Differential Cellular Balance of Olfactory and Vomeronasal Epithelia in a Transgenic BACHD Rat Model of Huntington’s Disease
Abstract
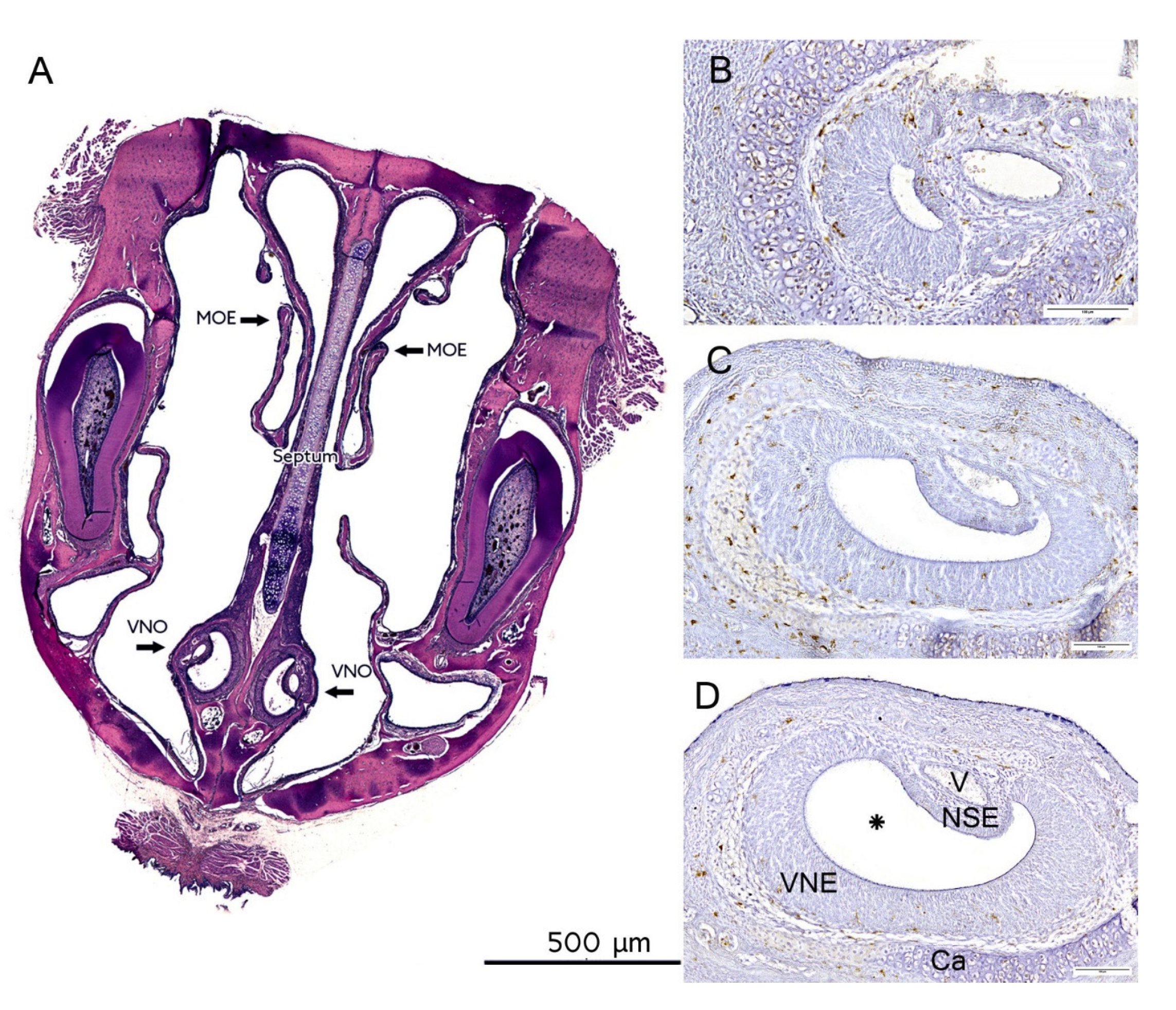
:1. Introduction
2. Results
2.1. Validation of the Bacterial Artificial Chromosome Huntington Disease (BACHD) Rat Model
2.2. Three-Month-Old BACHD Rats Have Olfactory Deficits
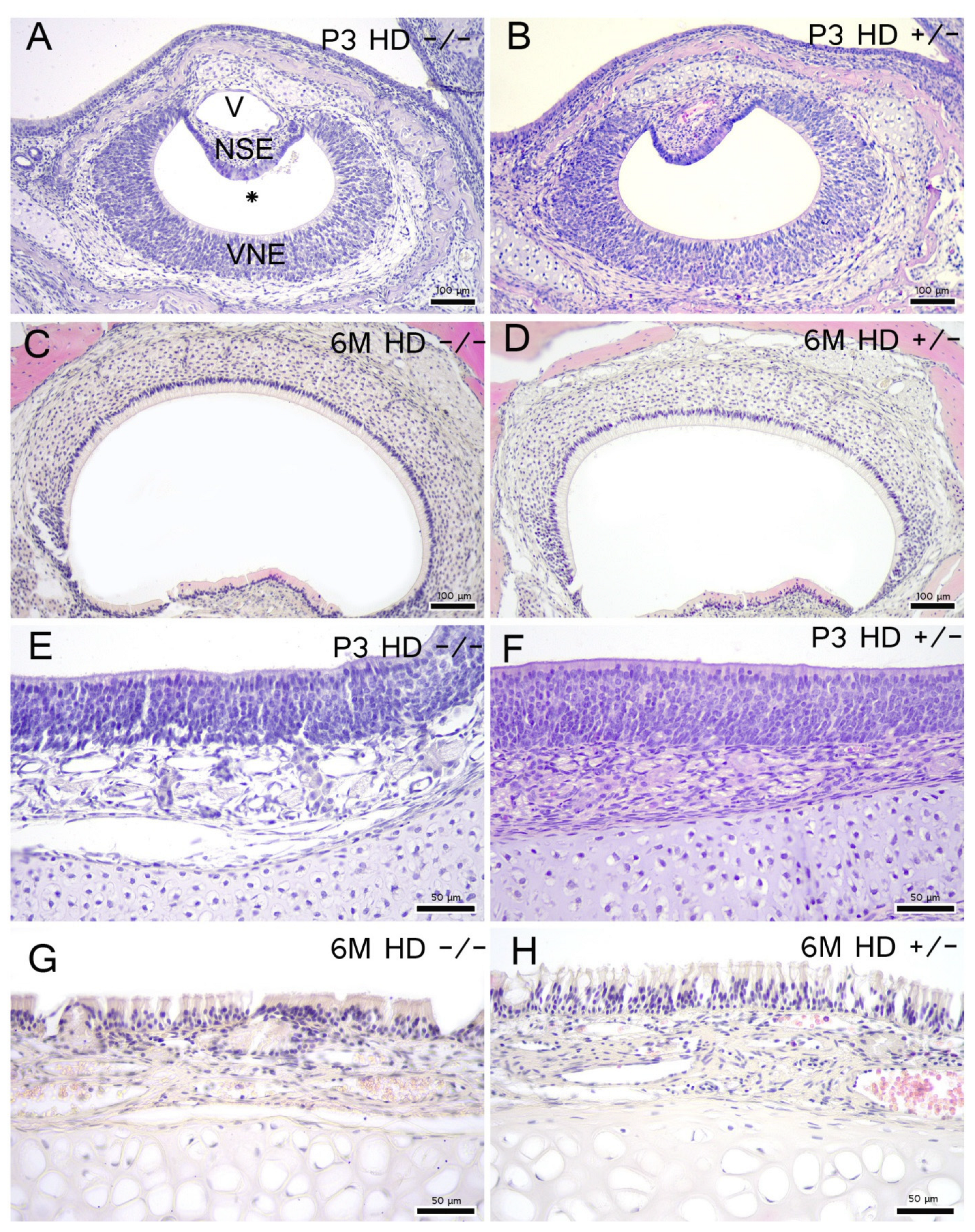
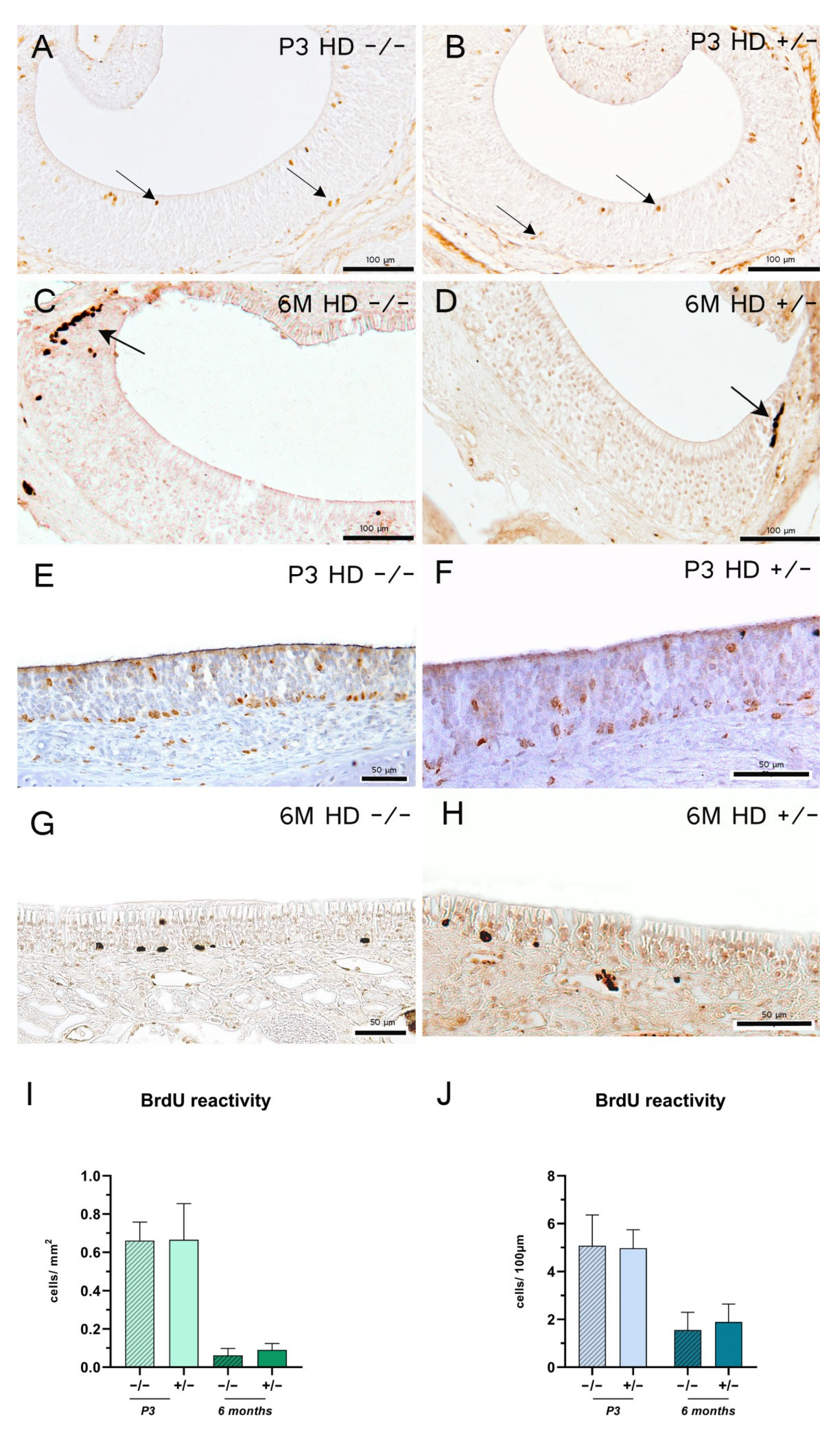
2.3. Morphology, Cell Proliferation and Apoptosis Are Independent of Genotype
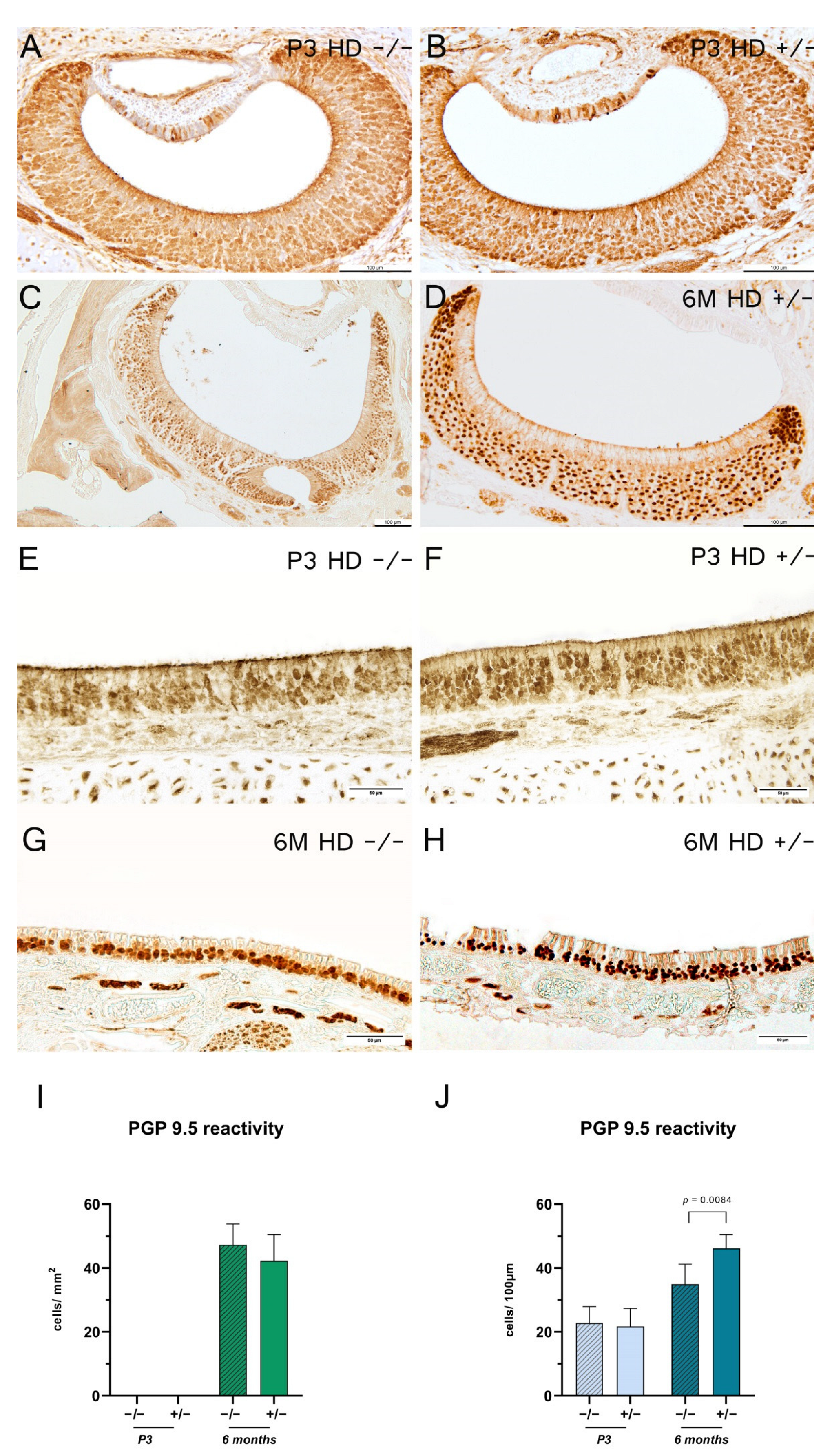
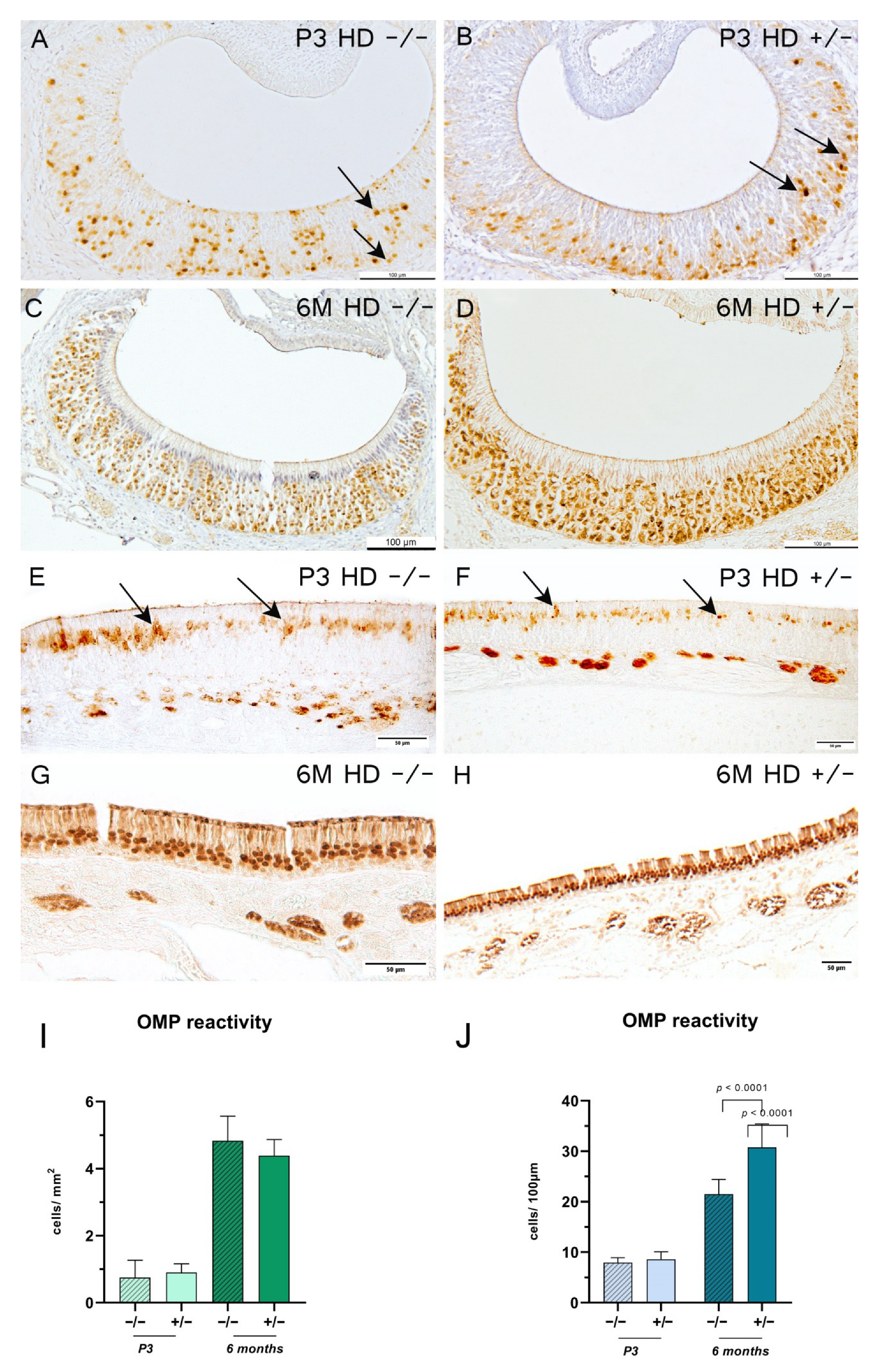
2.4. Transgenic Rats Revealed an Increase in Mature Olfactory Receptor Neurons in the Main Olfactory Epithelium
3. Discussion
3.1. Vomeronasal vs. Olfactory Epithelium: The Cellular Homeostasis Is Differentially Affected in BACHD Rats
3.2. Does the BACHD TG22 Line Have an Olfactory Phenotype? Comparison to Other BACHD Lines
3.3. Limitations of the Study
4. Materials and Methods
4.1. Animal Model
4.2. Olfaction Detection Ability Test: The Buried Pellet Test
4.3. BrdU Injections, Weight Measurement and Sample Preparation
4.4. Western Blot Analysis
4.5. Sample Selection
4.6. Histology and Immunohistochemistry
4.7. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wexler, A.; Wild, E.J.; Tabrizi, S.J. George Huntington: A legacy of inquiry, empathy and hope. Brain 2016, 139, 2326–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielcarek, M. Huntington’s disease is a multi-system disorder. Rare Dis. 2015, 3, e1058464. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. The Huntington’s Disease Collaborative Research Group: A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Brinkman, R.; Falush, D.; Paulsen, J.; Hayden, M.R.; on behalf of an International Huntington’s Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004, 65, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Scahill, R.I.; Durr, A.; Roos, R.A.C.; Leavitt, B.R.; Jones, R.; Landwehrmeyer, G.B.; Fox, N.C.; Johnson, H.; Hicks, S.L.; et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurol. 2011, 10, 31–42. [Google Scholar] [CrossRef]
