Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
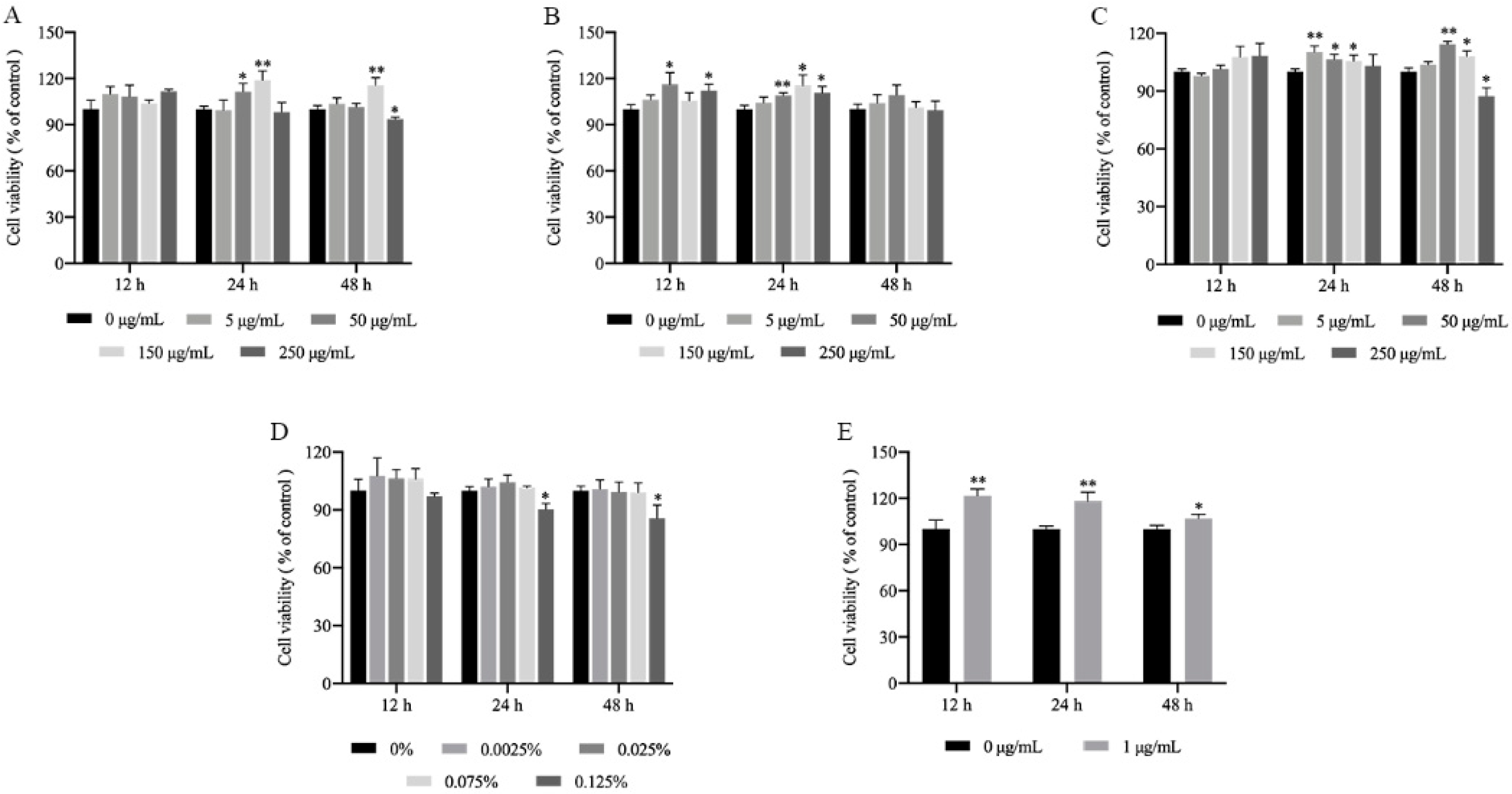
2.1. Cytotoxicity of the MLFs in RAW264.7 Cells
2.2. MLFs Decreased Nitric Oxide (NO) and Prostaglandin E2 (PGE2) Production in LPS-Induced RAW 264.7 Cells
2.3. MLFs Inhibited Inflammatory Cytokine Secretion in LPS-Induced RAW 264.7 Cells
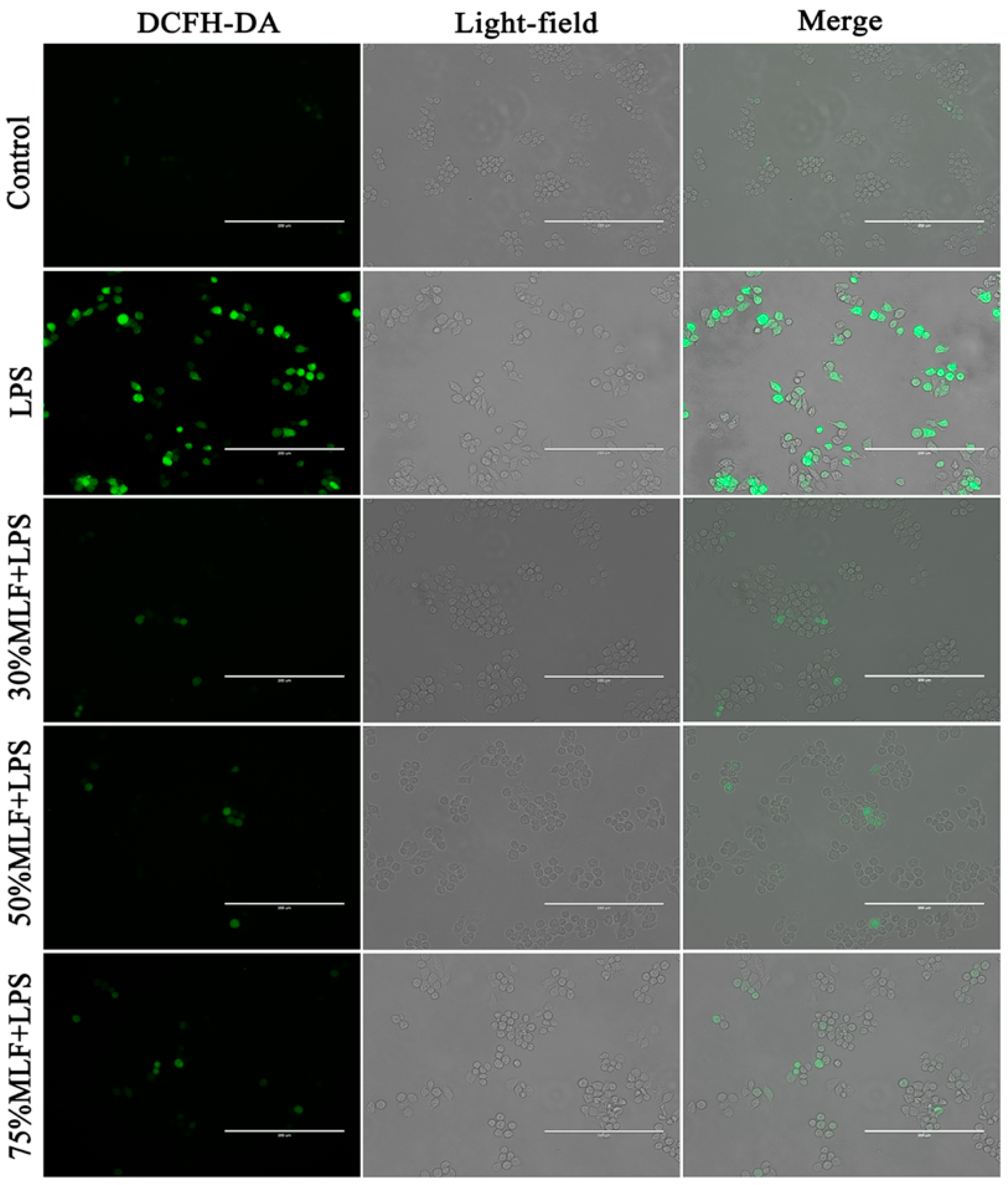
2.4. MLFs Inhibited ROS Production in LPS-Induced RAW 264.7 Cells
2.5. Antioxidant Activities of the MLFs
2.6. TFC of the MLFs
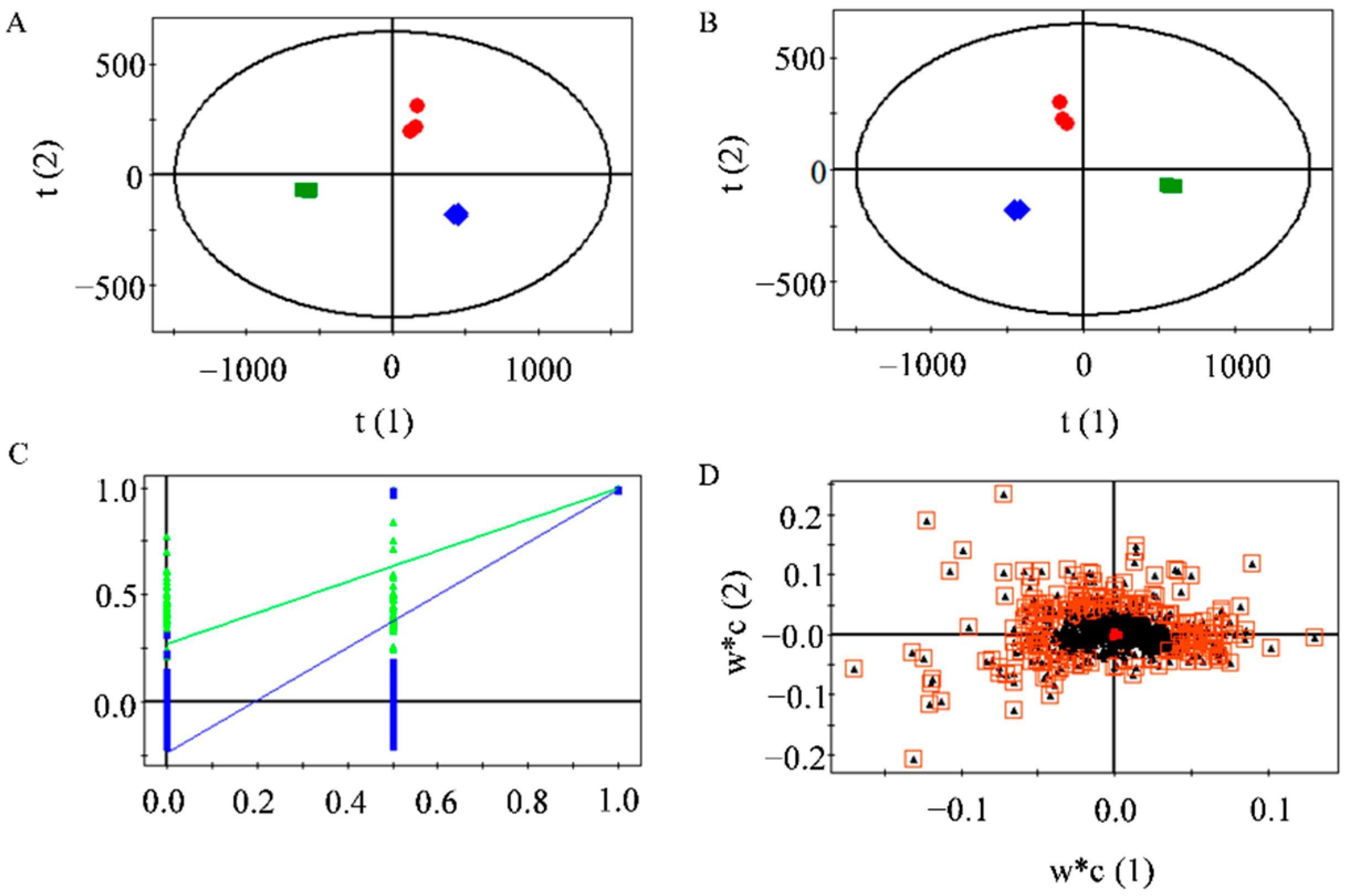
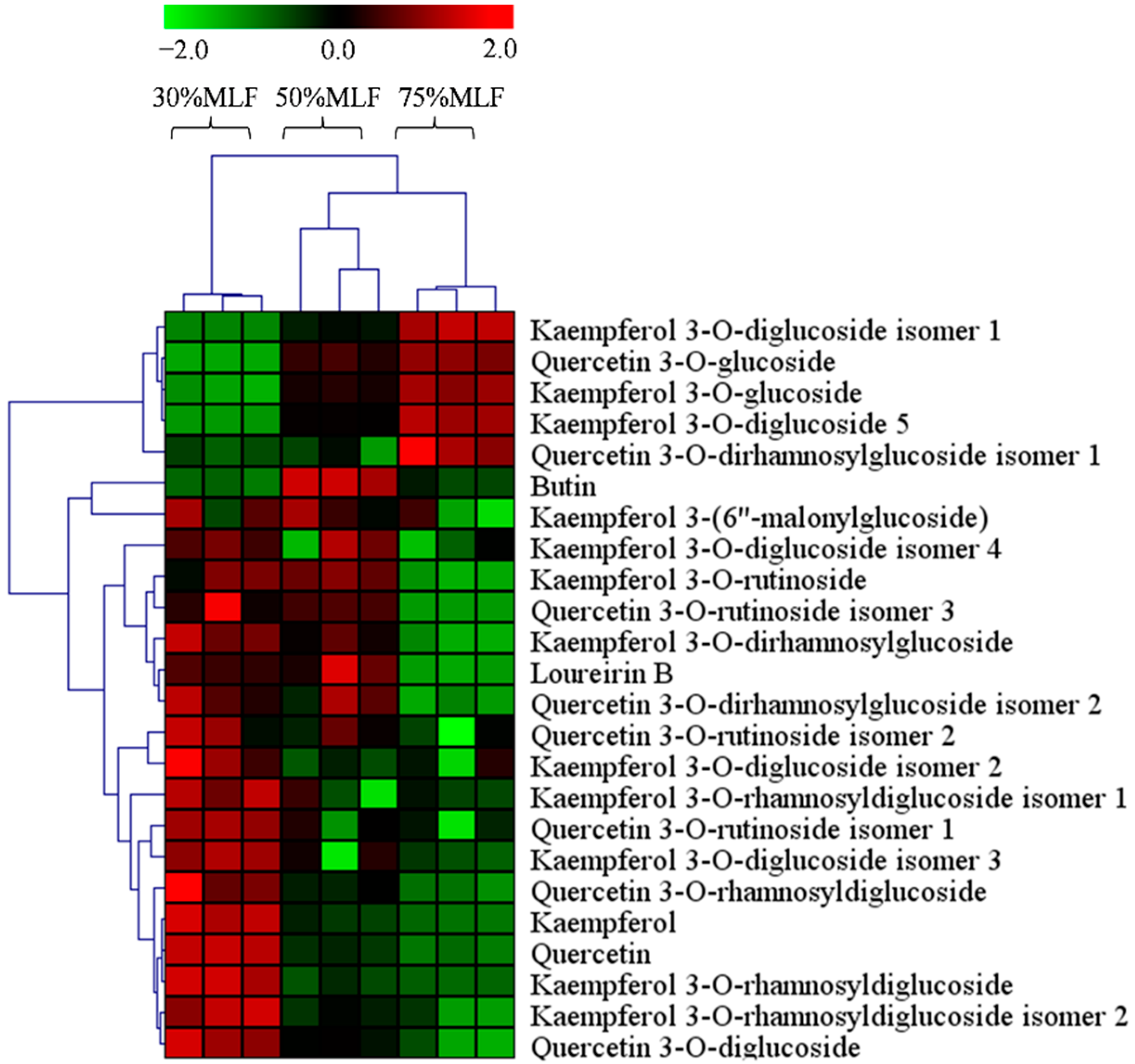
2.7. Differential Flavonoids between the MLFs
2.8. Treatment with 30%MLF Alleviated the Symptoms of DSS-Induced UC in Mice
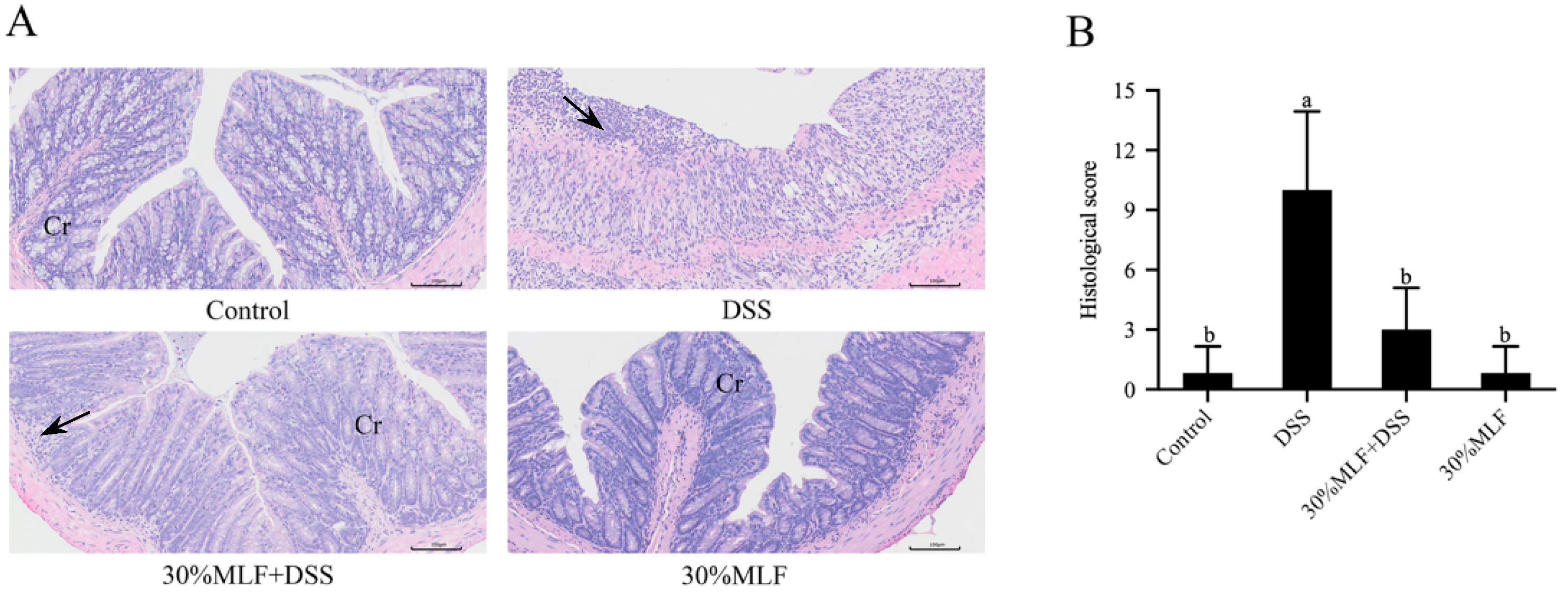
2.9. Treatment with 30%MLF Relieved the Morphological Damage Caused by DSS-Induced UC in Mice
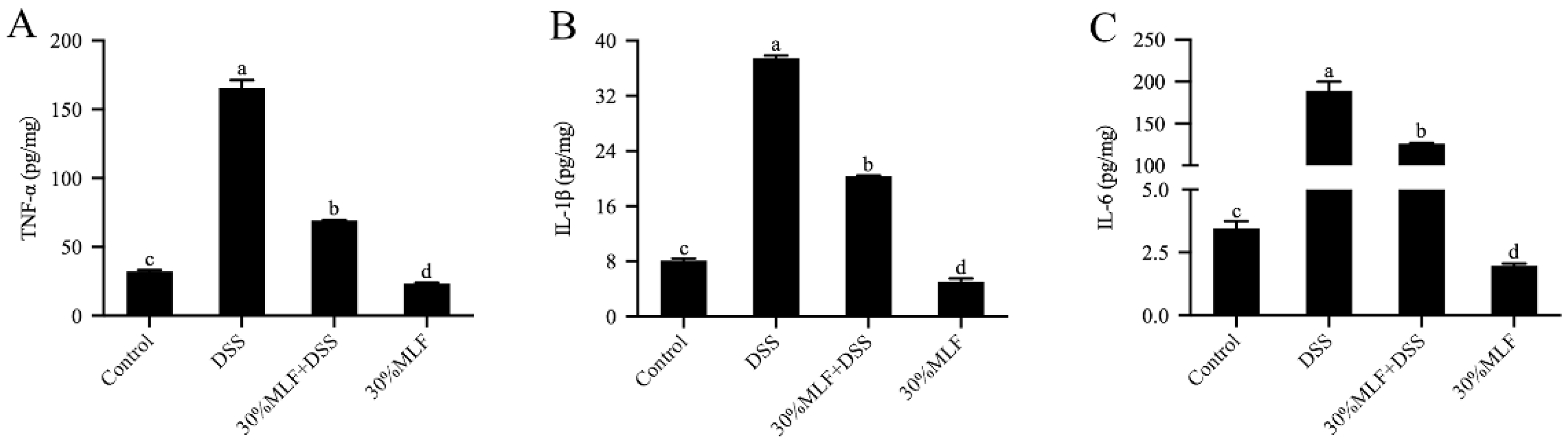
2.10. Treatment with 30%MLF Reduced Inflammatory Cytokine Secretion of DSS-Induced UC in Mice
2.11. Treatment with 30%MLF Inhibited the Toll-like Receptor 4 (TLR4)/Myeloid Differentiation Factor 88 (MyD88) Pathway Activation of DSS-Induced UC in Mice
3. Materials and Methods
3.1. MLF Extraction
3.2. Cell Cultures
3.3. Cell Viability Assay
3.4. NO Production Assay
3.5. ROS Level Detection
3.6. ELISA Test
3.7. RNA Extraction and RT-qPCR
3.8. Chemical Assays of Antioxidant Activity
3.8.1. DPPH Radical Scavenging Activity
3.8.2. Metal Ion Chelating Activity Assay
3.8.3. Assessment of Reducing Power
3.9. TFC Measurement
3.10. MLF Sample Preparation and Liquid Chromatography-Mass Spectrometry (LC-MS) Conditions
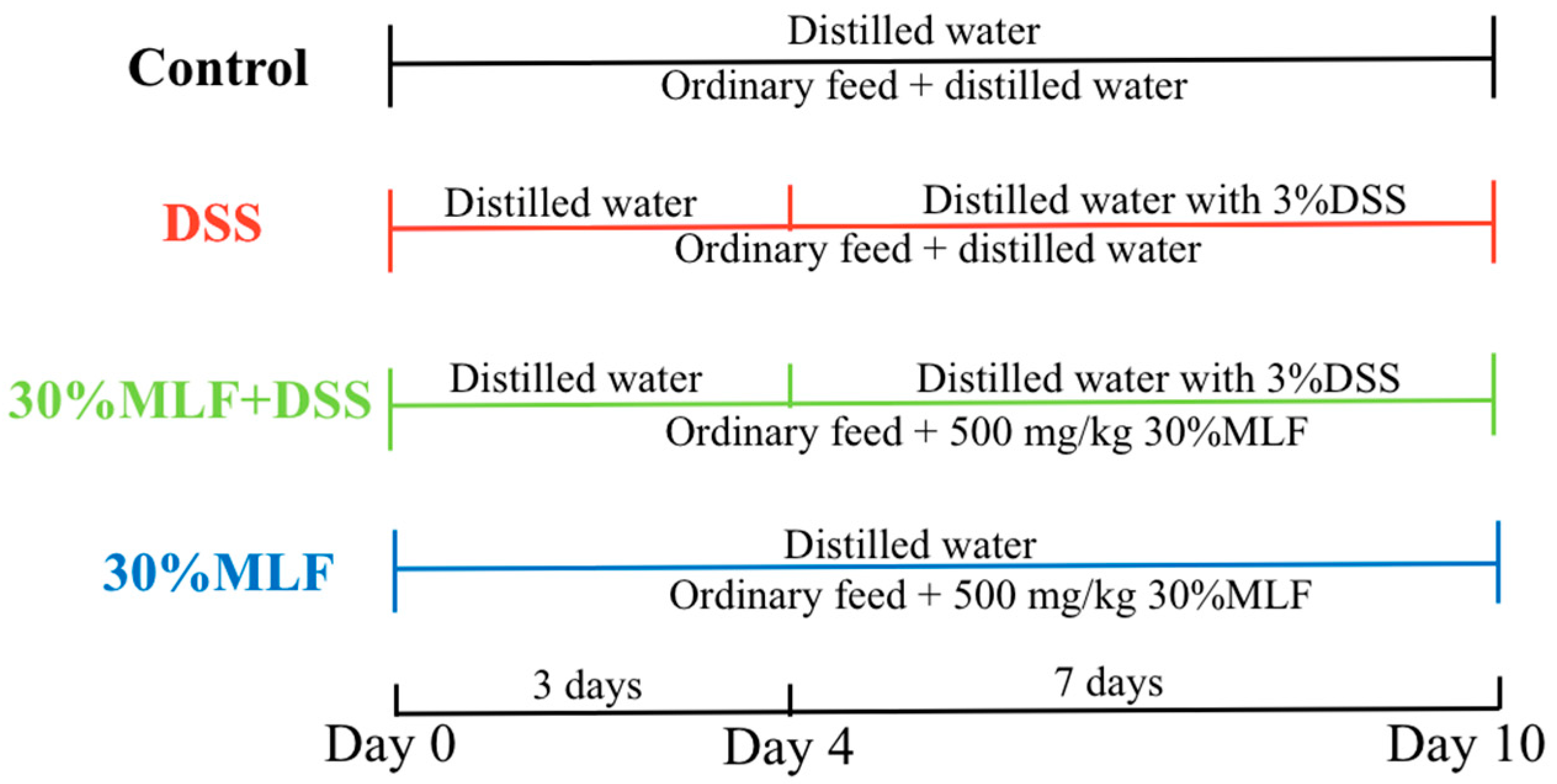
3.11. Induction of Colitis and Treatment
