genet. 2021, 29, 1894. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A.; Jędrzejczyk, I.; Winiecki, J. Gold nanoparticles and electromagnetic irradiation in tissue culture systems of bleeding heart: Biochemical, physiological, and (cyto)genetic effects. Plant Cell Tissue Organ Cult. 2022, 149, 715–734. [Google Scholar] [CrossRef]
- Schum, A. Mutation breeding in ornamentals: An efficient breeding method? Acta Hortic. 2003, 612, 47–60. [Google Scholar] [CrossRef]
- Jerzy, M.; Zalewska, M. Flower colour recurrence in chrysanthemum and gerbera mutants propagated in vitro with meristems and leaf explants. Acta Hortic. 1997, 417, 611–614. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Pavlov, Y.I. Theoretical analysis of mutation hotspots and their DNA sequence context specificity. Mutat. Res. 2003, 544, 65–85. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.S.; Souza, M.M. Genomic in situ hybridization in plants. Genet. Molec. Res. 2013, 12, 2953–2965. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Domeradzka-Gajda, K.; Nocuń, M.; Roszak, J.; Janasik, B.; Quarles, C.D., Jr.; Wąsowicz, W.; Grobelny, J.; Tomaszewska, E.; Celichowski, G.; Ranoszek-Soliwoda, K.; et al. A study on the in vitro percutaneous absorption of silver nanoparticles in combination with aluminum chloride, methyl paraben or di-n-butyl phthalate. Toxicol. Lett. 2017, 272, 38–48. [Google Scholar] [CrossRef]
- Pudlarz, A.; Czechowska, E.; Ranoszek-Soliwoda, K.; Tomaszewska, E.; Celichowski, G.; Grobelny, J.; Szemraj, J. Immobilization of recombinant human catalase on gold and silver nanoparticles. Appl. Biochem. Biotechnol. 2018, 185, 717–735. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative Biochemistry of the Flavonoids. Phytochemistry 1967, 6, 1569–1573. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Homaee, M.B.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic. Environ. Biotechnol. 2016, 57, 544–553. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. In Methods of Biochemical Analysis; Glick, D., Ed.; Willey: New York, NY, USA, 1954; pp. 357–425. [Google Scholar] [CrossRef]
- Nowogórska, A.; Patykowski, J. Selected reactive oxygen species and antioxidany enzymes in common bean after Pseudomonas syringae pv. phaseolicola and Botrytis cinerea infection. Acta Physiol. Plant. 2015, 37, 1725. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.G.K.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl. Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collard, B.C.Y.; Mackill, D.J. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. iMEC: Online Marker Efficiency Calculator. Appl. Plant Sci. 2018, 6, e1159. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.S. Mathematical model for studying genetic variation in terms of restriction nucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [Green Version]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]