- Vonsattel, J.P.G.; Difiglia, M. Huntington Disease. J. Neuropathol. Exp. Neurol. 1998, 57, 369–384. [Google Scholar] [CrossRef] [Green Version]
- Halliday, G.M.; McRitchie, D.A.; Macdonald, V.; Double, K.L.; Trent, R.J.; McCusker, E. Regional specificity of brain atrophy in Huntington’s disease. Exp. Neurol. 1998, 154, 663–672. [Google Scholar] [CrossRef]
- Vonsattel, J.P.G. Huntington disease models and human neuropathology: Similarities and differences. Acta Neuropathol. 2008, 115, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Ansari, K.; Johnson, A. Olfactory function in patients with Parkinson’s disease. J. Chronic Dis 1975, 28, 493–497. [Google Scholar] [CrossRef]
- Hovakimyan, M.; Meyer, A.; Lukas, J.; Luo, J.; Gudziol, V.; Hummel, T.; Rolfs, A.; Wree, A.; Witt, M. Olfactory deficits in Niemann-Pick type C1 (NPC1) disease. PLoS ONE 2013, 8, e82216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serby, M.; Larson, P.; Kalkstein, D. The nature and course of olfactory deficits in Alzheimer’s disease. Am. J. Psychiatry 1991, 148, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Influence of age and age-related diseases on olfactory function. Ann. N. Y. Acad. Sci. 1989, 561, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Barresi, M.; Ciurleo, R.; Giacoppo, S.; Foti Cuzzola, V.; Celi, D.; Bramanti, P.; Marino, S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012, 323, 16–24. [Google Scholar] [CrossRef]
- Moberg, P.J.; Pearlson, G.D.; Speedie, L.J.; Lipsey, J.R.; Strauss, M.E.; Folstein, S.E. Olfactory recognition: Differential impairments in early and late Huntington’s and Alzheimer’s diseases. J. Clin. Exp. Neuropsychol. 1987, 9, 650–664. [Google Scholar] [CrossRef]
- Nordin, S.; Paulsen, J.S.; Murphy, C. Sensory- and memory-mediated olfactory dysfunction in Huntington’s disease. J. Int. Neuropsychol. Soc. 1995, 1, 281–290. [Google Scholar] [CrossRef]
