Ameliorating Effects of TRIM67 against Intestinal Inflammation and Barrier Dysfunction Induced by High Fat Diet in Obese Mice
Abstract
:1. Introduction
2. Results
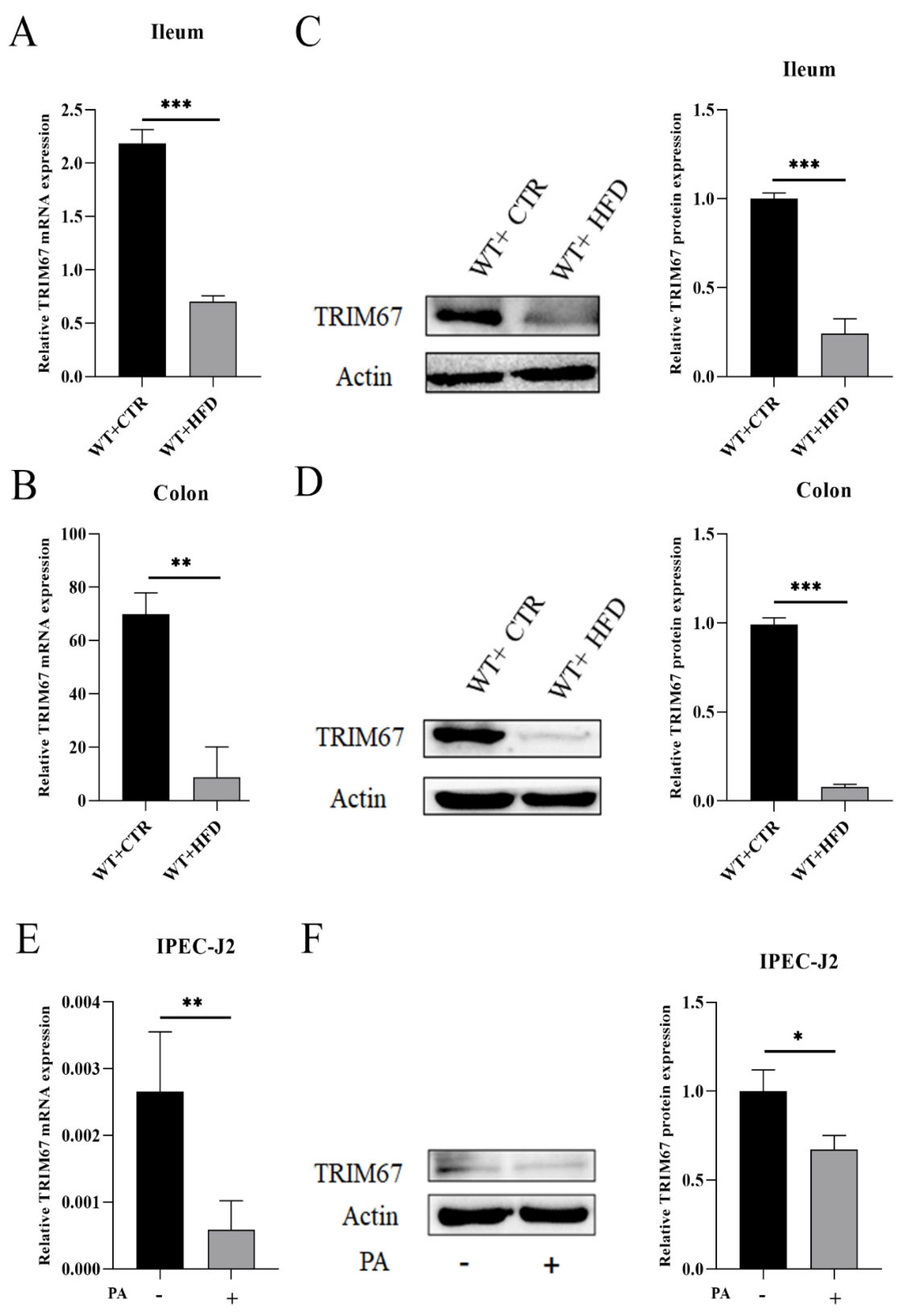
2.1. High Fat Diet (HFD) Suppresses the Expression of TRIM67
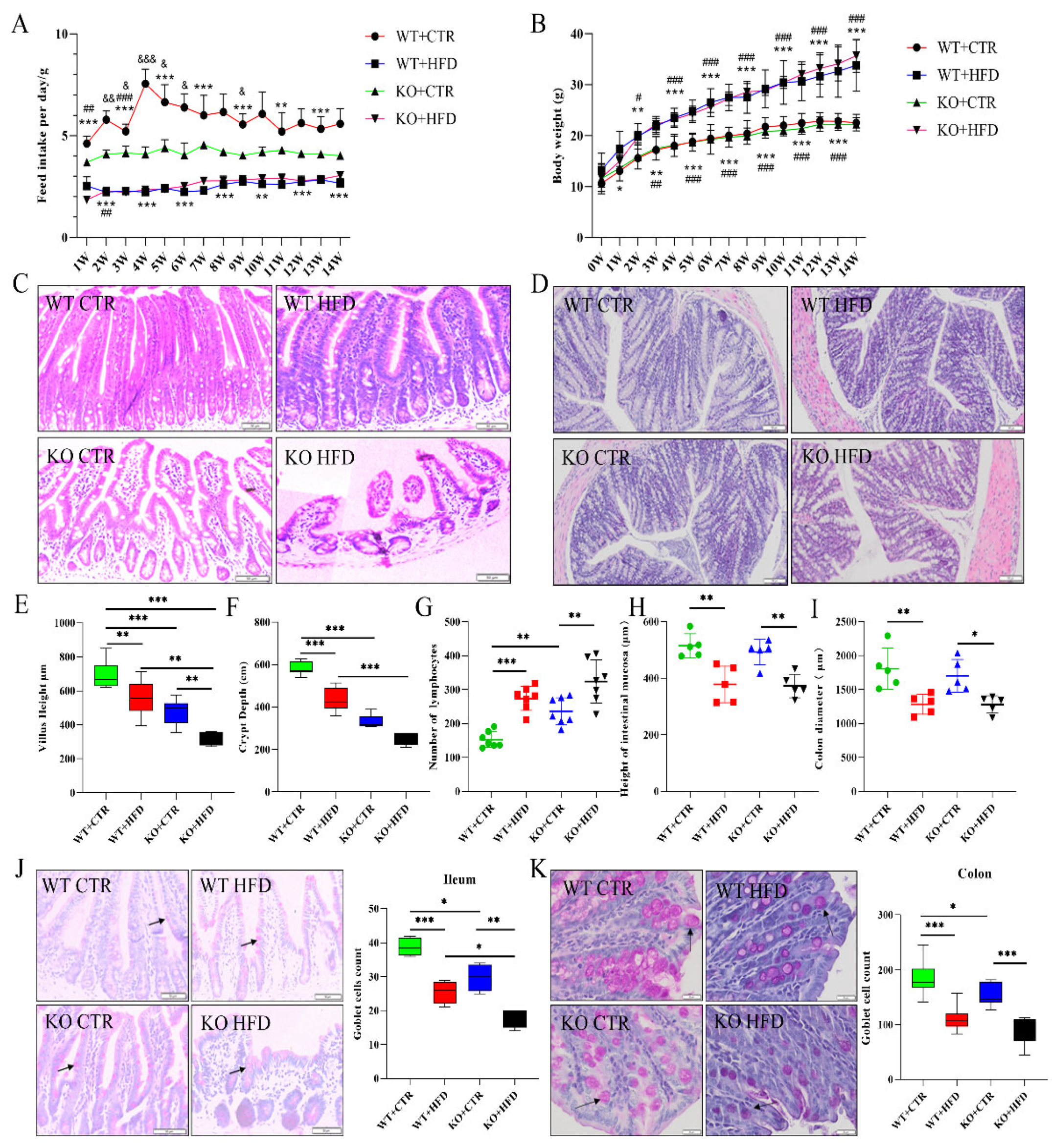
2.2. Deletion of TRIM67 Exacerbates the Pathological Damage of Intestine Induced by High Fat Diet (HFD)
2.3. TRIM67 Promotes Intestinal Barrier Integrity and Protects from the Worst Effects of HFD
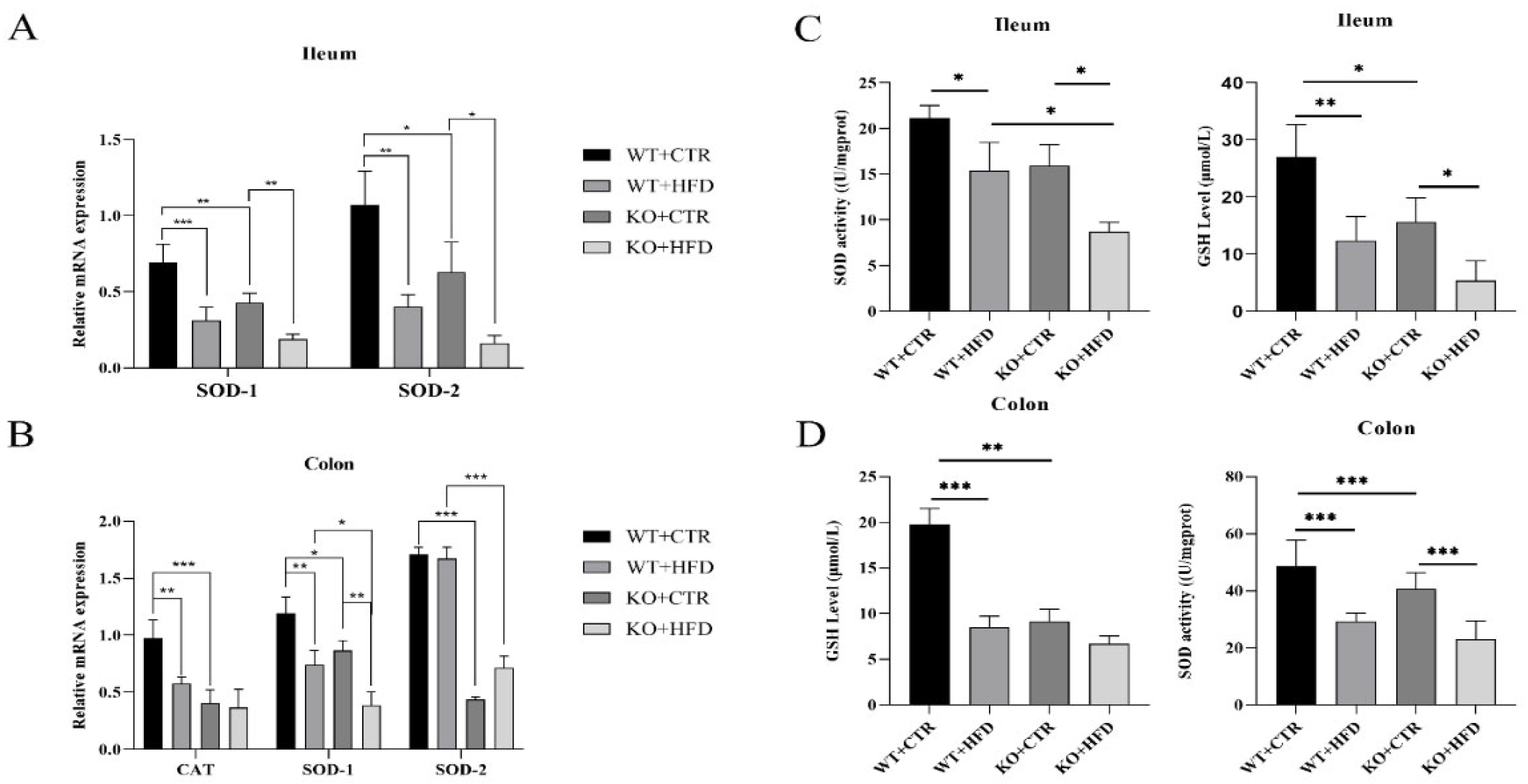
2.4. Deletion of TRIM67 can Suppress the Antioxidant Capacity in Ileum and Colon
2.5. Deletion of TRIM67 Exacerbate Intestinal Inflammation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Feed
4.3. Sample Collection
4.4. Blood Glucose, Blood Lipid and Blood LPS Concentration Detection
4.5. Quantitative Real-Time PCR
4.6. Western Blotting
4.7. Histopathology
4.8. Cell Culture
4.9. Oxidase Activity
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanasaki, K.; Koya, D. Biology of obesity: Lessons from animal models of obesity. J. Biomed. Biotechnol. 2011, 2011, 197636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Snider, A.J. Sphingolipids in high fat diet and obesity-related diseases. Mediat. Inflamm. 2015, 2015, 520618. [Google Scholar] [CrossRef] [Green Version]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Al Mushref, M.; Srinivasan, S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann. Transl. Med. 2013, 1, 14. [Google Scholar]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B. Improvement of colonic immune function with soy isoflavones in high-fat diet-induced obese rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Shi, Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013, 97, 1689–1697. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Sun, Y.; Du, J.; Li, X.; Xun, Z.; Li, Y.C. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G453–G462. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Towers, G.J. Inhibition of retroviral replication by members of the TRIM protein family. Intrinsic Immun. 2013, 29–66. [Google Scholar] [CrossRef]
- Reymond, A.; Meroni, G.; Fantozzi, A.; Merla, G.; Cairo, S.; Luzi, L.; Riganelli, D.; Zanaria, E.; Messali, S.; Cainarca, S. The tripartite motif family identifies cell compartments. EMBO J. 2001, 20, 2140–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajsbaum, R.; Stoye, J.P.; O’Garra, A. Type I interferon-dependent and-independent expression of tripartite motif proteins in immune cells. Eur. J. Immunol. 2008, 38, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, A.M.; Wlodarczyk, N.A.; Mackiewicz, A.; Czerwinska, P. The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cells 2020, 38, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, S. TRIM proteins and cancer. Nat. Rev. Cancer 2011, 11, 792–804. [Google Scholar] [CrossRef]
- Cox, T.C. The microtubule-associated CI subfamily of TRIM proteins and the regulation of polarized cell responses. TRIM/RBCC Proteins 2013, 105–118. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Z.; Meng, Q.; Liang, P.; Su, Z.; Wu, Y.; Huang, J.; Cui, J. TRIM14 Promotes Noncanonical NF-κB Activation by Modulating p100/p52 Stability via Selective Autophagy. Adv. Sci. 2020, 7, 1901261. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhao, Y.; Shi, L.; Li, W.; Chen, K.; Li, M.; Chen, X.; Zhang, H.; Li, T.; Matsuzawa-Ishimoto, Y. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J. Clin. Investig. 2020, 130, 2111–2128. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Huang, J.; Wong, C.C.; Zhai, J.; Li, C.; Wei, G.; Zhao, L.; Wang, G.; Wei, H. TRIM67 activates p53 to suppress colorectal cancer initiation and progression. Cancer Res. 2019, 79, 4086–4098. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, W.; Haeussler, K.; Nasrullah, U.; Pfeilschifter, J. Multifaceted Roles of TRIM Proteins in Colorectal Carcinoma. Int. J. Mol. Sci. 2020, 21, 7532. [Google Scholar] [CrossRef]
- Boyer, N.P.; Monkiewicz, C.; Menon, S.; Moy, S.S.; Gupton, S.L. Mammalian TRIM67 functions in brain development and behavior. Eneuro 2018, 48, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Liu, X.; Li, X.; Qian, S.; Chen, H.; Qian, P. TRIM67 Suppresses TNFalpha-Triggered NF-kB Activation by Competitively Binding Beta-TrCP to IkBa. Front. Immunol. 2022, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Pan, L.; Liao, H.; Yao, W.; Shen, N.; Chen, C.; Liu, D.; Ge, M. High-fat diet increased NADPH-oxidase-related oxidative stress and aggravated LPS-induced intestine injury. Life Sci. 2020, 253, 117539. [Google Scholar] [CrossRef]
- Li, L.-J.; Zheng, J.-C.; Kang, R.; Yan, J.-Q. Targeting Trim69 alleviates high fat diet (HFD)-induced hippocampal injury in mice by inhibiting apoptosis and inflammation through ASK1 inactivation. Biochem. Biophys. Res. Commun. 2019, 515, 658–664. [Google Scholar] [CrossRef]
- Alloush, J.; Weisleder, N. TRIM proteins in therapeutic membrane repair of muscular dystrophy. JAMA Neurol. 2013, 70, 928–931. [Google Scholar] [CrossRef] [Green Version]
- Dahl-Halvarsson, M.; Olive, M.; Pokrzywa, M.; Ejeskär, K.; Palmer, R.H.; Uv, A.E.; Tajsharghi, H. Drosophila model of myosin myopathy rescued by overexpression of a TRIM-protein family member. Proc. Natl. Acad. Sci. USA 2018, 115, E6566–E6575. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Nemoto, Y.; Takei, Y.; Morikawa, R.; Oshima, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Stutte, S. High-fat diet-derived free fatty acids impair the intestinal immune system and increase sensitivity to intestinal epithelial damage. Biochem. Biophys. Res. Commun. 2020, 522, 971–977. [Google Scholar] [CrossRef]
- Shi, C.; Li, H.; Qu, X.; Huang, L.; Kong, C.; Qin, H.; Sun, Z.; Yan, X. High fat diet exacerbates intestinal barrier dysfunction and changes gut microbiota in intestinal-specific ACF7 knockout mice. Biomed. Pharmacother. 2019, 110, 537–545. [Google Scholar] [CrossRef]
- Nakanishi, T.; Fukui, H.; Wang, X.; Nishiumi, S.; Yokota, H.; Makizaki, Y.; Tanaka, Y.; Ohno, H.; Tomita, T.; Oshima, T. Effect of a High-Fat Diet on the Small-Intestinal Environment and Mucosal Integrity in the Gut-Liver Axis. Cells 2021, 10, 3168. [Google Scholar] [CrossRef] [PubMed]
- Benoit, B.; Laugerette, F.; Plaisancié, P.; Géloën, A.; Bodennec, J.; Estienne, M.; Pineau, G.; Bernalier-Donadille, A.; Vidal, H.; Michalski, M.-C. Increasing fat content from 20 to 45 wt% in a complex diet induces lower endotoxemia in parallel with an increased number of intestinal goblet cells in mice. Nutr. Res. 2015, 35, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Villard, P.-H.; Dao, M.A.; Burcelin, R.; Champion, S.; Fouchier, F.; Savouret, J.-F.; Barra, Y.; Seree, E. Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol. Lett. 2010, 196, 161–167. [Google Scholar] [CrossRef]
- Tan, R.; Dong, H.; Chen, Z.; Jin, M.; Yin, J.; Li, H.; Shi, D.; Shao, Y.; Wang, H.; Chen, T. Intestinal microbiota mediates high-fructose and high-fat diets to induce chronic intestinal inflammation. Front. Cell. Infect. Microbiol. 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Shin, D.-M.; Chang, T.-H.; Morse, H.C. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatakeyama, S. TRIM family proteins: Roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight junction proteins and signaling pathways in cancer and inflammation: A functional crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Brooks, R.S.; Ciappio, E.D.; Kim, S.J.; Crott, J.W.; Bennett, G.; Greenberg, A.S.; Mason, J.B. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: A mechanism for obesity-associated colorectal cancer. J. Nutr. Biochem. 2012, 23, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-C.; Lee, H.-Y.; Kim, T.K.; Kim, M.-S.; Park, Y.M.; Kim, J.; Park, K.; Kweon, M.-N.; Kim, S.-H.; Bae, J.-W. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS ONE 2017, 12, e0187515. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.S.; Park, C.Y.; Seo, Y.K.; Woo, S.H.; Kim, D.Y.; Han, S.N. Vitamin D supplementation partially affects colonic changes in dextran sulfate sodium–induced colitis obese mice but not lean mice. Nutr. Res. 2019, 67, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. A J. Virtual Libr. 2009, 14, 2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, K.; Kakigawa, N.; Sekine, S.; Shitara, Y.; Horie, T. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother. Pharmacol. 2013, 72, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Zhao, J.; Xie, X.; Chen, T.; Yin, Y.; Zhai, R.; Wang, X.; An, W.; Li, J. Anthocyanins from the fruits of Lycium ruthenicum Murray improve high-fat diet-induced insulin resistance by ameliorating inflammation and oxidative stress in mice. Food Funct. 2021, 12, 3855–3871. [Google Scholar]
- Chen, F.; Li, M.; Fei, X.; Zhu, W.; Zhang, Z.; Shen, Y.; Mao, Y.; Zhu, Q.; Xu, J.; Zhou, W. Placental DNA methylation changes in the development of gestational diabetes mellitus. Nat. Portf. 2022. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.-C. Western blot: Technique, theory, and trouble shooting. North Am. J. Med. Sci. 2012, 4, 429. [Google Scholar]