| Concentration of AgNPs (A) | ‘Lilac Wonder’ | ‘Richmond’ | ||||||
|---|---|---|---|---|---|---|---|---|
| Week of Culture (B) | ||||||||
| First | Second | Third | Mean | First | Second | Third | Mean | |
| Chlorophyll a content (mg·g−1 FW) | ||||||||
| control object | 0.83 ± 0.11 ab | 0.50 ± 0.22 bc | 0.30 ± 0.16 d | 0.54 B | 0.72 ± 0.08 bc | 0.82 ± 0.20 ab | 0.55 ± 0.20 bc | 0.70 A |
| 50 mg·L−1 | 0.84 ± 0.05 a | 0.73 ± 0.22 a–c | 0.48 ± 0.13 cd | 0.68 A | 0.84 ± 0.13 ab | 0.78 ± 0.19 a–c | 0.46 ± 0.17 cd | 0.69 A |
| 100 mg·L−1 | 0.74 ± 0.11 a–c | 0.65 ± 0.18 a–c | 0.42 ± 0.29 cd | 0.60 AB | 1.09 ± 0.26 a | 0.46 ± 0.18 cd | 0.16 ± 0.06 d | 0.57 A |
| Mean | 0.80 A | 0.63 B | 0.40 C | 0.88 A | 0.69 B | 0.39 C | ||
| Chlorophyll b content (mg·g−1 FW) | ||||||||
| control object | 0.43 ± 0.07 ab | 0.18 ± 0.12 cd | 0.10 ± 0.05 d | 0.24 C | 0.47 ± 0.01 a | 0.45 ± 0.10 a | 0.21 ± 0.07 bc | 0.38 A |
| 50 mg·L−1 | 0.50 ± 0.03 a | 0.35 ± 0.10 ab | 0.17 ± 0.05 d | 0.34 A | 0.48 ± 0.06 a | 0.46 ± 0.11 a | 0.20 ± 0.09 bc | 0.38 A |
| 100 mg·L−1 | 0.42 ± 0.06 ab | 0.34 ± 0.11 bc | 0.15 ± 0.12 d | 0.30 AB | 0.55 ± 0.15 a | 0.25 ± 0.06 b | 0.06 ± 0.07 c | 0.29 B |
| Mean | 0.45 A | 0.29 B | 0.14 C | 0.50 A | 0.39 ± B | 0.16 C | ||
| Chlorophyll a/b ratio | ||||||||
| control object | 1.92 ± 0.06 cd | 3.45 ± 1.47 a–c | 3.64 ± 1.58 ab | 3.00 A | 1.55 ± 0.16 a | 1.83 ± 0.13 a | 2.61 ± 0.33 a | 2.00 A |
| 50 mg·L−1 | 1.67 ± 0.17 d | 2.11 ± 0.36 a–d | 2.88 ± 0.21 a–d | 2.22 B | 1.76 ± 0.15 a | 1.70 ± 0.07 a | 2.57 ± 0.85 a | 2.01 A |
| 100 mg·L−1 | 1.75 ± 0.14 cd | 1.99 ± 0.24 b–d | 3.74 ± 1.60 a | 2.49 AB | 2.00 ± 0.09 a | 1.76 ± 0.26 a | 15.91 ± 9.57 a | 6.56 A |
| Mean | 1.78 B | 2.52 B | 3.42 A | 1.77 A | 1.76 A | 7.03 A | ||
| Chlorophylls (a + b) content (mg·g−1 FW) | ||||||||
| control object | 1.26 ± 0.17 a | 0.68 ± 0.33 b–e | 0.39 ± 0.20 e | 0.78 B | 1.19 ± 0.09 a-c | 1.27 ± 0.29 a | 0.76 ± 0.27 bc | 1.07 A |
| 50 mg·L−1 | 1.34 ± 0.04a | 1.07 ± 0.30 ac | 0.66 ± 0.19 ce | 1.02 A | 1.32 ± 0.18 a | 1.23 ± 0.29 ab | 0.65 ± 0.25 de | 1.07 A |
| 100 mg·L−1 | 1.16 ± 0.17 ab | 0.99 ± 0.29 a–d | 0.57 ± 0.39 de | 0.91 AB | 1.64 ± 0.41 a | 0.71 ± 0.24 cd | 0.22 ± 0.13 e | 0.86 B |
| Mean | 1.25 A | 0.91 B | 0.54 C | 1.38 A | 1.07 B | 0.54 C | ||
| Carotenoids content (mg·g−1 FW) | ||||||||
| control object | 0.23 ± 0.04 a | 0.13 ± 0.05 b–d | 0.07 ± 0.03 d | 0.14 A | 0.20 ± 0.01 bc | 0.22 ± 0.05 ab | 0.12 ± 0.05 c–e | 0.18 AB |