- Bacon Moore, A.S.; Paulsen, J.S.; Murphy, C. A test of odor fluency in patients with Alzheimer’s and Huntington’s disease. J. Clin. Exp. Neuropsychol. 1999, 21, 341–351. [Google Scholar] [CrossRef]
- Lazic, S.E.; Goodman, A.O.G.; Grote, H.E.; Blakemore, C.; Morton, A.J.; Hannan, A.J.; van Dellen, A.; Barker, R.A. Olfactory abnormalities in Huntington’s disease: Decreased plasticity in the primary olfactory cortex of R6/1 transgenic mice and reduced olfactory discrimination in patients. Brain Res. 2007, 1151, 219–226. [Google Scholar] [CrossRef]
- Menalled, L.B.; Sison, J.D.; Dragatsis, I.; Zeitlin, S.; Chesselet, M.-F. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington’s disease with 140 CAG repeats. J. Comp. Neurol. 2003, 465, 11–26. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef]
- von Hörsten, S.; Schmitt, I.; Nguyen, H.P.; Holzmann, C.; Schmidt, T.; Walther, T.; Bader, M.; Pabst, R.; Kobbe, P.; Krotova, J.; et al. Transgenic rat model of Huntington’s disease. Hum. Mol. Genet. 2003, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Yu-Taeger, L.; Petrasch-Parwez, E.; Osmand, A.P.; Redensek, A.; Metzger, S.; Clemens, L.E.; Park, L.; Howland, D.; Calaminus, C.; Gu, X.; et al. A novel BACHD transgenic rat exhibits characteristic neuropathological features of Huntington disease. J. Neurosci. 2012, 32, 15426–15438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessard-Beaudoin, M.; Yu-Taeger, L.; Laroche, M.; Singer, E.; Riess, O.; Nguyen, H.H.P.; Graham, R.K. Olfactory bulb atrophy and caspase activation observed in the BACHD rat models of Huntington disease. Neurobiol. Dis. 2019, 125, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Lessard-Beaudoin, M.; Garcia-Miralles, M.; Kreidy, C.; Peachey, E.; Leavitt, B.R.; Pouladi, M.A.; Graham, R.K. Early deficits in olfaction are associated with structural and molecular alterations in the olfactory system of a Huntington disease mouse model. Hum. Mol. Genet. 2020, 29, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Funakoshi, T.; Unuma, K.; Uemura, K. Impairment of autophagy: From hereditary disorder to drug intoxication. Toxicology 2013, 311, 205–215. [Google Scholar] [CrossRef]
