Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications
Abstract
:1. Introduction
2. Results
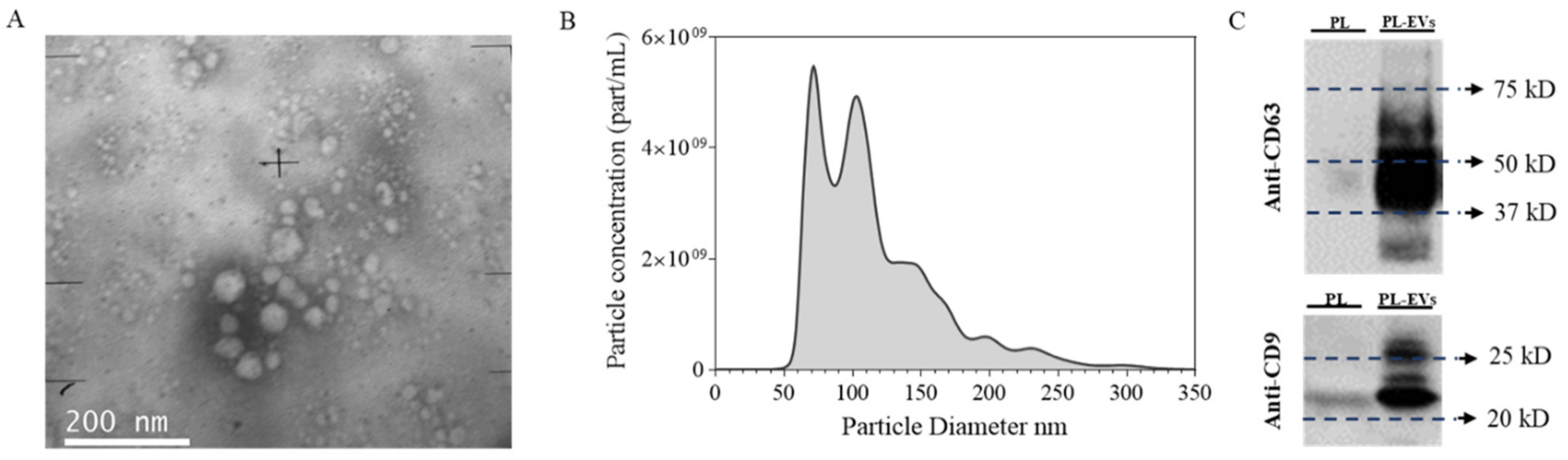
2.1. Platelet-Derived EV Characterization
2.2. PL and EVs’ Effect on ihGK and ihGF
2.3. HA Gel Characterization
2.4. In Vitro Effect of the Different HA Gels
3. Discussion
4. Materials and Methods
4.1. Human PL Preparation
4.2. EVs Isolation
4.3. Transmission Electron Microscopy (TEM)
4.4. Protein Quantification
4.5. Western Blot
4.6. Nanoparticle Tracking Analysis (NTA)
4.7. Hydrogel Preparations
4.8. Equilibrium Swelling Ratio Determination
4.9. EVs Release
4.10. Cell Culture
4.11. Wound Healing In Vitro Assay
4.12. Cell Cytotoxicity
4.13. Metabolic Activity
4.14. Gene Expression by Real-Time RT-PCR
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cells Mater. 2019, 38, 63–78. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Shin, M.K.; Lee, J.W.; Kim, Y., II; Kim, Y.O.; Seok, H.; Kim, N.I. The effects of platelet-rich clot releasate on the expression of MMP-1 and type I collagen in human adult dermal fibroblasts: PRP is a stronger MMP-1 stimulator. Mol. Biol. Rep. 2014, 41, 3–8. [Google Scholar] [CrossRef]
- Anitua, E.; Pino, A.; Orive, G. Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J. Wound Care 2016, 25, 680–687. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Merayo-Lloves, J.; de La Fuente, M.; Muruzabal, F.; Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates proliferation and migration of primary keratocytes and conjunctival fibroblasts and inhibits and reverts TGF-β1-induced myodifferentiation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6066–6073. [Google Scholar] [CrossRef]
- Cabral, J.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Extracellular vesicles as modulators of wound healing. Adv. Drug Deliv. Rev. 2018, 129, 394–406. [Google Scholar] [CrossRef]
- Lv, L.; Sheng, C.; Zhou, Y. Extracellular vesicles as a novel therapeutic tool for cell-free regenerative medicine in oral rehabilitation. J. Oral Rehabil. 2020, 47, 29–54. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Fais, S.; O’Driscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; Cordeiro da Silva, A.; Del Portillo, H.; El Andaloussi, S.; et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef]
- Penfornis, P.; Vallabhaneni, K.C.; Whitt, J.; Pochampally, R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int. J. Cancer 2016, 138, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglìo, S.R.; Baldini, N. Exosomes: Novel effectors of human platelet lysate activity. Eur. Cells Mater. 2014, 28, 137–151; discussion 151. [Google Scholar] [CrossRef]
- Brennan, M.; Layrolle, P.; Mooney, D.J. Biomaterials Functionalized with MSC Secreted Extracellular Vesicles and Soluble Factors for Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909125. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Müller, H.D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Babo, P.S.; Pires, R.L.; Santos, L.; Franco, A.; Rodrigues, F.; Leonor, I.; Reis, R.L.; Gomes, M.E. Platelet Lysate-Loaded Photocrosslinkable Hyaluronic Acid Hydrogels for Periodontal Endogenous Regenerative Technology. ACS Biomater. Sci. Eng. 2017, 3, 1359–1369. [Google Scholar] [CrossRef] [Green Version]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Lovisolo, F.; Carton, F.; Gino, S.; Migliario, M.; Renò, F. Platelet rich plasma-derived microvesicles increased in vitro wound healing. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9658–9664. [Google Scholar] [CrossRef]
- Häkkinen, L.; Uitto, V.J.; Larjava, H. Cell biology of gingival wound healing. Periodontol. 2000 2000, 24, 127–152. [Google Scholar] [CrossRef]
