Furcellaran Surface Deposition and Its Potential in Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of PET Films
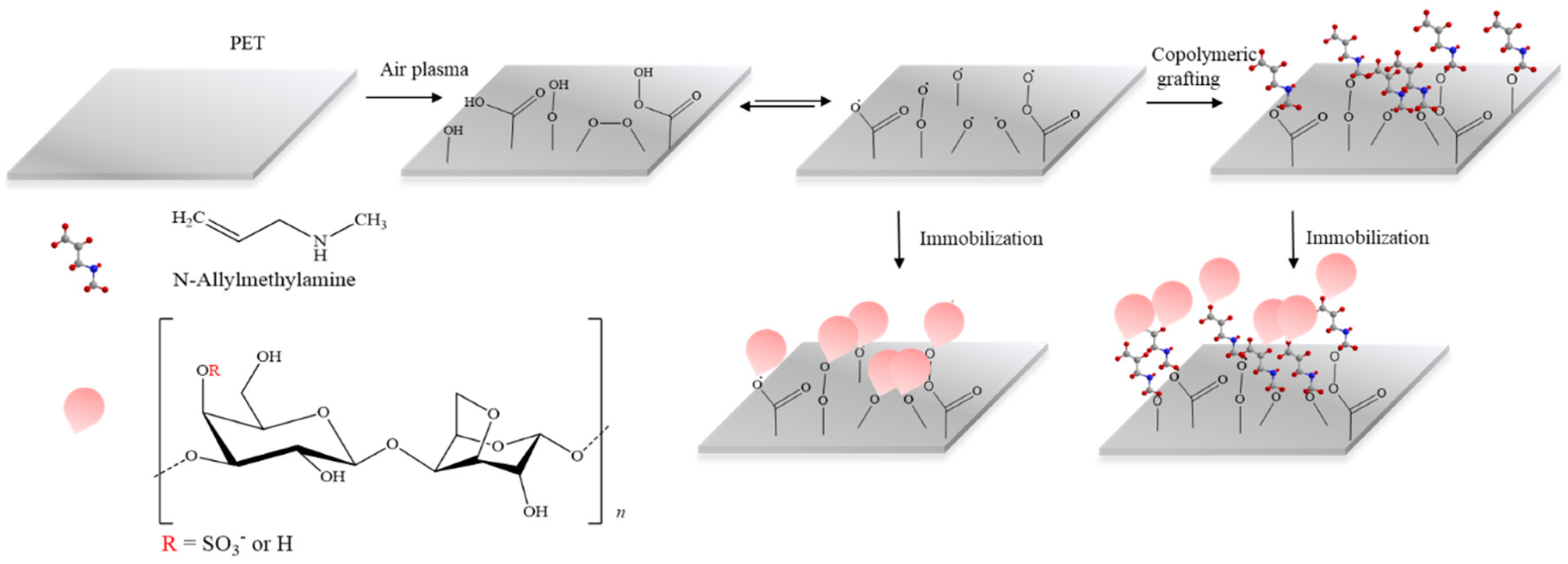
2.2. Plasma Surface Modification and MAAM Grafting
2.3. Furcerallan and κ-Carrageenan Immobilization
2.4. Contact Angle Measurement and Surface Energy Evaluation
2.5. X-ray Photoelectron Spectroscopy
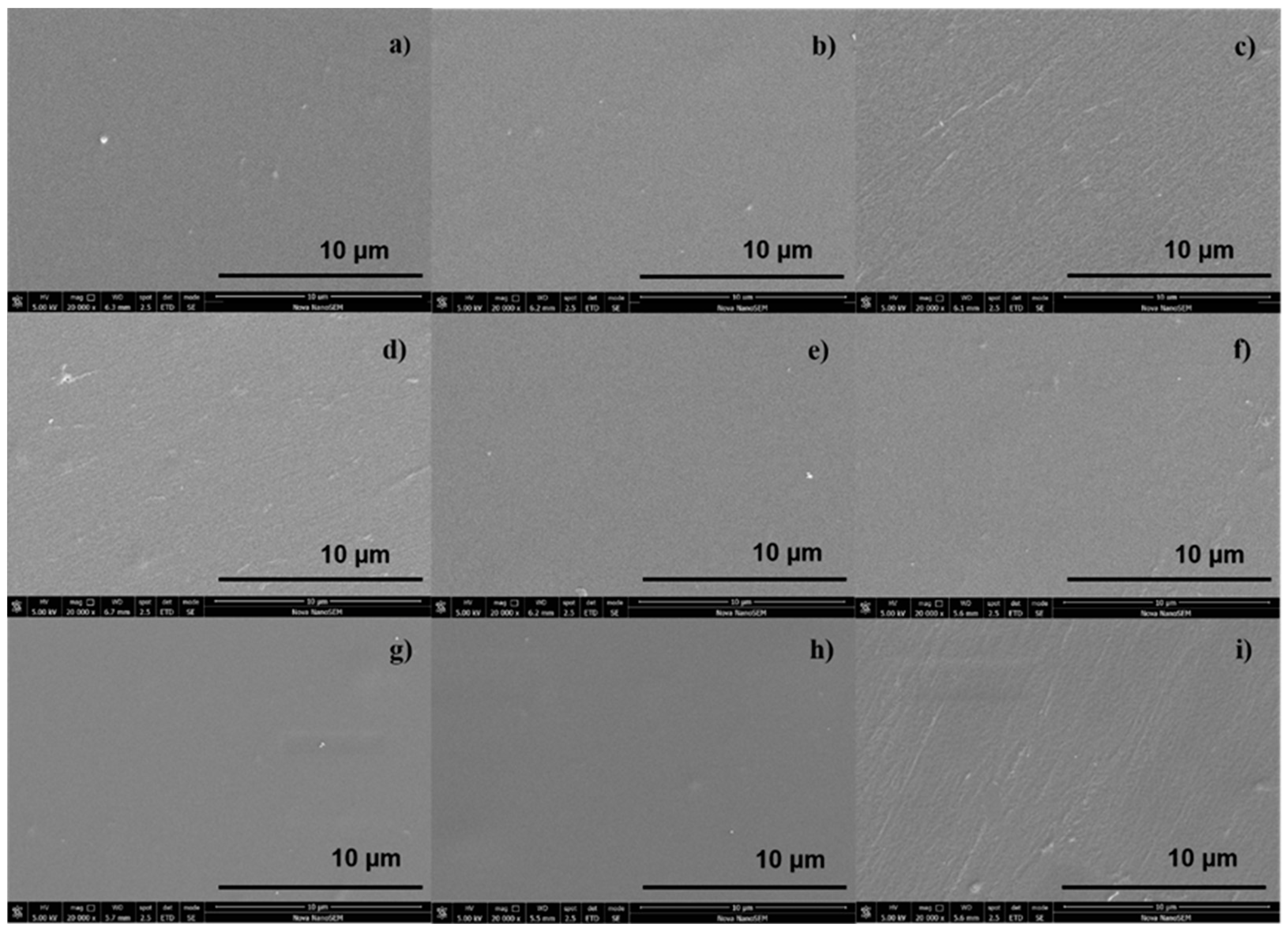
2.6. SEM Evaluation
2.7. Evaluation of Antibacterial Activity
2.8. Evaluation of Anticoagulant Activity
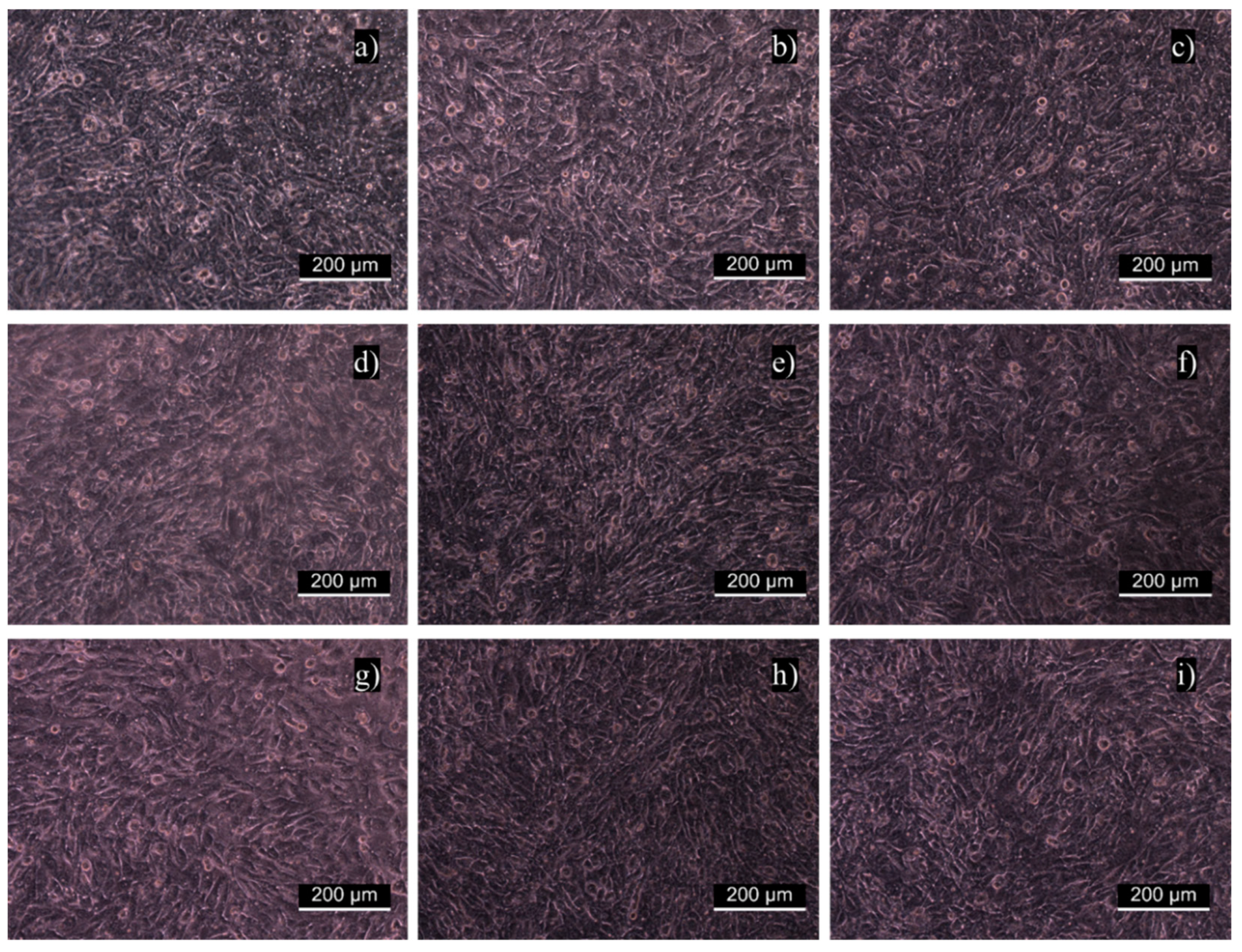
2.9. In Vitro Fibroblast Cytocompatibility
2.10. In Vitro Stem Cell Cytocompatibility
3. Results and Discussion
3.1. Surface Wettability Investigations
3.2. Surface Chemistry Investigations
3.3. SEM Imaging
3.4. Antibacterial Analysis
3.5. Anticoagulant Activity Assessment
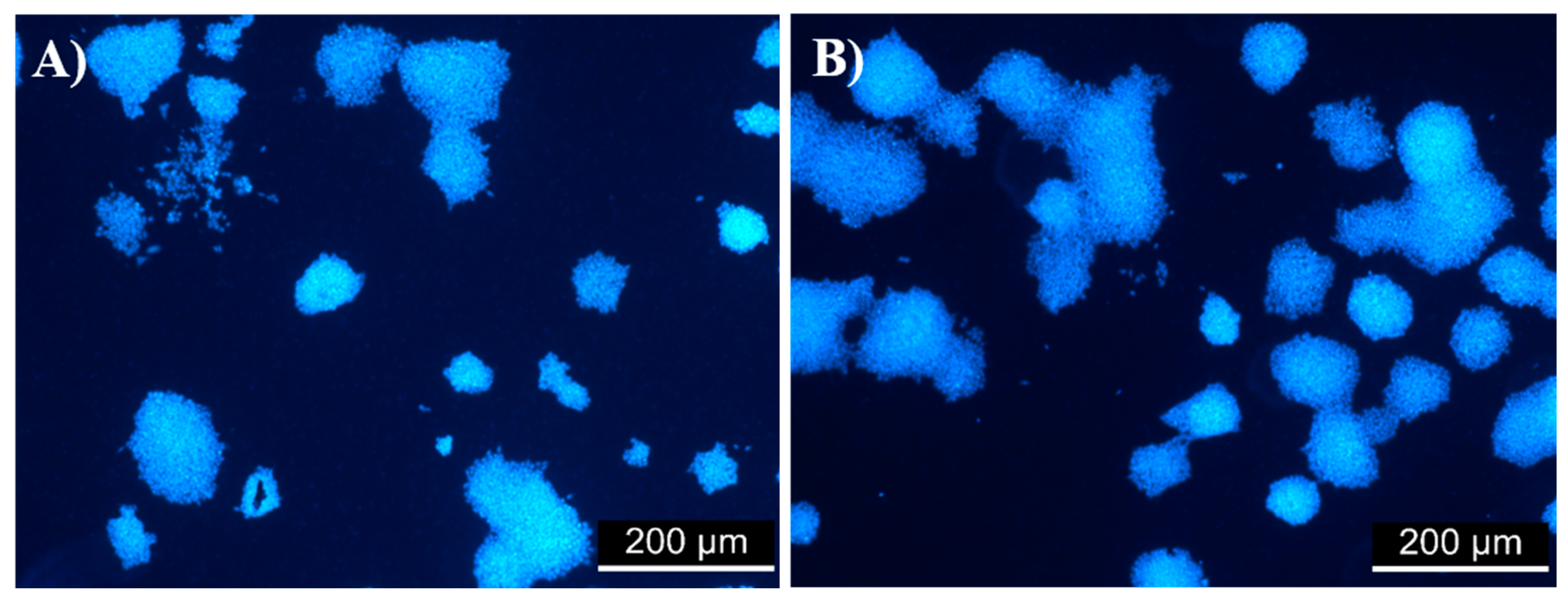
3.6. In Vitro Fibroblast Cytocompatibility
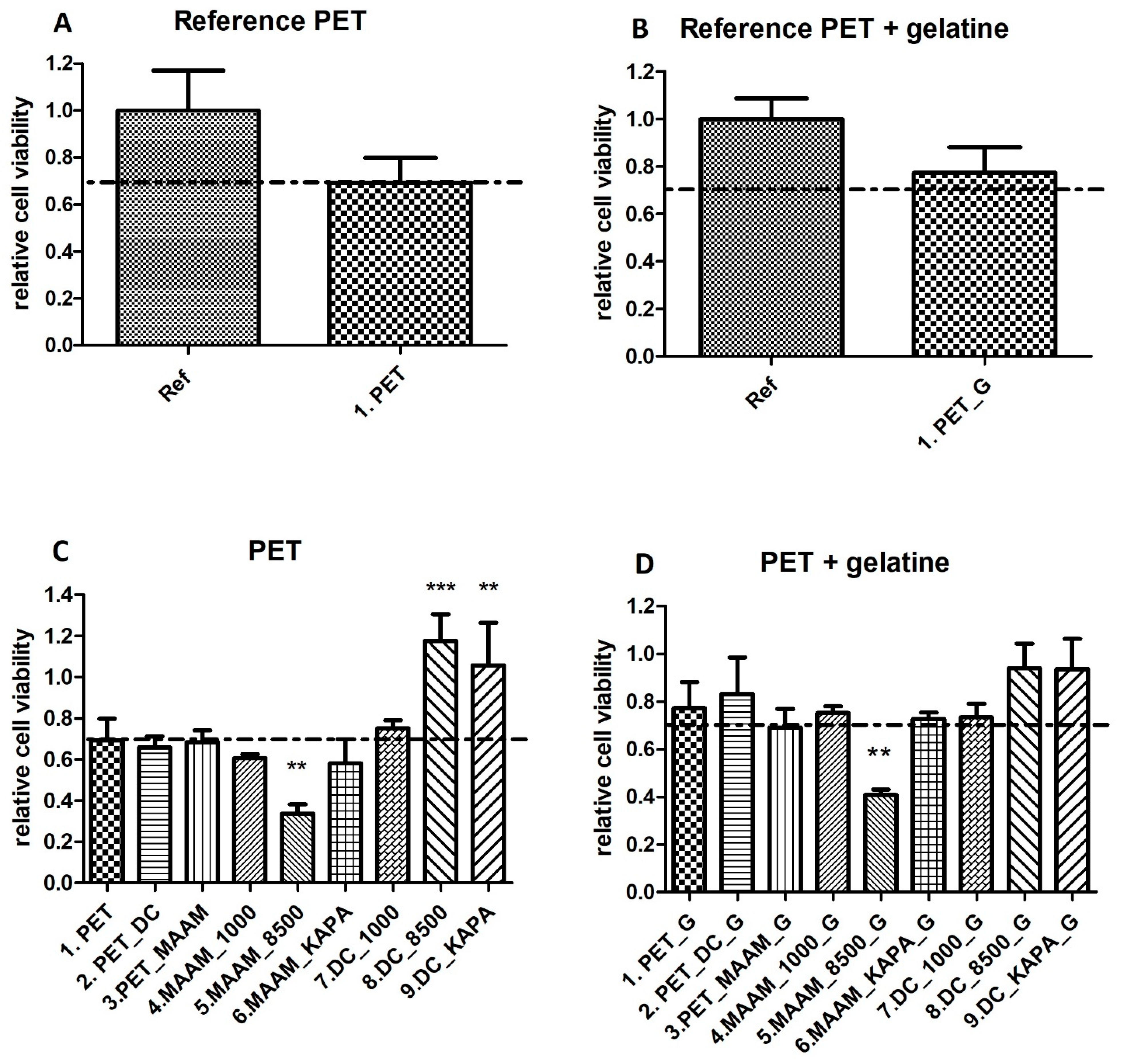
3.7. Evaluation of Cell Viability in the ES R1 Cell Line
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fasl, H.; Stana, J.; Stropnik, D.; Strnad, S.; Stana-Kleinschek, K.; Ribitsch, V. Improvement of the Hemocompatibility of PET Surfaces Using Different Sulphated Polysaccharides as Coating Materials. Biomacromolecules 2010, 11, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.I.; Zulkifli, N.; Selvakumaran, S.A.P.; Lazim, N.A.M. Bioactive Algal-Derived Polysaccharides: Multi-Functionalization, Therapeutic Potential and Biomedical Applications. CPD Curr. Pharm. Des. 2019, 25, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.-Y.; Hong, S.-C.; Kim, J.T.; Lee, J. Marine Polysaccharides: Therapeutic Efficacy and Biomedical Applications. Arch. Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and Carrageenases: Versatile Polysaccharides and Promising Marine Enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Silva, F.R.F.; Dore, C.M.P.G.; Marques, C.T.; Nascimento, M.S.; Benevides, N.M.B.; Rocha, H.A.O.; Chavante, S.F.; Leite, E.L. Anticoagulant Activity, Paw Edema and Pleurisy Induced Carrageenan: Action of Major Types of Commercial Carrageenans. Carbohydr. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of Sulfate Group in Red Seaweed Polysaccharides on Anticoagulant Activity and Cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. [Google Scholar] [CrossRef]
- van de Velde, F.; Rollema, H.S.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Tromp, R.H. Coil-Helix Transition of ?-Carrageenan as a Function of Chain Regularity. Biopolymers 2002, 65, 299–312. [Google Scholar] [CrossRef]
- Jamróz, E.; Para, G.; Jachimska, B.; Szczepanowicz, K.; Warszyński, P.; Para, A. Albumin–Furcellaran Complexes as Cores for Nanoencapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 880–884. [Google Scholar] [CrossRef]
- Laos, K.; Brownsey, G.; Ring, S. Interactions between Furcellaran and the Globular Proteins Bovine Serum Albumin and β-Lactoglobulin. Carbohydr. Polym. 2007, 67, 116–123. [Google Scholar] [CrossRef]
- Jamroz, E.; Konieczna-Molenda, A.; Para, A. Ternary Potato Starch-Furcellaran-Gelatin Film—A New Generation of Biodegradable Foils. Polimery 2017, 62, 673–679. [Google Scholar] [CrossRef]
- Alsharabasy, A.M.; Moghannem, S.A.; El-Mazny, W.N. Physical Preparation of Alginate/Chitosan Polyelectrolyte Complexes for Biomedical Applications. J. Biomater. Appl. 2016, 30, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.L.; Panitch, A. Physical Polymer Matrices Based on Affinity Interactions between Peptides and Polysaccharides. Biomacromolecules 2003, 4, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Janik, M.; Juszczak, L.; Kruk, T.; Kulawik, P.; Szuwarzyński, M.; Kawecka, A.; Khachatryan, K. Composite Biopolymer Films Based on a Polyelectrolyte Complex of Furcellaran and Chitosan. Carbohydr. Polym. 2021, 274, 118627. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A Comprehensive Review on Polyelectrolyte Complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The Verification of Intelligent Properties of Furcellaran Films with Plant Extracts on the Stored Fresh Atlantic Mackerel during Storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the Physical Properties, Antioxidant and Antimicrobial Activity of Ternary Potato Starch-Furcellaran-Gelatin Films Incorporated with Lavender Essential Oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran Nanocomposite Films: The Effect of Nanofillers on the Structural, Thermal, Mechanical and Antimicrobial Properties of Biopolymer Films. Carbohydr. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef]
- Anderson, J.M.; Miller, K.M. Biomaterial Biocompatibility and the Macrophage. Biomaterials 1984, 5, 5–10. [Google Scholar] [CrossRef]
- Hubbell, J.A. Biomaterials in Tissue Engineering. Biotechnology 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical Device-Induced Thrombosis: What Causes It and How Can We Prevent It? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface Modification and Endothelialization of Biomaterials as Potential Scaffolds for Vascular Tissue Engineering Applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [Green Version]
- Massia, S.P.; Stark, J.; Letbetter, D.S. Surface-Immobilized Dextran Limits Cell Adhesion and Spreading. Biomaterials 2000, 21, 2253–2261. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biomedical Polymers: Influence on Cell-Material Interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Ferreira, P.; Alves, P.; Coimbra, P.; Gil, M.H. Improving Polymeric Surfaces for Biomedical Applications: A Review. J Coat Technol. Res. 2015, 12, 463–475. [Google Scholar] [CrossRef]
- Drobota, M.; Trandabat, A.; Pislaru, M. Surface Modification of Poly(Ethylene Terephthalate) in Air Plasma. Acta Chem. Iasi 2019, 27, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Karakurt, I.; Ozaltin, K.; Vesela, D.; Lehocky, M.; Humpolíček, P.; Mozetič, M. Antibacterial Activity and Cytotoxicity of Immobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films. Polymers 2019, 11, 1186. [Google Scholar] [CrossRef] [Green Version]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Di Martino, A.; Karakurt, I.; Saha, P. Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers 2019, 11, 750. [Google Scholar] [CrossRef] [Green Version]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 Plasma Treatment on the Deposition of Chitosan onto Polyethylene Terephthalate (PET) Surfaces. Int. J. Adhes. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Lehocký, M.; Junkar, I.; Mozetič, M.; Kleinová, A.; Janigová, I.; Šlouf, M.; Bílek, F.; Chodák, I. A New Route for Chitosan Immobilization onto Polyethylene Surface. Carbohydr. Polym. 2012, 90, 1501–1508. [Google Scholar] [CrossRef]
- Popelka, A.; Kronek, J.; Novák, I.; Kleinová, A.; Mičušík, M.; Špírková, M.; Omastová, M. Surface Modification of Low-Density Polyethylene with Poly(2-Ethyl-2-Oxazoline) Using a Low-Pressure Plasma Treatment. Vacuum 2014, 100, 53–56. [Google Scholar] [CrossRef]
- Bílek, F.; Křížová, T.; Lehocký, M. Preparation of Active Antibacterial LDPE Surface through Multistep Physicochemical Approach: I. Allylamine Grafting, Attachment of Antibacterial Agent and Antibacterial Activity Assessment. Colloids Surf. B Biointerfaces 2011, 88, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ozaltin, K.; Lehocký, M.; Humpolíček, P.; Pelková, J.; Sáha, P. A New Route of Fucoidan Immobilization on Low Density Polyethylene and Its Blood Compatibility and Anticoagulation Activity. Int. J. Mol. Sci. 2016, 17, 908. [Google Scholar] [CrossRef] [PubMed]
- Martocq, L.; Douglas, T.E.L. Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization. Coatings 2021, 11, 983. [Google Scholar] [CrossRef]
- Bílek, F.; Sulovská, K.; Lehocký, M.; Sáha, P.; Humpolíček, P.; Mozetič, M.; Junkar, I. Preparation of Active Antibacterial LDPE Surface through Multistep Physicochemical Approach II: Graft Type Effect on Antibacterial Properties. Colloids Surf. B Biointerfaces 2013, 102, 842–848. [Google Scholar] [CrossRef]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Vesela, D.; Mozetic, M.; Novak, I.; Saha, P. Preparation of Active Antibacterial Biomaterials Based on Sparfloxacin, Enrofloxacin, and Lomefloxacin Deposited on Polyethylene. J. Appl. Polym. Sci. 2018, 135, 46174. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Luo, R.; Maitz, M.F.; Jing, F.; Sun, H.; Huang, N. The Covalent Immobilization of Heparin to Pulsed-Plasma Polymeric Allylamine Films on 316L Stainless Steel and the Resulting Effects on Hemocompatibility. Biomaterials 2010, 31, 2072–2083. [Google Scholar] [CrossRef]
- Charbonneau, C.; Ruiz, J.-C.; Lequoy, P.; Hébert, M.-J.; De Crescenzo, G.; Wertheimer, M.R.; Lerouge, S. Chondroitin Sulfate and Epidermal Growth Factor Immobilization after Plasma Polymerization: A Versatile Anti-Apoptotic Coating to Promote Healing Around Stent Grafts. Macromol. Biosci. 2012, 12, 812–821. [Google Scholar] [CrossRef]
- Subramaniam, A.; Sethuraman, S. Biomedical Applications of Nondegradable Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 301–308. ISBN 978-0-12-396983-5. [Google Scholar]
- Dhahri, M.; Abed, A.; Lajimi, R.H.; Mansour, M.B.; Gueguen, V.; Abdesselem, S.B.; Chaubet, F.; Letourneur, D.; Meddahi-Pellé, A.; Maaroufi, R.M. Grafting of Dermatan Sulfate on Polyethylene Terephtalate to Enhance Biointegration. J. Biomed. Mater. Res. Part A 2011, 98, 114–121. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Selvarajan, V.; Rhee, Y.H.; Kim, H.W.; Shah, S.I. Glow Discharge Plasma-Induced Immobilization of Heparin and Insulin on Polyethylene Terephthalate Film Surfaces Enhances Anti-Thrombogenic Properties. Mater. Sci. Eng. C 2009, 29, 796–805. [Google Scholar] [CrossRef]
- Gotoh, K.; Yasukawa, A.; Kobayashi, Y. Wettability Characteristics of Poly(Ethylene Terephthalate) Films Treated by Atmospheric Pressure Plasma and Ultraviolet Excimer Light. Polym. J. 2011, 43, 545–551. [Google Scholar] [CrossRef]
