Sphingosine-1-Phosphate-Triggered Expression of Cathelicidin LL-37 Promotes the Growth of Human Bladder Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Sphingosine Kinases 1/2 and hCAP-18/LL-37 Are Both Expressed in Human BC Tissues
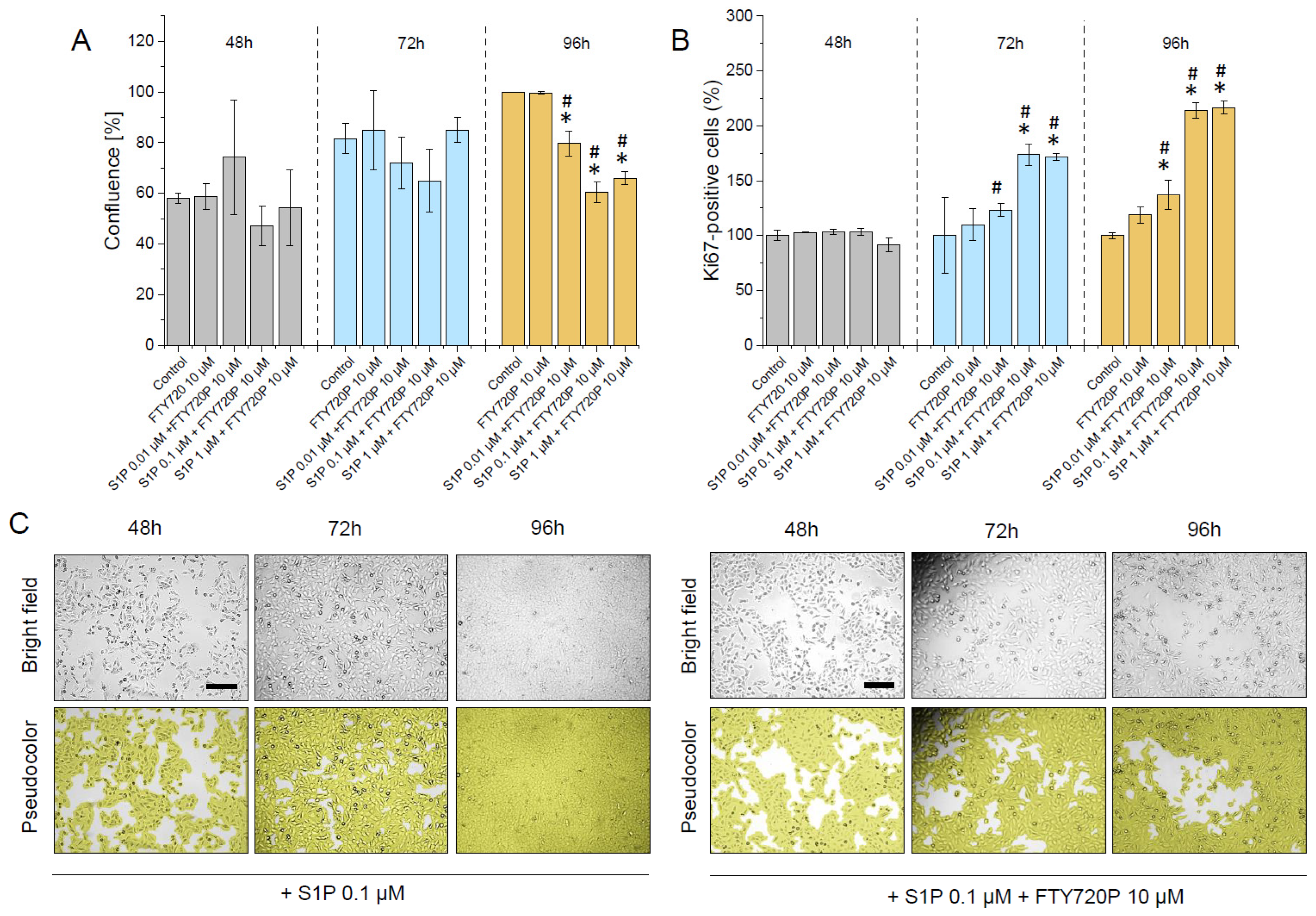
2.2. S1P Promotes the Proliferation of Human Bladder T24 Cells and This Effect Is Restricted by Pre-Treatment with FTY720P
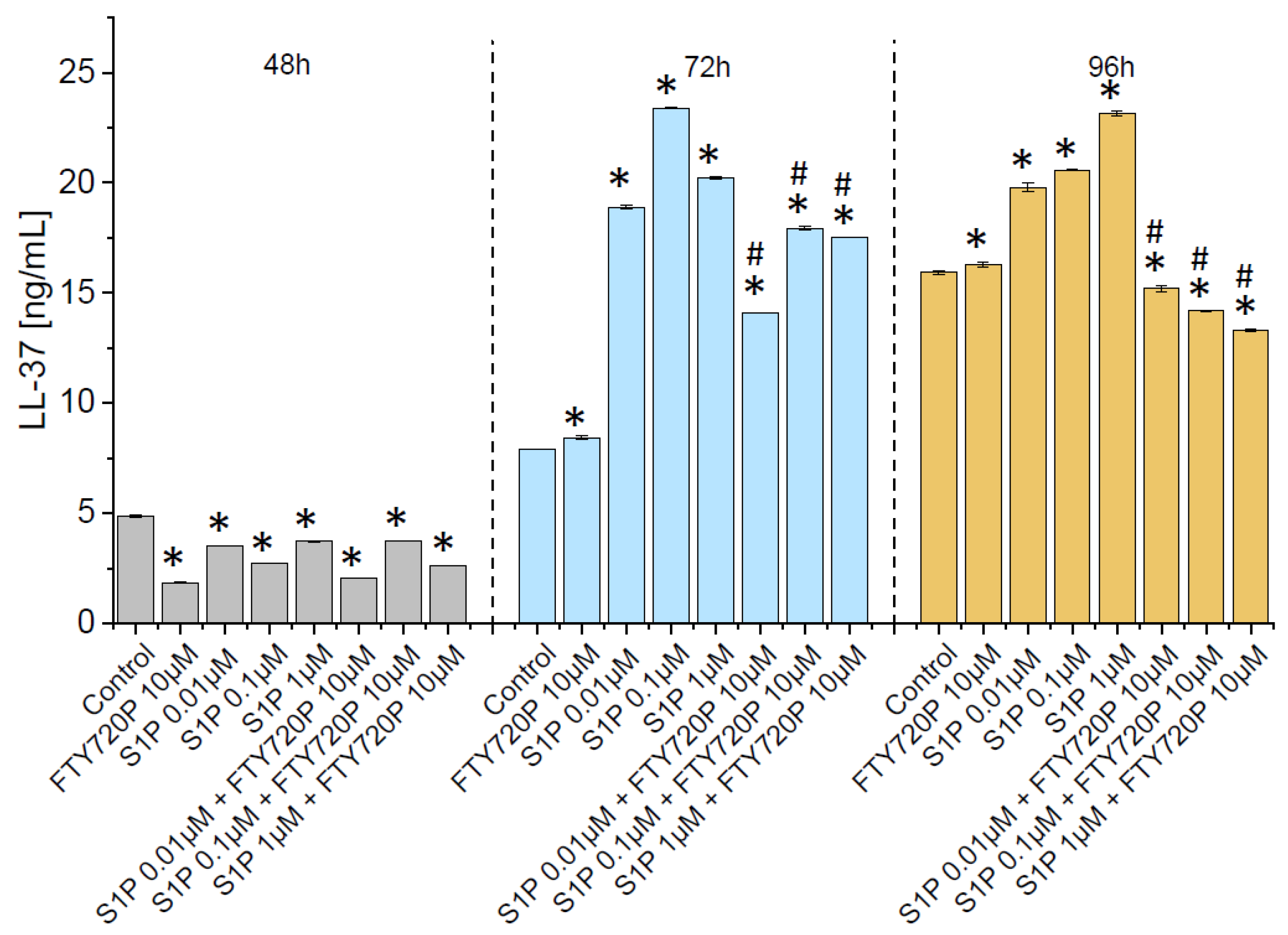
2.3. Release of Ll-37 from Human Bladder Cancer Cells Is Triggered by S1P
2.4. Exogenous Ll-37 Peptide Exerts Stimulatory Effect on Proliferation of Human Bladder Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Material
4.2. SphK1 and SphK2 Immunohistochemical Analysis
4.3. hCAP-18 Immunohistochemical Analysis
4.4. qRT-PCR Analysis
4.5. Cell Culture
4.6. Cell Counting and Confluence Assessment
4.7. Examination of LL-37 Production in T24 (ATCC® HTB-4™) Cells
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwab, S.R.; Cyster, J.G. Finding a way out: Lymphocyte egress from lymphoid organs. Nat. Immunol. 2007, 8, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Le Stunff, H.; Milstien, S.; Spiegel, S. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell. Biochem. 2004, 92, 882–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Sanchez, T.; Milne, G.L.; Morrow, J.D.; Hla, T.; Ferrer, F. S1P/S1P2 signaling induces cyclooxygenase-2 expression in Wilms tumor. J. Urol. 2009, 181, 1347–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, G.; Guba, M.; Ischenko, I.; Papyan, A.; Joka, M.; Schrepfer, S.; Bruns, C.J.; Jauch, K.W.; Heeschen, C.; Graeb, C. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J. Cell. Biochem. 2007, 101, 259–270. [Google Scholar] [CrossRef]
- Liang, J.; Nagahashi, M.; Kim, E.Y.; Harikumar, K.B.; Yamada, A.; Huang, W.C.; Hait, N.C.; Allegood, J.C.; Price, M.M.; Avni, D.; et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013, 23, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Hung, J.H.; Lu, Y.S.; Wang, Y.C.; Ma, Y.H.; Wang, D.S.; Kulp, S.K.; Muthusamy, N.; Byrd, J.C.; Cheng, A.L.; Chen, C.S. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008, 68, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Pchejetski, D.; Bohler, T.; Brizuela, L.; Sauer, L.; Doumerc, N.; Golzio, M.; Salunkhe, V.; Teissie, J.; Malavaud, B.; Waxman, J.; et al. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010, 70, 8651–8661. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.P.; Harris, C.C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer 2007, 121, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Shadpour, P.; Zamani, M.; Aghaalikhani, N.; Rashtchizadeh, N. Inflammatory cytokines in bladder cancer. J. Cell. Physiol. 2019, 234, 14489–14499. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.N.; Kim, S.K.; Mun, J.Y.; Choi, Y.D.; Leem, S.H.; Chu, I.S. Identification of an immunotherapy-responsive molecular subtype of bladder cancer. EBioMedicine 2019, 50, 238–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matulewicz, R.S.; Steinberg, G.D. Non-muscle-invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Rev. Urol. 2020, 22, 43–51. [Google Scholar] [PubMed]
- Westergren, D.O.; Gårdmark, T.; Lindhagen, L.; Chau, A.; Malmström, P.U. A Nationwide, Population Based Analysis of Patients with Organ Confined, Muscle Invasive Bladder Cancer Not Receiving Curative Intent Therapy in Sweden from 1997 to 2014. J. Urol. 2019, 202, 905–912. [Google Scholar] [CrossRef]
- Fumarola, S.; Cecati, M.; Sartini, D.; Ferretti, G.; Milanese, G.; Galosi, A.B.; Pozzi, V.; Campagna, R.; Morresi, C.; Emanuelli, M.; et al. Bladder Cancer Chemosensitivity is Affected by Paraoxonase-2 Expression. Antioxidants 2020, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Nesi, G.; Nobili, S.; Cai, T.; Caini, S.; Santi, R. Chronic inflammation in urothelial bladder cancer. Virchows Arch. 2015, 467, 623–633. [Google Scholar] [CrossRef]
- Brandau, S.; Suttmann, H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: A success story with room for improvement. Biomed Pharm. 2007, 61, 299–305. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G. APD: The Antimicrobial Peptide Database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Durr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucki, R.; Leszczynska, K.; Namiot, A.; Sokolowski, W. Cathelicidin LL-37: A multitask antimicrobial peptide. Arch. Immunol. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Scandurro, A.B. Tumors sound the alarmin(s). Cancer Res. 2008, 68, 6482–6485. [Google Scholar] [CrossRef] [Green Version]
- Piktel, E.; Niemirowicz, K.; Wnorowska, U.; Watek, M.; Wollny, T.; Gluszek, K.; Gozdz, S.; Levental, I.; Bucki, R. The Role of Cathelicidin LL-37 in Cancer Development. Arch. Immunol. Exp. 2016, 64, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Shaykhiev, R.; Beisswenger, C.; Kandler, K.; Senske, J.; Puchner, A.; Damm, T.; Behr, J.; Bals, R. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2005, 289, L842–L848. [Google Scholar] [CrossRef]
