Efficient Separation and Recovery of Petroleum Hydrocarbon from Oily Sludge by a Combination of Adsorption and Demulsification
Abstract
:1. Introduction
2. Results and Discussion
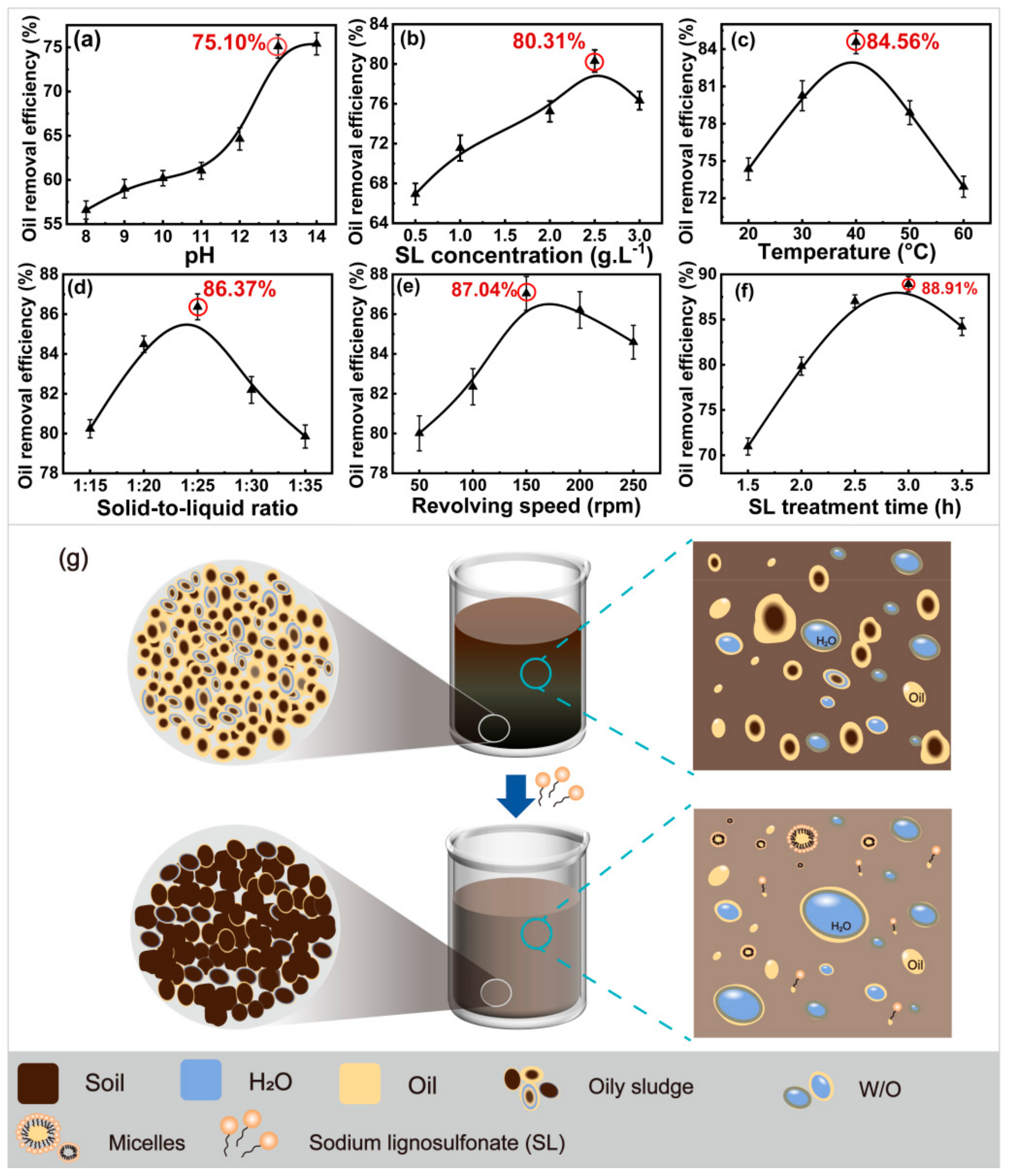
2.1. Effect of Adsorption on Oil Removal Efficiency
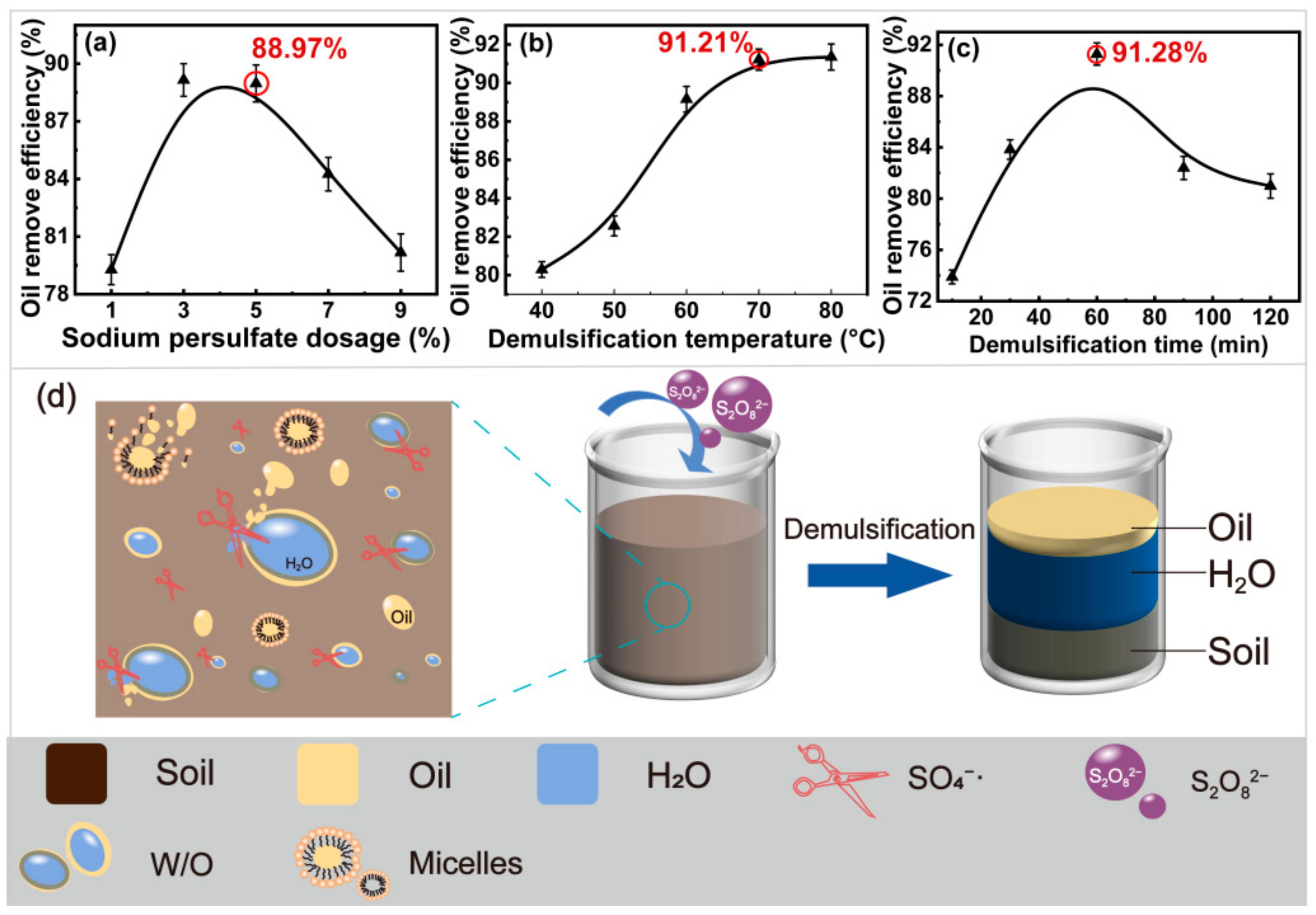
2.2. Effect of Demulsification on Oil Recovery
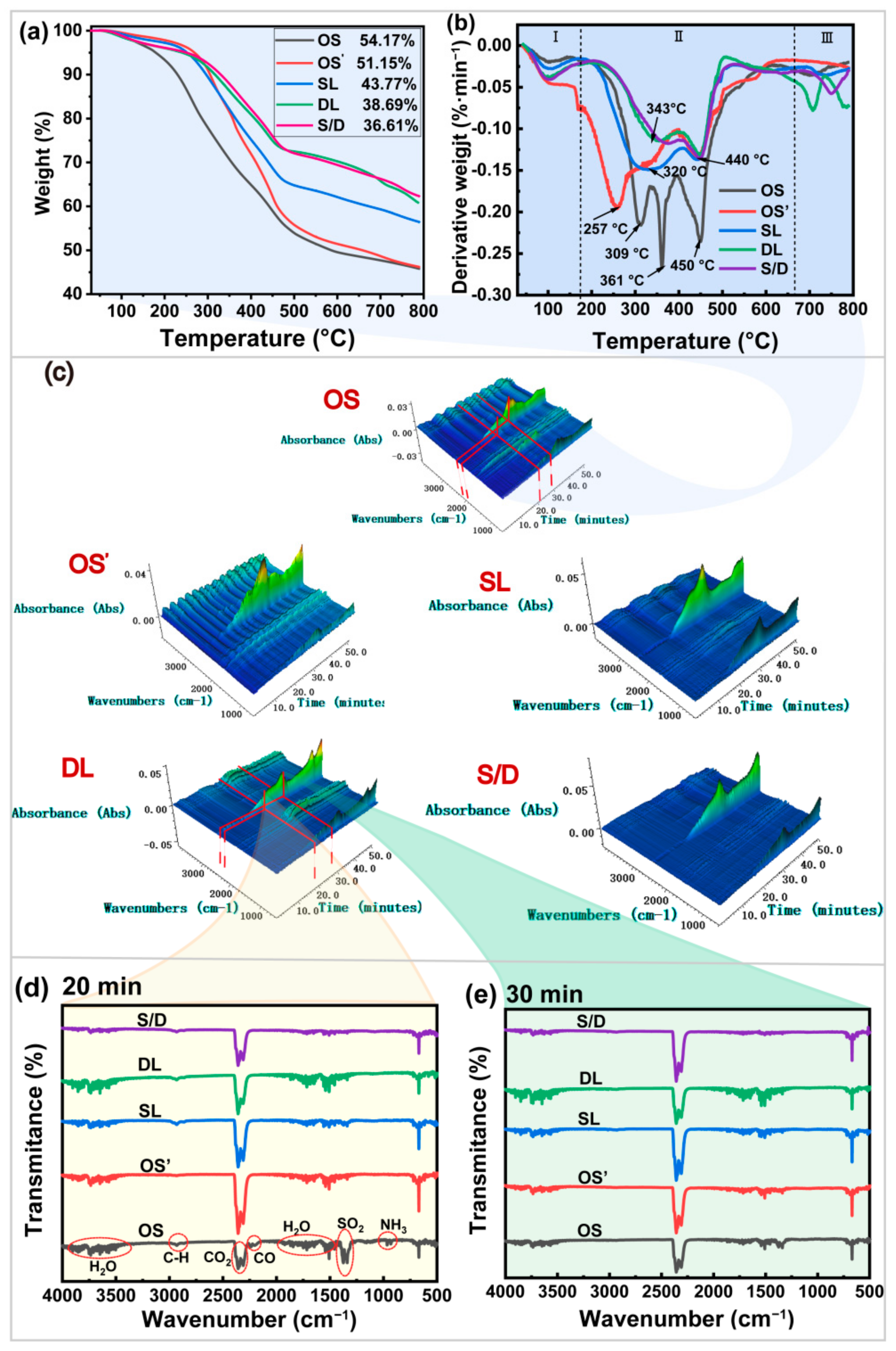
2.3. Apparent Morphology and Physicochemical Properties of Oily Sludge
2.4. Change of Composition of PHCs from the Oily Sludge
2.5. Removal Mechanism of PHCs from the Oily Sludge
3. Materials and Methods
3.1. Materials
3.2. Thermal Washing of Oily Sludge
3.3. Physicochemical Properties of Oily Sludge
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OS | Oily sludge |
| SL | Sodium lignosulfonate |
| DL | Sodium persulfate treatment |
| S/D | Sodium lignosulfonate-assisted sodium persulfate treatment |
References
- Hui, K.; Tang, J.; Lu, H.; Xi, B.; Qu, C.; Li, J. Status and prospect of oil recovery from oily sludge: A review. Arab. J. Chem. 2020, 13, 6523–6543. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Torkashvand, M.; Mohammad, M.; Alghoul, M.A.; Gasaymeh, S.S.; Sopian, K. Wastes from the petroleum industries as sustainable resource materials in construction sectors: Opportunities, limitations, and directions. J. Clean. Prod. 2021, 284, 125459. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Peng, K.; Zhao, X.; Xiong, Y.; Huang, X. A review of the interfacial stability mechanism of aging oily sludge: Heavy components, inorganic particles, and their synergism. J. Hazard. Mater. 2021, 415, 125624. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.J.; Feng, H.B.; He, P.W.; Li, J.B.; Hewage, K.; Sadiq, R. Comparative life-cycle assessment of traditional and emerging oily sludge treatment approaches. J. Clean. Prod. 2020, 251, 119594. [Google Scholar] [CrossRef]
- Teng, Q.; Zhang, D.; Yang, C. A review of the application of different treatment processes for oily sludge. Environ. Sci. Pollut. Res. Int. 2021, 28, 121–132. [Google Scholar] [CrossRef]
- Choudhury, S.P.; Dalasingh, B.; Haq, I.; Kalamdhad, A.S. Methane production and toxicity evaluation of petroleum refinery biosludge through optimization of different modes of heat. Process Saf. Environ. Prot. 2021, 154, 236–248. [Google Scholar] [CrossRef]