3.12. Assessment of DAI
3.13. Histological Analysis
3.14. Immunohistochemical Staining
3.15. Data Treatment and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of Inflammation: Mechanisms and Opportunity for Drug Development. Pharmacol. Ther. 2013, 139, 189–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Wang, W.; Zhang, R. Novel Natural Product Therapeutics Targeting Both Inflammation and Cancer. Chin. J. Nat. Med. 2017, 15, 401–416. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-Inflammatory Effect of Strawberry Extract against LPS-Induced Stress in RAW 264.7 Macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ďuračková, Z. Some Current Insights into Oxidative Stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Park, E.; Lee, S.-M.; Lee, J.e.; Kim, J.-H. Anti-Inflammatory Activity of Mulberry Leaf Extract through Inhibition of NF-κB. J. Funct. Foods 2013, 5, 178–186. [Google Scholar] [CrossRef]
- Warford, J.; Jones, Q.R.D.; Nichols, M.; Sullivan, V.; Rupasinghe, H.P.V.; Robertson, G.S. The Flavonoid-Enriched Fraction AF4 Suppresses Neuroinflammation and Promotes Restorative Gene Expression in a Mouse Model of Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2014, 268, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Sun, J.; Yan, J.; Li, C.; Lv, X. Anti-Inflammatory Effects of Isorhamnetin on LPS-Stimulated Human Gingival Fibroblasts by Activating Nrf2 Signaling Pathway. Microb. Pathog. 