- Ren, S.X.; Cheng, A.S.; To, K.F.; Tong, J.H.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef] [Green Version]
- Byfield, F.J.; Kowalski, M.; Cruz, K.; Leszczynska, K.; Namiot, A.; Savage, P.B.; Bucki, R.; Janmey, P.A. Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J. Immunol. 2011, 187, 6402–6409. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef] [Green Version]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandurska, K.; Berdowska, A.; Barczynska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Wu, W.K.; Wang, G.; Coffelt, S.B.; Betancourt, A.M.; Lee, C.W.; Fan, D.; Wu, K.; Yu, J.; Sung, J.J.; Cho, C.H. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int. J. Cancer 2010, 127, 1741–1747. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Park, K.; Elias, P.M.; Shin, K.O.; Lee, Y.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Saba, J.; Holleran, W.M.; Uchida, Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol. Cell. Biol. 2013, 33, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, S. Sphingosine and sphingosine 1-phosphate in cellular proliferation: Relationship with protein kinase C and phosphatidic acid. J. Lipid Mediat. 1993, 8, 169–175. [Google Scholar] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhang, H.; Zhang, L.; Cai, T.T.; Huang, D.J.; He, J.; Ni, H.H.; Zhou, F.J.; Zhang, X.S.; Li, J. Sphingosine 1 phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T cell expansion: Leading to poor survival in bladder cancer. Cell Death Dis. 2019, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.L.; Dynesen, P.; Larsen, P.; Jakobsen, L.; Andersen, P.S.; Frimodt-Møller, N. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect. Immun. 2014, 82, 1572–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babikir, I.H.; Abugroun, E.A.; Bilal, N.E.; Alghasham, A.A.; Abdalla, E.E.; Adam, I. The impact of cathelicidin, the human antimicrobial peptide LL-37 in urinary tract infections. BMC Infect. Dis. 2018, 18, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chromek, M.; Slamová, Z.; Bergman, P.; Kovács, L.; Podracká, L.; Ehrén, I.; Hökfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Leibovici, D.; Grossman, H.B.; Dinney, C.P.; Millikan, R.E.; Lerner, S.; Wang, Y.; Gu, J.; Dong, Q.; Wu, X. Polymorphisms in inflammation genes and bladder cancer: From initiation to recurrence, progression, and survival. J. Clin. Oncol. 2005, 23, 5746–5756. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Sukocheva, O.A. Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming. Int. J. Mol. Sci. 2018, 19, 420. [Google Scholar] [CrossRef] [Green Version]