| Gene | Primers (5′-3′) |
|---|---|
| β-Actin | F:AGAGGGAAATCGTGCGTGAC |
| R:CAATAGTGATGACCTGGCCGT | |
| IL-6 | F:CTTCCATCCAGTTGCCTTCTTG |
| R:AATTAAGCCTCCGACTTGTGAAG | |
| TNF-α | F:ACGGCATGGATCTCAAAGAC |
| R:GTGGGTGAGGAGCACGTAG | |
| TRIM67 | F:GGCGAAGGAGTTTCTGGTTC |
| R:TAGCTTCAGGGTGCAGTGATT | |
| IL-4 | F:CTTCCAAGGTGCTTCGCATA |
| R:GATGAATCCAGGCATCGAAA | |
| IL-10 | F:AAGGGTTACTTGGGTTGCCA |
| R:CCTGGGGCATCACTTCTACC | |
| IL-1β | F:CCCCAGGGCATGTTAAGGAG |
| R:TCTTGGCCGAGGACTAAGGA | |
| IL-2 | F:CCTGAGCAGGATGGAGAATTACA |
| R:TCCAGAACATGCCGCAGAG | |
| SOD1 | F:AACCAGTTGTGTTGTCAGGAC |
| R:CCACCATGTTTCTTAGAGTGAGG | |
| SOD2 | F:TGGACAAACCTGAGCCCTAAG |
| R:CCCAAAGTCACGCTTGATAGC | |
| CAT | F:TGGCACACTTTGACAGAGAGC |
| R:CCTTTGCCTTGGAGTATCTGG | |
| Claudin-1 | F:TGGTAATTGGCATCCTGCTG |
| R:CAGCCATCCACATCTTCTGC | |
| Occludin | F:GTACCCACCAGTGACCAACA |
| R:GTTGCTGGAGCTTAGCCTGT | |
| ZO-1 | F:CGAGGCATCATCCCAAATAAGAAC |
| R:TCCAGAAGTCTGCCCGATCAC | |
| R:GACGCTTATGTTGTTGCTGATGGC |
| Gene | Primers (5′-3′) |
|---|---|
| GADPH | F:TCACCAGGGCTGCTTTTA |
| R:TTGCCGTGGGTGGAATCATA | |
| IL-6 | F:TGGGTTCAATCAGGAGACCT |
| R:CAGCCTCGACATTTCCCTTA | |
| TRIM67 | F:CACAAGGCCCAACTGTCTCA |
| R:ACCAGAGCATCACACTGAGC | |
| TNF-α | F:TCCTCACTCACACCATCAGC |
| R:GCCCAGATTCAGCAAAGTCC | |
| IL-1β | F:CCAAAGAGGGACATGGAGAA |
| R:GGGCTTTTGTTCTGCTTGAG | |
| IL-10 | F:CTGCCTCCCACTTTCTCTTG |
| R:TCAAAGGGGCTCCCTAGTTT |
| Name | Company | Country | NO. |
|---|---|---|---|
| Anti-IL-6 Antibody | huabio | China | EM170414 1:1000 |
| IL1β Rabbit pAb | ABclonal | China | A1112 1:1000 |
| Claudin 1 Rabbit Polyclonal Antibody | Beyotime | China | AF6504 1:1000 |