2018, 120, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical Ethanol Extraction of Flavonoids from Moringa Oleifera Leaf and Evaluation of Antioxidant Activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A Review of Bioactive Compounds and Advanced Processing Technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In Vitro Biological Effects of Phenolic Compounds from Morus Alba Root Bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, Bioactivities and Future Prospects of Mulberry Leaves: A Review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New Potential Phytotherapeutics Obtained from White Mulberry (Morus Alba L.) Leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant Flavonol Glycosides in Mulberry (Morus Alba L.) Leaves Isolated Based on LDL Antioxidant Activity. Food Chem. 2006, 97, 25–31. [Google Scholar] [CrossRef]
- Kim, G.-N.; Jang, H.-D. Flavonol Content in the Water Extract of the Mulberry (Morus Alba L.) Leaf and Their Antioxidant Capacities. J. Food Sci. 2011, 76, C869–C873. [Google Scholar] [CrossRef]
- Park, S.-K.; Wong, Z.; Park, S.H.; Vu, K.V.; Bang, K.B.; Piyachaturawat, P.; Myint, T.; Hilmi, I.; Park, D.-I. Extraintestinal Manifestation of Inflammatory Bowel Disease in Asian Patients: A Multinational Study. Dig. Liver Dis. 2021, 53, 196–201. [Google Scholar] [CrossRef]
- Chang, K.-W.; Kuo, C.-Y. 6-Gingerol Modulates Proinflammatory Responses in Dextran Sodium Sulfate (DSS)-Treated Caco-2 Cells and Experimental Colitis in Mice through Adenosine Monophosphate-Activated Protein Kinase (AMPK) Activation. Food Funct. 2015, 6, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, W.; Fang, X.; Chen, H.; Han, Y.; Liu, R.; Niu, B.; Gao, H. Structural Characterization of a Polysaccharide from Bamboo (Phyllostachys Edulis) Shoot and Its Prevention Effect on Colitis Mouse. Food Chem. 2022, 387, 132807. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; El Allam, A.; Aboulaghras, S.; Jaouadi, I.; Bakrim, S.; El Omari, N.; Shariati, M.A.; Miftakhutdinov, A.; Wilairatana, P.; Mubarak, M.S.; et al. Inflammatory Auto-Immune Diseases of the Intestine and Their Management by Natural Bioactive Compounds. Biomed. Pharmacother. 2022, 151, 113158. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Wang, G.-Y.; Lin, J.-H.; Yen, G.-C. Antioxidant and Anti-Inflammatory Activities and Bioactive Compounds of the Leaves of Trichodesma Khasianum Clarke. Ind. Crops Prod. 2020, 151, 112447. [Google Scholar] [CrossRef]
- Ma, Y.; He, Y.; Yin, T.; Chen, H.; Gao, S.; Hu, M. Metabolism of Phenolic Compounds in LPS-Stimulated Raw264.7 Cells Can Impact Their Anti-Inflammatory Efficacy: Indication of Hesperetin. J. Agric. Food Chem. 2018, 66, 6042–6052. [Google Scholar] [CrossRef]
- Kim, N.Y.; Cheong, S.H.; Lee, K.J.; Sok, D.-E.; Kim, M.R. Anti-Inflammatory Effects of Ribes Diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model. Antioxidants 2020, 9, 622. [Google Scholar] [CrossRef]
- Yu, T.; Lee, Y.J.; Yang, H.M.; Han, S.; Kim, J.H.; Lee, Y.; Kim, C.; Han, M.H.; Kim, M.-Y.; Lee, J.; et al. Inhibitory Effect of Sanguisorba Officinalis Ethanol Extract on NO and PGE2 Production Is Mediated by Suppression of NF-κB and AP-1 Activation Signaling Cascade. J. Ethnopharmacol. 2011, 134, 11–17. [Google Scholar] [CrossRef]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-Inflammatory Properties of the Marine Plant Posidonia Oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.; Jo, M.; Seo, Y.; Park, N.; Kim, G.