- Dosler, S.; Karaaslan, E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [Green Version]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krotz, F.; Zahler, S.; Gloe, T.; Issbrucker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Mader, J.S.; Mookherjee, N.; Hancock, R.E.; Bleackley, R.C. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol. Cancer Res. 2009, 7, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.X.; Shen, J.; Cheng, A.S.; Lu, L.; Chan, R.L.; Li, Z.J.; Wang, X.J.; Wong, C.C.; Zhang, L.; Ng, S.S.; et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Waterman, R.S.; Florez, L.; Honer zu Bentrup, K.; Zwezdaryk, K.J.; Tomchuck, S.L.; LaMarca, H.L.; Danka, E.S.; Morris, C.A.; Scandurro, A.B. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int. J. Cancer 2008, 122, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Jimenez, C.I.; Sandstedt, B.; Borregaard, N.; Tham, E.; Sorensen, O.E.; Weber, G.; Stahle, M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int. J. Cancer 2005, 114, 713–719. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.J.; Choi, J.M.; Lee, K.H.; Kim, T.Y.; Cho, B.K.; Jung, J.Y.; Chung, K.Y.; Cho, D.; Park, H.J. The antimicrobial peptide human cationic antimicrobial protein-18/cathelicidin LL-37 as a putative growth factor for malignant melanoma. Br. J. Dermatol. 2010, 163, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Von Haussen, J.; Koczulla, R.; Shaykhiev, R.; Herr, C.; Pinkenburg, O.; Reimer, D.; Wiewrodt, R.; Biesterfeld, S.; Aigner, A.; Czubayko, F.; et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer 2008, 59, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Murakami, M.; Iimura, M.; Cole, S.P.; Horibe, Y.; Ohtake, T.; Obonyo, M.; Gallo, R.L.; Eckmann, L.; Kagnoff, M.F. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 2003, 125, 1613–1625. [Google Scholar] [CrossRef]
- Geng, T.; Sutter, A.; Harland, M.D.; Law, B.A.; Ross, J.S.; Lewin, D.; Palanisamy, A.; Russo, S.B.; Chavin, K.D.; Cowart, L.A. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J. Lipid Res. 2015, 56, 2359–2371. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.L.; Yarla, N.S.; Menschikowski, M.; Sukocheva, O.A. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J. Stem Cells 2018, 10, 119–133. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukocheva, O.; Wadham, C.; Holmes, A.; Albanese, N.; Verrier, E.; Feng, F.; Bernal, A.; Derian, C.K.; Ullrich, A.; Vadas, M.A.; et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: The role of sphingosine kinase-1. J. Cell Biol. 2006, 173, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Kitayama, J.; Yamaguchi, H.; Yamashita, H.; Mori, K.; Watanabe, T.; Yatomi, Y.; Nagawa, H. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett. 2004, 577, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, A.; Zhang, W.; Lee, J.F.; An, J.; Ekambaram, P.; Liu, J.; Honn, K.V.; Klinge, C.M.; Lee, M.J. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int. J. Oncol. 2012, 40, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, M.G.; Vanetti, C.; Samarani, M.; Aureli, M.; Bassi, R.; Sonnino, S.; Giussani, P. Cross-talk between sphingosine-1-phosphate and EGFR signaling pathways enhances human glioblastoma cell invasiveness. FEBS Lett. 2018, 592, 949–961. [Google Scholar] [CrossRef] [Green Version]





| Gene | Primer Direction | Sequence 5′->3′ (DNA) | Annealing Temperature (°C) |
|---|---|---|---|
| hCAP-18/LL-37 | forward | GAAGACCCAAAGGAATGGCC | 53.8 |
| reverse | CAGAGCCCAGAAGCCTGAGC | 57.9 | |
| SPHK1 | forward | GCTGGCAGCTTCCTTGAACCAT | 56.7 |
| reverse | GTGTGCAGAGACAGCAGGTTCA | 56.7 | |
| SPHK2 | forward | GAGGAAGCTGTGAAGATGCCTG | 56.7 |
| reverse | GAGCAGTTGAGCAACAGGTCGA | 56.7 | |
| GAPDH | forward | GTCTCCTCTGACTTCAACAGCG | 56.7 |
| reverse | ACCACCCTGTTGCTGTAGCCAA | 56.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollny, T.; Wnorowska, U.; Piktel, E.; Suprewicz, Ł.; Król, G.; Głuszek, K.; Góźdź, S.; Kopczyński, J.; Bucki, R. Sphingosine-1-Phosphate-Triggered Expression of Cathelicidin LL-37 Promotes the Growth of Human Bladder Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7443. https://doi.org/10.3390/ijms23137443
Wollny T, Wnorowska U, Piktel E, Suprewicz Ł, Król G, Głuszek K, Góźdź S, Kopczyński J, Bucki R. Sphingosine-1-Phosphate-Triggered Expression of Cathelicidin LL-37 Promotes the Growth of Human Bladder Cancer Cells. International Journal of Molecular Sciences. 2022; 23(13):7443. https://doi.org/10.3390/ijms23137443
Chicago/Turabian StyleWollny, Tomasz, Urszula Wnorowska, Ewelina Piktel, Łukasz Suprewicz, Grzegorz Król, Katarzyna Głuszek, Stanisław Góźdź, Janusz Kopczyński, and Robert Bucki. 2022. "Sphingosine-1-Phosphate-Triggered Expression of Cathelicidin LL-37 Promotes the Growth of Human Bladder Cancer Cells" International Journal of Molecular Sciences 23, no. 13: 7443. https://doi.org/10.3390/ijms23137443