- Hamidi, Y.; Ataei, S.A.; Sarrafi, A. A simple, fast and low-cost method for the efficient separation of hydrocarbons from oily sludge. J. Hazard. Mater. 2021, 413, 125328. [Google Scholar] [CrossRef]
- Zubaidy, E.A.H.; Abouelnasr, D.M. Fuel recovery from waste oily sludge using solvent extraction. Process Saf. Environ. Prot. 2010, 88, 318–326. [Google Scholar] [CrossRef]
- Sivagami, K.; Tamizhdurai, P.; Mujahed, S.; Nambi, I. Process optimization for the recovery of oil from tank bottom sludge using microwave pyrolysis. Process Saf. Environ. Prot. 2021, 148, 392–399. [Google Scholar] [CrossRef]
- Chen, P.; Yin, D.; Song, P.F.; Liu, Y.Y.; Cai, L.K.; Wang, H.L.; Zhang, L.H. Demulsification and oil recovery from oil-in-water cutting fluid wastewater using electrochemical micromembrane technology. J. Clean. Prod. 2020, 244, 118698. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, L.; Hou, W. Solid effect in chemical cleaning treatment of oily sludge. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 38–42. [Google Scholar] [CrossRef]
- Ma, C.; Tchameni, A.P.; Pan, L.; Su, C.; Zhou, C. A thermothickening polymer as a novel flocculant for oily wastewater treatment. Sep. Sci. Technol. 2019, 55, 123–134. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.E.H.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Jing, G.; Chen, T.; Luan, M. Studying oily sludge treatment by thermo chemistry. Arab. J. Chem. 2016, 9, S457–S460. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Qi, D.M.; Zhang, D.; Fu, K.J.; Li, Y.; Zhao, H.T. Fabrication of recyclable multi-responsive magnetic nanoparticles for emulsified oil-water separation. J. Clean. Prod. 2020, 255, 120293. [Google Scholar] [CrossRef]
- Smits, J.; Giri, R.P.; Shen, C.; Mendonça, D.; Murphy, B.; Huber, P. Synergistic and competitive adsorption of hydrophilic nanoparticles and oil-soluble surfactants at the oil–water interface. Langmuir 2021, 37, 5659–5672. [Google Scholar] [CrossRef]
- Davis, C.R.; Martinez, C.J.; Howarter, J.A.; Erk, K.A. Diffusion-controlled spontaneous emulsification of water-soluble oils via micelle swelling. Langmuir 2020, 36, 7517–7527. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Wang, G.; Li, S.; Han, Z.; Ren, L. Switchable wettability surface with chemical ctability and antifouling properties for controllable oil–water separation. Langmuir 2019, 35, 4498–4508. [Google Scholar] [CrossRef]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef]