- Nikoloudaki, G.; Creber, K.; Hamilton, D.W. Wound healing and fibrosis: A contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 2020, 318, C1065–C1077. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Lagosz-Cwik, K.B.; Wielento, A.; Lipska, W.; Kantorowicz, M.; Darczuk, D.; Kaczmarzyk, T.; Gibbs, S.; Potempa, J.; Grabiec, A.M. hTERT-immortalized gingival fibroblasts respond to cytokines but fail to mimic primary cell responses to Porphyromonas gingivalis. Sci. Rep. 2021, 11, 10770. [Google Scholar] [CrossRef]
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells. Bone Jt. Res. 2020, 9, 667–674. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Anitua, E.; Prado, R.; Orive, G. Allogeneic Platelet-Rich Plasma: At the Dawn of an Off-the-Shelf Therapy? Trends Biotechnol. 2017, 35, 91–93. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Shu, X.Z.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Prestwich, G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C. Wound healing in the oral mucosa. In Oral Mucosa in Health and Disease: A Concise Handbook; Springer: Cham, Switzerland, 2018; pp. 77–90. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of quercitrin as a potential therapeutic agent for periodontal applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef]
- Melrose, J. Glycosaminoglycans in Wound Healing. Bone Tissue Regen. Insights 2016, 7, BTRI.S38670. [Google Scholar] [CrossRef] [Green Version]
- Satish, L.; Kathju, S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol. Res. Pract. 2010, 2010, 790234. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Anitua, E.; Prado, R.; Azkargorta, M.; Rodriguez-Suárez, E.; Iloro, I.; Casado-Vela, J.; Elortza, F.; Orive, G. High-throughput proteomic characterization of plasma rich in growth factors (PRGF-Endoret)-derived fibrin clot interactome. J. Tissue Eng. Regen. Med. 2015, 9, E1–E12. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Van der Pol, E.; Harrison, P. From platelet dust to gold dust: Physiological importance and detection of platelet microvesicles. Platelets 2017, 28, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Otahal, A.; Kuten-Pella, O.; Kramer, K.; Neubauer, M.; Lacza, Z.; Nehrer, S.; De Luna, A. Functional repertoire of EV-associated miRNA profiles after lipoprotein depletion via ultracentrifugation and size exclusion chromatography from autologous blood products. Sci. Rep. 2021, 11, 5823. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, L.; Pils, V.; Bobbili, M.R.; Lämmermann, I.; Perrotta, I.; Grillenberger, T.; Schwestka, J.; Weiß, K.; Pum, D.; Arcalis, E.; et al. Extracellular Vesicles in Human Skin: Cross-Talk from Senescent Fibroblasts to Keratinocytes by miRNAs. J. Investig. Dermatol. 2019, 139, 2425–2436.e5. [Google Scholar] [CrossRef] [Green Version]
- Rubert, M.; Alonso-Sande, M.; Monjo, M.; Ramis, J.M. Evaluation of Alginate and Hyaluronic Acid for Their Use in Bone Tissue Engineering. Biointerphases 2012, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Florit, M.; Xing, R.; Ramis, J.M.; Taxt-Lamolle, S.; Haugen, H.J.; Lyngstadaas, S.P.; Monjo, M. Human gingival fibroblasts function is stimulated on machined hydrided titanium zirconium dental implants. J. Dent. 2014, 42, 30–38. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Extracellular Matrix of Animals. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Ivanovski, S.; Haase, H.R.; Bartold, P.M. Isolation and characterization of fibroblasts derived from regenerating human periodontal defects. Arch. Oral Biol. 2001, 46, 679–688. [Google Scholar] [CrossRef]
- Bartold, P.M.; Narayanan, A.S. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol. 2000 2006, 40, 29–49. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Serezani, A.P.M.; Bozdogan, G.; Sehra, S.; Walsh, D.; Krishnamurthy, P.; Sierra Potchanant, E.A.; Nalepa, G.; Goenka, S.; Turner, M.J.; Spandau, D.F.; et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J. Allergy Clin. Immunol. 2017, 139, 142–151.e5. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Erikss, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [Green Version]