-D. Anti-Inflammatory Activity of β-Thymosin Peptide Derived from Pacific Oyster (Crassostrea Gigas) on NO and PGE2 Production by Down-Regulating NF-κB in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M. Nitric Oxide: From Good to Bad. Ann. Vasc. Dis. 2018, 11, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Hibbs, J.B.; Taintor, R.R.; Vavrin, Z.; Rachlin, E.M. Nitric Oxide: A Cytotoxic Activated Macrophage Effector Molecule. Biochem. Biophys. Res. Commun. 1988, 157, 87–94. [Google Scholar] [CrossRef]
- Martín, M.C.; Martinez, A.; Mendoza, J.L.; Taxonera, C.; Díaz-Rubio, M.; Fernández-Arquero, M.; de la Concha, E.G.; Urcelay, E. Influence of the Inducible Nitric Oxide Synthase Gene (NOS2A) on Inflammatory Bowel Disease Susceptibility. Immunogenetics 2007, 59, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Rao, C.V. INOS-Selective Inhibitors for Cancer Prevention: Promise and Progress. Future Med. Chem. 2012, 4, 2193–2204. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.S.; Mann, M.; DuBois, R.N. The Role of Cyclooxygenases in Inflammation, Cancer, and Development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam Honey Inhibits the Production of Proinflammatory, Mediators NO, PGE 2, TNF-α, and IL-6 in Carrageenan-Induced Acute Paw Edema in Rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The Inhibitory Effects of Gelam Honey and Its Extracts on Nitric Oxide and Prostaglandin E2 in Inflammatory Tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar] [CrossRef]
- Ren, J.; Su, D.; Li, L.; Cai, H.; Zhang, M.; Zhai, J.; Li, M.; Wu, X.; Hu, K. Anti-Inflammatory Effects of Aureusidin in LPS-Stimulated RAW264.7 Macrophages via Suppressing NF-κB and Activating ROS- and MAPKs-Dependent Nrf2/HO-1 Signaling Pathways. Toxicol. Appl. Pharmacol. 2020, 387, 114846. [Google Scholar] [CrossRef]