- Chen, G.; Cheng, C.; Zhang, J.; Sun, Y.; Hu, Q.; Qu, C.; Dong, S. Synergistic effect of surfactant and alkali on the treatment of oil sludge. J. Pet. Sci. Eng. 2019, 183, 106420. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Wang, Z.; Dai, X.; Chen, M.; Zhang, J. Novel lignosulfonate/N,N-dimethylacrylamide/γ-methacryloxypropyl trimethoxy silane graft copolymer as a filtration reducer for water-based drilling fluids. J. Appl. Polym. Sci. 2019, 137, 48274. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Islam, M.A.; Sadrzadeh, M. Industrial waste lignin as an antifouling coating for the treatment of oily wastewater: Creating wealth from waste. J. Clean. Prod. 2020, 256, 120304. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, M.; Liu, L.; Qin, C.; Qin, B.; Deng, N.; Liang, C.; Yao, S. Mechanism and Characteristics of Oil Recovery from Oily Sludge by Sodium Lignosulfonate Treatment. ACS Omega 2021, 6, 25819–25827. [Google Scholar] [CrossRef] [PubMed]
- Benmouhoub, C.; Turmine, M.; Kadri, A.; Pailleret, A. Influence of dodecylsulfate adsorption on the stability of cerium oxide nanoparticle-based colloidal aqueous dispersions. Langmuir 2020, 36, 14563–14572. [Google Scholar] [CrossRef]
- Li, P.Q.; Lu, J.; Yang, R.F.; Liu, Y.J. Comparison of Structures and Properties of Lignosulfonate in Different Pulping Waste Liquors; World Scientific Publ Co Pte Ltd.: Singapore, 2017; pp. 273–278. [Google Scholar]
- Liao, X.; Zhao, D.; Yan, X.; Huling, S.G. Identification of persulfate oxidation products of polycyclic aromatic hydrocarbon during remediation of contaminated soil. J. Hazard. Mater. 2014, 276, 26–34. [Google Scholar] [CrossRef]
- Bouzid, I.; Maire, J.; Brunol, E.; Caradec, S.; Fatin-Rouge, N. Compatibility of surfactants with activated-persulfate for the selective oxidation of PAH in groundwater remediation. J. Environ. Chem. Eng. 2017, 5, 6098–6106. [Google Scholar] [CrossRef]
- Lominchar, M.A.; Santos, A.; de Miguel, E.; Romero, A. Remediation of aged diesel contaminated soil by alkaline activated persulfate. Sci. Total Environ. 2018, 62, 41–48. [Google Scholar] [CrossRef]
- Natarajan, A.; Kuznicki, N.; Harbottle, D.; Masliyah, J.; Zeng, H.; Xu, Z. Understanding mechanisms of asphaltene adsorption from organic solvent on mica. Langmuir 2014, 30, 9370–9377. [Google Scholar] [CrossRef]