| 50 mg·L−1 | 0.21 ± 0.02 a | 0.17 ± 0.05 a–c | 0.11 ± 0.03 b–d | 0.16 A | 0.23 ± 0.03 ab | 0.23 ± 0.04 ab | 0.11 ± 0.05 de | 0.19 A |
| 100 mg·L−1 | 0.19 ± 0.03 ab | 0.16 ± 0.04 a–c | 0.09 ± 0.06 cd | 0.15A | 0.28 ± 0.08 a | 0.13 ± 0.03 cd | 0.05 ± 0.01 e | 0.15 B |
| Mean | 0.21 A | 0.15 B | 0.09 C | 0.24 A | 0.19 B | 0.09 C | ||
| Chlorophylls/carotenoids ratio | ||||||||
| control object | 5.61 ± 0.25 a | 5.13 ± 0.90 a | 6.20 ± 2.29 a | 5.65 A | 6.01 ± 0.59 a | 5.85 ± 0.34 a | 6.24 ± 0.56 a | 6.03 A |
| 50 mg·L−1 | 6.40 ± 0.32 a | 6.49 ± 0.89 a | 5.80 ± 0.33 a | 6.23 A | 5.80 ± 0.24 a | 5.26 ± 0.42 a | 6.00 ± 1.60 a | 5.68 A |
| 100 mg·L−1 | 6.22 ± 0.09 a | 6.18 ± 0.40 a | 6.36 ± 2.43 a | 6.25 A | 5.88 ± 0.19 a | 5.22 ± 0.48 a | 4.84 ± 2.99 a | 5.31 A |
| Mean | 6.08 A | 5.93 A | 6.12 A | 5.90 A | 5.44 A | 5.69 A | ||
| Anthocyanins content (mg·g−1 FW) | ||||||||
| control object | 0.28 ± 0.02 a | 0.18 ± 0.08 a | 0.13 ± 0.07 a | 0.20 A | 0.23 ± 0.06 ab | 0.23 ± 0.03 ab | 0.14 ± 0.04 c | 0.20 A |
| 50 mg·L−1 | 0.25 ± 0.04 a | 0.14 ± 0.04 a | 0.11 ± 0.02 a | 0.17 A | 0.25 ± 0.04 a | 0.16 ± 0.02 bc | 0.18 ± 0.06 a–c | 0.20 A |
| 100 mg·L−1 | 0.25 ± 0.03 a | 0.24 ± 0.07 a | 0.12 ± 0.02 a | 0.20 A | 0.25 ± 0.02 a | 0.16 ± 0.05 bc | 0.16 ± 0.04 bc | 0.19 A |
| Mean | 0.26 A | 0.19 A | 0.12 A | 0.24 A | 0.18 B | 0.16 B | ||
| Phenolic compounds content (mg·g−1 FW) | ||||||||
| control object | 9.54 ± 2.24 ab | 6.72 ± 1.79 b–d | 6.32 ± 2.37 cd | 7.53 AB | 6.09 ± 2.47 a | 9.21 ± 1.03 a | 7.04 ± 3.39 a | 7.45 A |
| 50 mg·L−1 | 7.67 ± 0.14 a–d | 6.79 ± 1.63 b–d | 5.52 ± 1.12 d | 6.66 B | 9.66 ± 2.25 a | 7.77 ± 3.69 a | 9.13 ± 1.91 a | 8.85 A |
| 100 mg·L−1 | 9.09 ± 1.40 a–c | 10.32 ± 1.06 a | 6.11 ± 1.50 cd | 8.51 A | 6.78 ± 1.84 a | 8.03 ± 3.27 a | 9.88 ± 1.49 a | 8.23 A |
| Mean | 8.77 A | 7.94 A | 5.98 B | 7.51 A | 8.34 A | 8.68 A | ||
| Concentration of AgNPs | No. of Shoots Transferred to Rooting Medium | No. (%) of Acclimatized and Ex Vitro Cultivated Shoots | No. (%) of Flowering Shoots | No. of Mutants | Frequency of Mutants (%) | No. of Mutations | Frequency of Mutations (%) |
|---|---|---|---|---|---|---|---|
| ‘Lilac Wonder’ | |||||||
| standard | 25 | 25 (100 a) | 24 (96.0 a) | 0 | 0 | 0 | 0 |
| control object | 100 | 59 (59 b) | 44 (74.6 a) | 0 | 0 | 0 | 0 |
| 50 mg·L−1 | 92 | 52 (56.5 b) | 43 (82.7 a) | 1 | 2.3 | 1 | 2.3 |
| 100 mg·L−1 | 55 | 55 (100 a) | 50 (90.9 a) | 5 | 10 | 5 | 10 |
| ‘Richmond’ | |||||||
| standard | 25 | 25 (100 a) | 17 (68.0 a) | 0 | 0 | 0 | 0 |
| control object | 81 | 81 (100 a) | 73 (90.1 a) | 1 | 1.4 | 1 | 1.4 |
| 50 mg·L−1 | 17 | 17 (100 a) | 14 (82.3 a) | 0 | 0 | 0 | 0 |
| 100 mg·L−1 | 1 | 1 (100 a) | 1 (100 a) | 0 | 0 | 0 | 0 |