| Gen | Primer Sequence (5′−3′) | Product Size (bp) | Function | Ref. |
|---|---|---|---|---|
| Collagen I α1 (COL1A1) NM_000088.3 | Sense: CCTGACGCACGGCCAAGAGG Antisense: GGCAGGGCTCGGGTTTCCAC | 122 | COL1A1 is one of the main components of the extracellular matrix. | [44] |
| Decorin (DCN)> NM_001920.3 | Sense: ATCTCAGCTTTGAGGGCTCC Antisense: GCCTCTCTGTTGAAACGGTC | 146 | DCN is a component of the extracellular matrix. However, DCN is also related to quiescence and growth inhibition [45]. | [44,45] |
| Matrix metalloproteinase-1 (MMP-1) NM_002421.3 | Sense: TGTCAGGGGAGATCATCGGGAC Antisense: TGGCCGAGTTATGAGCTGCA | 177 | MMP1 is an enzyme that degrades collagen proteins. | [46] |
| Tissue inhibitor of metalloproteinases 1 (TIMP-1) NM_003254.2 | Sense: TTCCGACCTCGTCATCAGGG Antisense: TAGACGAACCGGATGTCAGC | 144 | TIMP-1 inhibits MMP-1 and it is involved in extracellular matrix remodeling. | [47] |
| α-Smooth muscle actin 2 (ACTA2) NM_001141945.1 | Sense: TAAGACGGGAATCCTGTGAAGC Antisense: TGTCCCATTCCCACCATCAC | 184 | It promotes collagen production, but it may induce myofibroblast differentiation and scar formation. | [34] |
| Transforming growth factor-β1 (TGF-B1) NM_000660.4 | Sense: TGTCACCGGAGTTGTGCGGC Antisense: GGCCGGTAGTGAACCCGTTG | 131 | It promotes COL1A1 production and enhances cell proliferation. | [34] |
| Endothelin-1 (EDN) NM_001955.4 | Sense: ACGGCGGGGAGAAACCCACT Antisense: ACGGAACAACGTGCTCGGGA | 147 | It promotes COL1A1 production and enhances cell proliferation. | [34] |
| Fibronectin (FN1) NM_001365522.2 | Sense: CGGAGAGACAGGAGGAAATAGCCCT Antisense: TTGCTGCTTGCGGGGCTGTC | 150 | Fibronectin is a component of the extracellular matrix that induces wound healing and cell adhesion, migration, and differentiation. | [48] |
| Vimentin (VIM) NM_003380.5 | Sense: GGCCGCCTGCAGGATGAGATTC Antisense: CAGAGAAATCCTGCTCTCCTCGC | 153 | VIM is associated with the cytoskeleton reorganization and induces cell proliferation and migration during the regeneration process in keratinocytes. | [49] |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) NM_002046.3 | Sense: TGCACCACCAACTGCTTAGC Antisense: AAGGGACTTCCTGTAACAA | 87 | Housekeeping gene. | |
| Beta-actin (ACTBL2) NM_001101.3 | Sense: CTGGAACGGTGAAGGTGACA Antisense: AAGGGACTTCCTGTAACAA | 140 | Housekeeping gene. | |
| 18S ribosomal RNA (18S rRNA) NR_146156.1 | Sense: GTAACCCGTTGAACCCCATT Antisense: CCATCCAATCGGTAGTAGCG | 151 | Housekeeping gene. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antich-Rosselló, M.; Munar-Bestard, M.; Forteza-Genestra, M.A.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications. Int. J. Mol. Sci. 2022, 23, 7668. https://doi.org/10.3390/ijms23147668
Antich-Rosselló M, Munar-Bestard M, Forteza-Genestra MA, Calvo J, Gayà A, Monjo M, Ramis JM. Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications. International Journal of Molecular Sciences. 2022; 23(14):7668. https://doi.org/10.3390/ijms23147668
Chicago/Turabian StyleAntich-Rosselló, Miquel, Marta Munar-Bestard, Maria Antònia Forteza-Genestra, Javier Calvo, Antoni Gayà, Marta Monjo, and Joana M. Ramis. 2022. "Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications" International Journal of Molecular Sciences 23, no. 14: 7668. https://doi.org/10.3390/ijms23147668
APA StyleAntich-Rosselló, M., Munar-Bestard, M., Forteza-Genestra, M. A., Calvo, J., Gayà, A., Monjo, M., & Ramis, J. M. (2022). Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications. International Journal of Molecular Sciences, 23(14), 7668. https://doi.org/10.3390/ijms23147668