- Chen, F.; Luo, Z.; Liu, G.; Yang, Y.; Zhang, S.; Ma, J. Remediation of electronic waste polluted soil using a combination of persulfate oxidation and chemical washing. J. Environ. Manag. 2017, 204, 170–178. [Google Scholar] [CrossRef]
- Yan, M.; Yang, D.; Deng, Y.; Chen, P.; Zhou, H.; Qiu, X. Influence of pH on the behavior of lignosulfonate macromolecules in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 50–58. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Zhou, Y.; Chen, J.; Zhan, P.; Yang, G.; Wu, Z. Assembly of lignin-based colloidal particles: Effects of cationic surfactants, molecular weight, and solvent on morphology. RSC Adv. 2020, 10, 18594–18600. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manag. 2017, 199, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, M.; Guo, C.; Abel, S.; Yi, X.; Lu, G.; Yang, C.; Dang, Z. Competitive solubilization of low-molecular-weight polycyclic aromatic hydrocarbons mixtures in single and binary surfactant micelles. Chem. Eng. J. 2014, 244, 522–530. [Google Scholar] [CrossRef]
- Kontturi, A.-K.; Kontturi, K.; Niinikoski, P.; Murtomäki, L.; Springborg, J.; Wang, D.-N.; Christensen, S.B. An experimental study of the effect of temperature on effective charge numbers and diffusion coefficients of lignosulfonate. Acta Chem. Scand. 1992, 46, 941–948. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, M.; Yang, D.; Qiu, X. Effects of concentration and temperature on the rheological behavior of concentrated sodium lignosulfonate (NaLS) solutions. Holzforschung 2015, 69, 265–271. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lee, S.; Lee, J.W. Does SDS micellize under methane hydrate-forming conditions below the normal Krafft point? J. Colloid Interface Sci. 2007, 315, 313–318. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, S.; Zhou, J.; Zhang, Z. A novel combined system using Na2S2O8/urea to simultaneously remove SO2 and NO in marine diesel engine exhaust. J. Hazard. Mater. 2020, 399, 123069. [Google Scholar] [CrossRef]
- Duan, M.; Wang, X.; Fang, S.; Zhao, B.; Li, C.; Xiong, Y. Treatment of daqing oily sludge by thermochemical cleaning method. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 272–278. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, L. Solubilization of pyrene by anionic-nonionic mixed surfactants. J. Hazard. Mater. 2004, 109, 213–220. [Google Scholar] [CrossRef]