- Schwob, J.E.; Jang, W.; Holbrook, E.H.; Lin, B.; Herrick, D.B.; Peterson, J.N.; Hewitt Coleman, J. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J. Comp. Neurol. 2017, 525, 1034–1054. [Google Scholar] [CrossRef] [Green Version]
- Witt, M.; Thiemer, R.; Meyer, A.; Schmitt, O.; Wree, A. Main Olfactory and Vomeronasal Epithelium Are Differently Affected in Niemann-Pick Disease Type C1. Int. J. Mol. Sci. 2018, 19, 3563. [Google Scholar] [CrossRef] [Green Version]
- Brann, J.H.; Firestein, S.J. A lifetime of neurogenesis in the olfactory system. Front. Neurosci. 2014, 8, 182. [Google Scholar] [CrossRef]
- Witt, M.; Woźniak, W. Structure and function of the vomeronasal organ. Adv. Otorhinolaryngol. 2006, 63, 70–83. [Google Scholar] [CrossRef]
- Gratzner, H.G.; Leif, R.C.; Ingram, D.J.; Castro, A. The use of antibody specific for bromodeoxyuridine for the immunofluorescent determination of DNA replication in single cells and chromosomes. Exp. Cell Res. 1975, 95, 88–94. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 1994, 269, 30761–30764. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A.; et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.O.; Barber, P.C.; Hamid, Q.A.; Power, B.F.; Dhillon, A.P.; Rode, J.; Day, I.N.; Thompson, R.J.; Polak, J.M. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br. J. Exp. Pathol. 1988, 69, 91–104. [Google Scholar] [PubMed]
- Margolis, F.L. Olfactory marker protein (OMP). Scand. J. Immunol. 1982, 9, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Bock, P.; Rohn, K.; Beineke, A.; Baumgärtner, W.; Wewetzer, K. Site-specific population dynamics and variable olfactory marker protein expression in the postnatal canine olfactory epithelium. J. Anat. 2009, 215, 522–535. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Paulsen, J.S. Early Detection of Huntington’s Disease. Future Neurol. 2010, 5, 85–104. [Google Scholar] [CrossRef] [Green Version]