| Anti-Occludin antibody | abcam | Britain | ab216327 1:1000 |
| IL10 antibody | genetex | America | GTX632359 1:5000 |
| Rabbit Anti-IL-4 antibody | Bioss | China | bs-0581R 1:1000 |
| Rabbit Anti-TNF alpha antibody | Bioss | China | bs-2081R 1:1000 |
| TRIM67 Polyclonal Antibody | Proteintech | America | 24369-1-AP 1:1000 |
| β-Actin Rabbit mAb | ABclonal | China | AC026 1:100,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Jahangir, A.; He, J.; Huang, C.; Xia, Y.; Jia, L.; Wei, X.; Pan, T.; Du, Y.; Mu, B.; et al. Ameliorating Effects of TRIM67 against Intestinal Inflammation and Barrier Dysfunction Induced by High Fat Diet in Obese Mice. Int. J. Mol. Sci. 2022, 23, 7650. https://doi.org/10.3390/ijms23147650
Luo Q, Jahangir A, He J, Huang C, Xia Y, Jia L, Wei X, Pan T, Du Y, Mu B, et al. Ameliorating Effects of TRIM67 against Intestinal Inflammation and Barrier Dysfunction Induced by High Fat Diet in Obese Mice. International Journal of Molecular Sciences. 2022; 23(14):7650. https://doi.org/10.3390/ijms23147650
Chicago/Turabian StyleLuo, Qihui, Asad Jahangir, Junbo He, Chao Huang, Yu Xia, Lanlan Jia, Xiaoli Wei, Ting Pan, Yanni Du, Bin Mu, and et al. 2022. "Ameliorating Effects of TRIM67 against Intestinal Inflammation and Barrier Dysfunction Induced by High Fat Diet in Obese Mice" International Journal of Molecular Sciences 23, no. 14: 7650. https://doi.org/10.3390/ijms23147650
APA StyleLuo, Q., Jahangir, A., He, J., Huang, C., Xia, Y., Jia, L., Wei, X., Pan, T., Du, Y., Mu, B., Gong, H., Liu, W., Ur-Rehman, S., Pan, K., & Chen, Z. (2022). Ameliorating Effects of TRIM67 against Intestinal Inflammation and Barrier Dysfunction Induced by High Fat Diet in Obese Mice. International Journal of Molecular Sciences, 23(14), 7650. https://doi.org/10.3390/ijms23147650