| Concentration of AgNPs | Inflorescence Characteristic | Content of Pigments in Ligulate Florets (mg·g−1 FW) | |||
|---|---|---|---|---|---|
| Color | RHSCC Color Code | Shape | Anthocyanins | Carotenoids | |
| ‘Lilac Wonder’ | |||||
| Standard | pink | 70C/69A * | full, semi-ball | 0.65 | - |
| Individual no. 1 (50 mg·L−1 AgNPs) | light pink with a burgundy-gold stripe | 69B/69D172D/163C | full, semi-ball | 0.39 | 0.40 |
| Individual no. 2 (100 mg·L−1 AgNPs) | burgundy-gold | 173A/163B | full, semi-ball | 0.60 | 0.34 |
| Individual no. 3 (100 mg·L−1 AgNPs) | burgundy-gold | 173A/163B | full, semi-ball | 0.77 | 0.46 |
| Individual no. 4 (100 mg·L−1 AgNPs) | light burgundy-gold | 172D/163C | full, semi-ball | 1.26 | 0.44 |
| Individual no. 5 (100 mg·L−1 AgNPs) | light burgundy-gold | 172D/163C | full, semi-ball | 1.37 | 0.51 |
| Individual no. 6 (100 mg·L−1 AgNPs) | burgundy-gold | 173A/163B | full, semi-ball | 0.78 | 0.36 |
| ‘Richmond’ | |||||
| Standard | purple pink | 71B/75D | full, flat | 0.84 | - |
| Individual no. 1 (0 mg·L−1 AgNPs) | purple pink | 71B/75D | full, irregular | 1.18 | - |
| Cv. | Primer Symbol | Primer Sequence 5′ → 3′ | No. of Bands | No. of Loci | Total Polymorphic loci [%] | No. (%) of Polymorphic Plants | No. of Genotypes | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mono. | Poly. | Spec. | |||||||
| RAPD | ||||||||||
| LW R | R1 | GGG AAT TCG G | 588 | 12 | 0 | 12 | 0 | 100 | 23 (27.1) | 2 |
| 399 | 7 | 2 | 5 | 0 | 71.4 | 17 (25) | 3 | |||
| LW | R2 | GAC CGC TTG T | 425 | 5 | 5 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| R | 528 | 9 | 7 | 2 | 0 | 22.2 | 18 (26.5) | 3 | ||
| LW | R3 | GCT GCC TCA GG | 510 | 6 | 6 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| R | 414 | 8 | 6 | 2 | 0 | 25 | 3 (4.4) | 2 | ||
| LW | R4 | TAC CCA GGA GCG | 509 | 7 | 4 | 2 | 1 | 42.9 | 1 (1.2) | 2 |
| R | 476 | 10 | 6 | 4 | 0 | 40.0 | 15 (22.1) | 5 | ||
| LW | R5 | CAA TCG CCG T | 170 | 2 | 2 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| R | 101 | 8 | 0 | 8 | 0 | 100 | 17 (25) | 4 | ||
| LW R | ∑ | 2202 1918 | 32 42 | 17 21 | 14 21 | 1 0 | - - | 24 (28.2) 18 (26.5) | 3 13 | |
| LW R | mean from a single primer | 440.4 383.3 | 6.4 8.4 | 3.4 4.2 | 2.8 4.2 | 0.2 0.0 | 28.6 51.7 | - - | - - | |
| SCoT | ||||||||||
| LW R | S1 | CAA CAA TGG CTA CCA CCG | 595 | 7 | 7 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| 413 | 12 | 3 | 4 | 5 | 75.0 | 3 (4.4) | 3 | |||
| LW R | S2 | CAA CAA TGG CTA CCA CCT | 595 | 7 | 7 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| 478 | 8 | 7 | 1 | 0 | 12.5 | 2 (2.9) | 2 | |||
| LW R | S3 | CAA CAA TGG CTA CCA CGT | 595 | 7 | 7 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| 277 | 6 | 4 | 2 | 0 | 33.3 | 4 (5.9) | 3 | |||