- Tracey, K.J.; Beutler, B.; Lowry, S.F.; Merryweather, J.; Wolpe, S.; Milsark, I.W.; Hariri, R.J.; Fahey, T.J.; Zentella, A.; Albert, J.D.; et al. Shock and Tissue Injury Induced by Recombinant Human Cachectin. Science 1986, 234, 470–474. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.-Y.; He, G.; Ali, S.R.; Holzer, R.G.; Österreicher, C.H.; Takahashi, H.; Karin, M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Takashiba, S.; Naruishi, K.; Murayama, Y. Perspective of Cytokine Regulation for Periodontal Treatment: Fibroblast Biology. J. Periodontol. 2003, 74, 103–110. [Google Scholar] [CrossRef]
- Silva, B.; Biluca, F.C.; Mohr, E.T.B.; Caon, T.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Costa, A.C.O. Effect of Mimosa Scabrella Bentham Honeydew Honey on Inflammatory Mediators. J. Funct. Foods 2020, 72, 104034. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Haque, M.A.; Bukhari, S.N.A. Immunomodulatory Effects of Tinospora Crispa Extract and Its Major Compounds on the Immune Functions of RAW 264.7 Macrophages. Int. Immunopharmacol. 2018, 60, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Romero-Castillo, L.; El Brahmi, N.; Manzanares, D.; Mignani, S.; Majoral, J.-P.; Ceña, V. Neutral High-Generation Phosphorus Dendrimers Inhibit Macrophage-Mediated Inflammatory Response In Vitro and In Vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E7660–E7669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Guo, Q.; Liang, Z.; Wang, M.; Wang, B.