- Barber, P.C.; Raisman, G. Cell division in the vomeronasal organ of the adult mouse. Brain Res. 1978, 141, 57–66. [Google Scholar] [CrossRef]
- Weiler, E.; Apfelbach, R.; Farbman, A.I. The vomeronasal organ of the male ferret. Chem Senses 1999, 24, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Weiler, E.; Benali, A. Olfactory epithelia differentially express neuronal markers. J. Neurocytol. 2005, 34, 217–240. [Google Scholar] [CrossRef]
- Cuschieri, A.; Bannister, L.H. The development of the olfactory mucosa in the mouse: Light microscopy. J. Anat. 1975, 119, 277–286. [Google Scholar] [PubMed]
- Weiler, E.; McCulloch, M.A.; Farbman, A.I. Proliferation in the vomeronasal organ of the rat during postnatal development. Eur. J. Neurosci. 1999, 11, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Schwob, J.E. Neural regeneration and the peripheral olfactory system. Anat. Rec. 2002, 269, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Wree, A.; Günther, R.; Holzmann, C.; Schmitt, O.; Rolfs, A.; Witt, M. Increased Regenerative Capacity of the Olfactory Epithelium in Niemann-Pick Disease Type C1. Int. J. Mol. Sci. 2017, 18, 777. [Google Scholar] [CrossRef] [Green Version]
- Mackay-Sim, A.; Kittel, P.W. On the Life Span of Olfactory Receptor Neurons. Eur. J. Neurosci. 1991, 3, 209–215. [Google Scholar] [CrossRef]
- Buschhüter, D.; Smitka, M.; Puschmann, S.; Gerber, J.C.; Witt, M.; Abolmaali, N.D.; Hummel, T. Correlation between olfactory bulb volume and olfactory function. NeuroImage 2008, 42, 498–502. [Google Scholar] [CrossRef]
- Smith, C.G. Pathologic change in olfactory nasal mucosa on Albino Rats with "stunted" olfactory bulbs. Arch. Otolaryngol Head Neck Surg 1937, 25, 131–143. [Google Scholar] [CrossRef]
- Onoda, N. Monoclonal antibody immunohistochemistry of degenerative and renewal patterns in rabbit olfactory receptor neurons following unilateral olfactory bulbectomy. Neuroscience 1988, 26, 1013–1022. [Google Scholar] [CrossRef]