- Abdulraheim, A.M. Green polymeric surface active agents for crude oil demulsification. J. Mol. Liq. 2018, 271, 329–341. [Google Scholar] [CrossRef]
- Peng, S.; Wu, W.; Chen, J. Removal of PAHs with surfactant-enhanced soil washing: Influencing factors and removal effectiveness. Chemosphere 2011, 82, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Burlatsky, S.F.; Atrazhev, V.V.; Dmitriev, D.V.; Sultanov, V.I.; Timokhina, E.N.; Ugolkova, E.A.; Tulyani, S.; Vincitore, A. Surface tension model for surfactant solutions at the critical micelle concentration. J. Colloid Interface Sci. 2013, 393, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, X.; Wang, Y.; Li, X.; Wang, Z.; Yang, Z.; Li, C.; Yun, J. Application of chemical hot washing method for oily solid waste treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 042040. [Google Scholar] [CrossRef]
- Mora, V.C.; Madueno, L.; Peluffo, M.; Rosso, J.A.; Del Panno, M.T.; Morelli, I.S. Remediation of phenanthrene-contaminated soil by simultaneous persulfate chemical oxidation and biodegradation processes. Environ. Sci. Pollut. Res. Int. 2014, 21, 7548–7556. [Google Scholar] [CrossRef] [PubMed]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of base activation of persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef]
- Rodionova, G.; Keleşoğlu, S.; Sjöblom, J. AC field induced destabilization of water-in-oil emulsions based on North Sea acidic crude oil. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 60–66. [Google Scholar] [CrossRef]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Guan, R.P.; Wu, Z.B.; Jiang, L.B.; Li, Y.F.; Chen, X.H.; Zeng, G.M. Effective treatment of oily scum via catalytic wet persulfate oxidation process activated by Fe2+. J. Environ. Manag. 2018, 217, 411–415. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; Liu, D.; Li, Z.; Guo, S.; Zhu, W.; Shi, N.; Wen, F.; Dong, J. Insight into essential channel effect of pore structures and hydrogen bonds on the solvent extraction of oily sludge. J. Hazard. Mater. 2020, 389, 121826. [Google Scholar] [CrossRef]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Bioinspired surfaces with superamphiphobic properties: Concepts, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1707415. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, D.; Lin, M.; Yang, Z.; Dong, Z. Effect of resins, waxes and asphaltenes on water-oil interfacial properties and emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2016, 507, 1–6. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Gao, F.; Zhou, J.; Cen, K. The slurrying properties of slurry fuels made of petroleum coke and petrochemical sludge. Fuel Processing Technol. 2012, 104, 57–66. [Google Scholar] [CrossRef]
- Seka, M.A.; Verstraete, W. Test for assessing shear sensitivity of activated sludge flocs: A feasibility study. Water Res. 2003, 37, 3327–3334. [Google Scholar] [CrossRef]
- Pensini, E.; Harbottle, D.; Yang, F.; Tchoukov, P.; Li, Z.; Kailey, I.; Behles, J.; Masliyah, J.; Xu, Z. Demulsification mechanism of asphaltene-stabilized water-in-oil emulsions by a polymeric ethylene oxide–propylene oxide demulsifier. Energy Fuels 2014, 28, 6760–6771. [Google Scholar] [CrossRef]
- Zheng, X.; Ying, Z.; Cui, J.; Wang, B.; Chen, J.; Zhang, Q. Simultaneous dewatering and recovering oil from high-viscosity oily sludge through the combination process of demulsification, viscosity reduction, and centrifugation. Energy Fuels 2017, 31, 14401–14407. [Google Scholar] [CrossRef]
- Nichols, E.G.; Musella, J. Differences in PAH desorption and sediment organic matter composition between non-vegetated and recently vegetated fuel-oiled sediments. Int. J. Phytoremediation 2009, 11, 463–478. [Google Scholar] [CrossRef]
- Liu, C.; Wu, B.; Chen, X.E. Sulfate radical-based oxidation for sludge treatment: A review. Chem. Eng. J. 2018, 335, 865–875. [Google Scholar] [CrossRef]
- Nie, F.; He, D.; Guan, J.; Zhang, K.; Meng, T.; Zhang, Q. The influence of abundant calcium oxide addition on oil sand pyrolysis. Fuel Processing Technol. 2017, 155, 216–224. [Google Scholar] [CrossRef]
- Do, L.D.; Singh, V.; Chen, L.; Kibbey, T.C.G.; Gollahalli, S.R.; Sabatini, D.A. Algae, canola, or palm oils—Diesel microemulsion fuels: Phase behaviors, viscosity, and combustion properties. Int. J. Green Energy 2011, 8, 748–767. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, C.; Jiang, Q.; Wang, Y.; Wu, D. Pyrolysis model of oil sand using thermogravimetric analysis. J. Therm. Anal. Calorim. 2013, 116, 499–509. [Google Scholar] [CrossRef]