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Wang, J.; Ma, C.; Kang, W. Anti-Inflammatory and Antioxidant Effects of Chaetoglobosin Vb in LPS-Induced RAW264.7 Cells: Achieved via the MAPK and NF-κB Signaling Pathways. Food Chem. Toxicol. 2021, 147, 111915. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative Stress: A Major Pathogenesis and Potential Therapeutic Target of Antioxidative Agents in Parkinson’s Disease and Alzheimer’s Disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, 10. [Google Scholar] [CrossRef] [Green Version]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The Role of Oxidative Stress and Inflammation in Cardiovascular Aging. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Pirvulescu, M.M.; Gan, A.-M.; Stan, D.; Simion, V.; Calin, M.; Butoi, E.; Tirgoviste, C.I.; Manduteanu, I. Curcumin and a Morus Alba Extract Reduce Pro-Inflammatory Effects of Resistin in Human Endothelial Cells. Phytother. Res. 2011, 25, 1737–1742. [Google Scholar] [CrossRef]
- Geesin, J.C.; Gordon, J.S.; Berg, R.A. Retinoids Affect Collagen Synthesis through Inhibition of Ascorbate-Induced Lipid Peroxidation in Cultured Human Dermal Fibroblasts. Arch. Biochem. Biophys. 1990, 278, 350–355. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Larson, R.A. The Antioxidants of Higher Plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wei, H.; Zhang, Y.; Guo, Z.; Qiu, Y.; Wen, L.; Xie, Z. Structural Characterization of Lanzhou Lily (Lilium Davidii Var. Unicolor) Polysaccharides and Determination of Their Associated Antioxidant Activity. J. Sci. Food Agric. 2020, 100, 5603–5616. [Google Scholar] [CrossRef]