- van Raamsdonk, J.M.; Gibson, W.T.; Pearson, J.; Murphy, Z.; Lu, G.; Leavitt, B.R.; Hayden, M.R. Body weight is modulated by levels of full-length huntingtin. Hum. Mol. Genet. 2006, 15, 1513–1523. [Google Scholar] [CrossRef]
- Gray, M.; Shirasaki, D.I.; Cepeda, C.; André, V.M.; Wilburn, B.; Lu, X.-H.; Tao, J.; Yamazaki, I.; Li, S.-H.; Sun, Y.E.; et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J. Neurosci. 2008, 28, 6182–6195. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, J.L.; Surmeier, D.J. Corticostriatal synaptic adaptations in Huntington’s disease. Curr. Opin. Neurobiol. 2015, 33, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrasate, M.; Mitra, S.; Schweitzer, E.S.; Segal, M.R.; Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 2004, 431, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu-Taeger, L.; Bonin, M.; Stricker-Shaver, J.; Riess, O.; Nguyen, H.H.P. Dysregulation of gene expression in the striatum of BACHD rats expressing full-length mutant huntingtin and associated abnormalities on molecular and protein levels. Neuropharmacology 2017, 117, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Zlebnik, N.E.; Gildish, I.; Sesia, T.; Fitoussi, A.; Cole, E.A.; Carson, B.P.; Cachope, R.; Cheer, J.F. Motivational Impairment is Accompanied by Corticoaccumbal Dysfunction in the BACHD-Tg5 Rat Model of Huntington’s Disease. Cereb. Cortex 2019, 29, 4763–4774. [Google Scholar] [CrossRef]
- Manfré, G.; Doyère, V.; Bossi, S.; Riess, O.; Nguyen, H.P.; El Massioui, N. Impulsivity trait in the early symptomatic BACHD transgenic rat model of Huntington disease. Behav. Brain Res. 2016, 299, 6–10. [Google Scholar] [CrossRef]