- Liu, W.J.; Shao, Z.G.; Xu, Y. Emission characteristics of nitrogen and sulfur containing pollutants during the pyrolysis of oily sludge with and without catalysis. J. Hazard. Mater. 2021, 401, 123820. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, X.; Yan, X.; Huling, S.G.; Chai, T.; Tao, H. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. J. Hazard. Mater. 2013, 254, 228–235. [Google Scholar] [CrossRef]
- Mousavi, S.-P.; Hemmati-Sarapardeh, A.; Norouzi-Apourvari, S.; Jalalvand, M.; Schaffie, M.; Ranjbar, M. Toward mechanistic understanding of wettability alteration in calcite and dolomite rocks: The effects of resin, asphaltene, anionic surfactant, and hydrophilic nano particles. J. Mol. Liq. 2021, 321, 114672. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Jiao, Y.; Sun, Z.; Yang, X.; Cheng, Z.; Bai, Q.; Zhang, Y.; Wang, K.; Shao, L. Constructing scalable superhydrophobic membranes for ultrafast water-oil separation. ACS Nano 2021, 15, 3500–3508. [Google Scholar] [CrossRef] [PubMed]
- Delgado, N.; Ysambertt, F.; Chávez, G.; Bravo, B.; García, D.E.; Santos, J. Valorization of kraft Lignin of different molecular weights as surfactant agent for the oil Industry. Waste Biomass Valoriz. 2018, 10, 3383–3395. [Google Scholar] [CrossRef]
- Kök, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Crude oil characterization using TGA-DTA, TGA-FTIR and TGA-MS techniques. J. Pet. Sci. Eng. 2017, 154, 537–542. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, M.; Ma, Y.; Liu, L.; Qin, C.; Huang, H.; Zhang, Z.; Liang, C.; Yao, S. Efficient Separation and Recovery of Petroleum Hydrocarbon from Oily Sludge by a Combination of Adsorption and Demulsification. Int. J. Mol. Sci. 2022, 23, 7504. https://doi.org/10.3390/ijms23147504
Yao M, Ma Y, Liu L, Qin C, Huang H, Zhang Z, Liang C, Yao S. Efficient Separation and Recovery of Petroleum Hydrocarbon from Oily Sludge by a Combination of Adsorption and Demulsification. International Journal of Molecular Sciences. 2022; 23(14):7504. https://doi.org/10.3390/ijms23147504
Chicago/Turabian StyleYao, Mingzhu, Yun Ma, Lu Liu, Chengrong Qin, Haibo Huang, Zhiwei Zhang, Chen Liang, and Shuangquan Yao. 2022. "Efficient Separation and Recovery of Petroleum Hydrocarbon from Oily Sludge by a Combination of Adsorption and Demulsification" International Journal of Molecular Sciences 23, no. 14: 7504. https://doi.org/10.3390/ijms23147504