- van Oss, C.J.; Wu, W.; Docoslis, A.; Giese, R.F. The Interfacial Tensions with Water and the Lewis Acid–Base Surface Tension Parameters of Polar Organic Liquids Derived from Their Aqueous Solubilities. Colloids Surf. B Biointerfaces 2001, 20, 87–91. [Google Scholar] [CrossRef]
- Ozaltin, K.; Lehocký, M.; Kuceková, Z.; Humpolíček, P.; Sáha, P. A Novel Multistep Method for Chondroitin Sulphate Immobilization and Its Interaction with Fibroblast Cells. Mater. Sci. Eng. C 2017, 70, 94–100. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, Characterization and Antibacterial Activity of Oxidized κ-Carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Daheriya, P. Kappa-Carrageenan/PVA Filmswith Antibacterial Properties: Part 1. Optimization of Preparation Conditions and Preliminary Drug Release Studies. J. Macromol. Sci. Part A 2014, 51, 286–295. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 2021, 3. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; De Yao, K. Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the barents sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef]
- Olson, S. Identification of Critical Molecular Interactions Mediating Heparin Activation of Antithrombin Implications for the Design of Improved Heparin Anticoagulants. Trends Cardiovasc. Med. 2002, 12, 198–205. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of Mouse Fibroblasts on Hexamethyldisiloxane Surfaces with Wide Range of Wettability. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.-Y.; Lee, J.-H.; Kim, H.-S.; Kim, H.-H.; Jeon, Y.-J. Cell proliferation effect of brown marine algae extracts on Mouse Fibroblast. J. Mar. Biosci. Biotechnol. 2015, 7, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Popa, E.; Carvalho, P.; Dias, A.; Santos, T.; Santo, V.; Marques, A.; Viegas, C.; Gomes, M.; Reis, R.L. Evaluation of the in Vitro and in Vivo Biocompatibility of Carrageenan-Based Hydrogels. J. Biomed. Mater. Res. Part A 2014, 102, 4087–4097. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In Vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phytother. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]