- Manfré, G.; Clemensson, E.K.H.; Kyriakou, E.I.; Clemensson, L.E.; van der Harst, J.E.; Homberg, J.R.; Nguyen, H.P. The BACHD Rat Model of Huntington Disease Shows Specific Deficits in a Test Battery of Motor Function. Front. Behav. Neurosci. 2017, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Clemensson, E.K.H.; Clemensson, L.E.; Fabry, B.; Flunkert, S.; Riess, O.; Wronski, R.; Nguyen, H.P. Further investigation of phenotypes and confounding factors of progressive ratio performance and feeding behavior in the BACHD rat model of Huntington disease. PLoS ONE 2017, 12, e0173232. [Google Scholar] [CrossRef] [Green Version]
- Novati, A.; Yu-Taeger, L.; Gonzalez Menendez, I.; Quintanilla Martinez, L.; Nguyen, H.P. Sexual behavior and testis morphology in the BACHD rat model. PLoS ONE 2018, 13, e0198338. [Google Scholar] [CrossRef] [Green Version]
- Novati, A.; Manfré, G.; Flunkert, S.; van der Harst, J.E.; Homberg, J.R.; Wronski, R.; Nguyen, H.P. Validation of behavioral phenotypes in the BACHD rat model. Behav. Brain Res. 2020, 393, 112783. [Google Scholar] [CrossRef]
- Lehmkuhl, A.M.; Dirr, E.R.; Fleming, S.M. Olfactory assays for mouse models of neurodegenerative disease. J. Vis. Exp. 2014, 90, e51804. [Google Scholar] [CrossRef] [Green Version]
- Schackel, S.; Pauly, M.-C.; Piroth, T.; Nikkhah, G.; Döbrössy, M.D. Donor age dependent graft development and recovery in a rat model of Huntington’s disease: Histological and behavioral analysis. Behav. Brain Res. 2013, 256, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dover, R.; Patel, K. Improved methodology for detecting bromodeoxyuridine in cultured cells and tissue sections by immunocytochemistry. Histochemistry 1994, 102, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Onda, K.; Davis, R.L.; Shibuya, M.; Wilson, C.B.; Hoshino, T. Correlation between the bromodeoxyuridine labeling index and the MIB-1 and Ki-67 proliferating cell indices in cerebral gliomas. Cancer 1994, 74, 1921–1926. [Google Scholar] [CrossRef]
- Ohta, Y.; Ichimura, K. Globosal basal cells are identified as proliferating cells in mouse olfactory epithelium. Ann. Otol. Rhinol. Laryngol. 2001, 110, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Margolis, F.L. Immunological studies of the rat olfactory marker protein. J. Neurochem. 1975, 24, 1101–1106. [Google Scholar] [CrossRef]