| LW R | S4 | ACG ACA TGG CGA CCA ACG | 850 | 10 | 10 | 0 | 0 | 0.0 | 0 (0.0) | 1 |
| 551 | 10 | 7 | 3 | 0 | 30.0 | 11 (16.2) | 4 | |||
| LW R | S5 | ACG ACA TGG CGA CCA TCG | 680 | 12 | 4 | 4 | 4 | 66.7 | 1 (1.2) | 2 |
| 409 | 7 | 6 | 0 | 1 | 14.3 | 1 (1.5) | 2 | |||
| LW R | ∑ | 3315 2128 | 43 43 | 35 27 | 4 10 | 4 6 | - - | 1 (1.2) 17 (25) | 2 11 | |
| LW R | mean from a single primer | 663 425.6 | 8.6 8.6 | 7.0 5.4 | 0.8 2.0 | 0.8 1.2 | 13.3 33.0 | - - | - - | |
| Cv. | Primer | H | PIC | E | MI | D | R |
|---|---|---|---|---|---|---|---|
| RAPD | |||||||
| LW | R-1 | 0.49 | 0.37 | 6.92 | 0.003 | 0.67 | 6.49 |
| R | 0.27 | 0.40 | 5.87 | 0.003 | 0.30 | 2.26 | |
| LW | R-2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.24 | 0.41 | 7.76 | 0.003 | 0.26 | 0.59 | |
| LW | R-3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.36 | 0.38 | 6.09 | 0.004 | 0.42 | 0.18 | |
| LW | R-4 | 0.20 | 0.18 | 7.99 | 0.002 | 0.21 | 0.07 |
| R | 0.42 | 0.35 | 7.00 | 0.004 | 0.51 | 0.71 | |
| LW | R-5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.30 | 0.40 | 1.48 | 0.001 | 0.97 | 1.74 | |
| LW | mean | 0.14 | 0.11 | 2.98 | 0.001 | 0.18 | 1.31 |
| R | 0.32 | 0.39 | 5.64 | 0.003 | 0.49 | 1.10 | |
| SCoT | |||||||
| LW | S-1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.50 | 0.27 | 6.07 | 0.004 | 0.74 | 0.32 | |
| LW | S-2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.21 | 0.37 | 7.03 | 0.003 | 0.23 | 0.06 | |
| LW | S-3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.44 | 0.30 | 4.07 | 0.004 | 0.54 | 0.15 | |
| LW | S-4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 0.31 | 0.35 | 8.10 | 0.004 | 0.34 | 0.74 | |
| LW | S-5 | 0.44 | 0.35 | 8.0 | 0.004 | 0.56 | 0.19 |
| R | 0.24 | 0.37 | 6.02 | 0.003 | 0.26 | 0.03 | |
| LW | mean | 0.09 | 0.07 | 1.6 | 0.001 | 0.11 | 0.04 |
| R | 0.34 | 0.33 | 6.26 | 0.004 | 0.42 | 0.26 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tymoszuk, A.; Kulus, D. Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum. Int. J. Mol. Sci. 2022, 23, 7406. https://doi.org/10.3390/ijms23137406
Tymoszuk A, Kulus D. Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum. International Journal of Molecular Sciences. 2022; 23(13):7406. https://doi.org/10.3390/ijms23137406
Chicago/Turabian StyleTymoszuk, Alicja, and Dariusz Kulus. 2022. "Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum" International Journal of Molecular Sciences 23, no. 13: 7406. https://doi.org/10.3390/ijms23137406
APA StyleTymoszuk, A., & Kulus, D. (2022). Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum. International Journal of Molecular Sciences, 23(13), 7406. https://doi.org/10.3390/ijms23137406