- Perestrelo, A.R.; Grenha, A.; Rosa da Costa, A.M.; Belo, J.A. Locust Bean Gum as an Alternative Polymeric Coating for Embryonic Stem Cell Culture. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 336–344. [Google Scholar] [CrossRef]

| Contact Angles (°) for Liquids | Surface Free Energy Parameters (mJ/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| SAMPLE | θw | θd | θf | γs | γsLW | γsAB | γs+ | γs− |
| PET | 63.27 ± 3.08 | 26.63 ± 0.54 | 53.72 ± 0.95 | 50.17 | 45.55 | 4.62 | 0.24 | 22.10 |
| PET_DC | 30.25 ± 3.40 | 25.57 ± 0.61 | 8.97 ± 1.15 | 57.64 | 45.95 | 11.72 | 0.88 | 38.80 |
| PET_MAAM | 33.71 ± 1.83 | 30.76 ± 0.38 | 12.22 ± 2.62 | 57.08 | 43.90 | 13.18 | 1.22 | 35.70 |
| MAAM_1000 | 40.94 ± 1.74 | 27.78 ± 2.91 | 17.8 ± 2.81 | 56.28 | 45.11 | 11.17 | 1.07 | 29.27 |
| MAAM_8500 | 47.33 ± 1.95 | 32.02 ± 2.36 | 20.18 ± 2.05 | 55.32 | 43.36 | 11.96 | 1.59 | 22.52 |
| MAAM_KAPA | 49.19 ± 2.0 | 28.16 ± 1.15 | 18.73 ± 2.28 | 56.11 | 44.98 | 11.13 | 1.55 | 20.02 |
| DC_1000 | 36.75 ± 1.35 | 18.06 ± 1.55 | 12.08 ± 2.03 | 57.98 | 48.33 | 9.65 | 0.71 | 32.62 |
| DC_8500 | 47.08 ± 2.25 | 21.67 ± 0.80 | 10.99 ± 1.46 | 58.35 | 47.27 | 11.07 | 1.49 | 20.61 |
| DC_KAPA | 46.71 ± 0.99 | 26.82 ± 1.94 | 11.28 ± 1.69 | 57.72 | 45.48 | 12.24 | 1.79 | 20.97 |
| SAMPLE | C | N | O | S |
|---|---|---|---|---|
| PET | 74.6 | 25.4 | ||
| PET_DC | 69.3 | 1.5 | 29.3 | |
| PET_MAAM | 69.8 | 2.1 | 28.1 | |
| MAAM_8500 | 73.7 | 1.0 | 25.3 | |
| MAAM_1000 | 74.6 | 1.0 | 24.5 | |
| MAAM_KAPA | 74.2 | 0.9 | 24.8 | 0.2 |
| DC_1000 | 70.0 | 29.7 | 0.3 | |
| DC_8500 | 73.9 | 0.6 | 25.5 | 0.1 |
| DC_KAPA | 72.7 | 0.7 | 26.5 | 0.1 |
| SAMPLE | Staphylococcus aureus CCM 2020 | Escherichia coli CCM 4517 | ||
|---|---|---|---|---|
| N (cfu/cm2) | R | N (cfu/cm2) | R | |
| PET | 1.1 × 104 | 0 | 9.8 × 105 | 0 |
| PET_DC | 1.1 × 104 | −0.02 | 8.9 × 105 | 0.05 |
| PET_MAAM | 1.1 × 104 | −0.02 | 8.3 × 105 | 0.07 |