| VNE | MOE | |||
|---|---|---|---|---|
| Genotype | +/− | −/− | +/− | −/− |
| Animal number (n) | 6 | 6 | 6 | 5 |
| Mean | 24.67 | 32.50 | 1.40 × 10−5 | 1.25 × 10−5 |
| Standard deviation | 11.66 | 29.67 | 7.98 × 10−6 | 6.27 × 10−6 |
| Test performed | Mann–Whitney test | Unpaired t-test (two-tailed) | ||
| Significance level | ns | ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysewski, L.-M.; Power Guerra, N.; Glatzel, A.; Holzmann, C.; Antipova, V.; Schmitt, O.; Yu-Taeger, L.; Nguyen, H.P.; Wree, A.; Witt, M. Differential Cellular Balance of Olfactory and Vomeronasal Epithelia in a Transgenic BACHD Rat Model of Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 7625. https://doi.org/10.3390/ijms23147625
Krysewski L-M, Power Guerra N, Glatzel A, Holzmann C, Antipova V, Schmitt O, Yu-Taeger L, Nguyen HP, Wree A, Witt M. Differential Cellular Balance of Olfactory and Vomeronasal Epithelia in a Transgenic BACHD Rat Model of Huntington’s Disease. International Journal of Molecular Sciences. 2022; 23(14):7625. https://doi.org/10.3390/ijms23147625
Chicago/Turabian StyleKrysewski, Lina-Marielle, Nicole Power Guerra, Annika Glatzel, Carsten Holzmann, Veronica Antipova, Oliver Schmitt, Libo Yu-Taeger, Huu Phuc Nguyen, Andreas Wree, and Martin Witt. 2022. "Differential Cellular Balance of Olfactory and Vomeronasal Epithelia in a Transgenic BACHD Rat Model of Huntington’s Disease" International Journal of Molecular Sciences 23, no. 14: 7625. https://doi.org/10.3390/ijms23147625
APA StyleKrysewski, L.-M., Power Guerra, N., Glatzel, A., Holzmann, C., Antipova, V., Schmitt, O., Yu-Taeger, L., Nguyen, H. P., Wree, A., & Witt, M. (2022). Differential Cellular Balance of Olfactory and Vomeronasal Epithelia in a Transgenic BACHD Rat Model of Huntington’s Disease. International Journal of Molecular Sciences, 23(14), 7625. https://doi.org/10.3390/ijms23147625