| MAAM_1000 | 1.2 × 104 | −0.04 | 5.8 × 105 | 0.22 |
| MAAM_8500 | 1.2 × 104 | −0.05 | 8.6 × 105 | 0.05 |
| MAAM_KAPA | 4.6 × 103 | 0.38 | 9.5 × 105 | 0.01 |
| DC_1000 | 8.1 × 103 | 0.14 | 8.2 × 105 | 0.07 |
| DC_8500 | 2.4 × 104 | −0.34 | 7.1 × 105 | 0.14 |
| DC_KAPA | 8.6 × 103 | 0.11 | 5.8 × 105 | 0.12 |
| SAMPLE | PT (s) | aPTT (s) | TT (s) |
|---|---|---|---|
| PET | 12.0 | 29.1 | 18.0 |
| PET_DC | 12.6 | 32.8 | 20.1 |
| PET_MAAM | 12.6 | 30.5 | 18.5 |
| MAAM_1000 | 12.1 | 28.8 | 18.4 |
| MAAM_8500 | 12.4 | 30.6 | 18.6 |
| MAAM_KAPA | 12.3 | 29.7 | 18.9 |
| DC_1000 | 13.0 | 32.5 | 18.7 |
| DC_8500 | 13.3 | 33.4 | 19.7 |
| DC_KAPA | 12.3 | 33.9 | 20.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štěpánková, K.; Ozaltin, K.; Pelková, J.; Pištěková, H.; Karakurt, I.; Káčerová, S.; Lehocky, M.; Humpolicek, P.; Vesel, A.; Mozetic, M. Furcellaran Surface Deposition and Its Potential in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 7439. https://doi.org/10.3390/ijms23137439
Štěpánková K, Ozaltin K, Pelková J, Pištěková H, Karakurt I, Káčerová S, Lehocky M, Humpolicek P, Vesel A, Mozetic M. Furcellaran Surface Deposition and Its Potential in Biomedical Applications. International Journal of Molecular Sciences. 2022; 23(13):7439. https://doi.org/10.3390/ijms23137439
Chicago/Turabian StyleŠtěpánková, Kateřina, Kadir Ozaltin, Jana Pelková, Hana Pištěková, Ilkay Karakurt, Simona Káčerová, Marian Lehocky, Petr Humpolicek, Alenka Vesel, and Miran Mozetic. 2022. "Furcellaran Surface Deposition and Its Potential in Biomedical Applications" International Journal of Molecular Sciences 23, no. 13: 7439. https://doi.org/10.3390/ijms23137439
APA StyleŠtěpánková, K., Ozaltin, K., Pelková, J., Pištěková, H., Karakurt, I., Káčerová, S., Lehocky, M., Humpolicek, P., Vesel, A., & Mozetic, M. (2022). Furcellaran Surface Deposition and Its Potential in Biomedical Applications. International Journal of Molecular Sciences, 23(13), 7439. https://doi.org/10.3390/ijms23137439