- Duh, P.-D. Antioxidant Activity of Burdock (Arctium Lappa Linné): Its Scavenging Effect on Free-Radical and Active Oxygen. J. Am. Oil Chem. Soc. 1998, 75, 455–461. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant Activity of Water Extract of Harng Jyur (Chrysanthemum Morifolium Ramat). LWT—Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.-P.; Liu, B.-Y. Antidepressant-like Effects and Mechanisms of Flavonoids and Related Analogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Iwashina, T. The Structure and Distribution of the Flavonoids in Plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Salcedo, E.M.; Tassotti, M.; Del Rio, D.; Hernández, F.; Martínez, J.J.; Mena, P. (Poly)Phenolic Fingerprint and Chemometric Analysis of White (Morus Alba L.) and Black (Morus Nigra L.) Mulberry Leaves by Using a Non-Targeted UHPLC–MS Approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Takahashi, M.; Katsube, T.; Koyama, A.; Itamura, H. Effects of Applied Nitrogen Amounts on the Functional Components of Mulberry (Morus Alba L.) Leaves. J. Agric. Food Chem. 2016, 64, 6923–6929. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of Extraction Method to Obtain a Phenolic Compounds-Rich Extract from Moringa Oleifera Lam Leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural Products and Anti-Inflammatory Activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus Alba L. Nature’s Functional Tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Shiwaku, K.; Katsube, T.; Kitajima, K.; Anuurad, E.; Yamasaki, M.; Yamane, Y. Mulberry (Morus Alba L.) Leaves and Their Major Flavonol Quercetin 3-(6-Malonylglucoside) Attenuate Atherosclerotic Lesion Development in LDL Receptor-Deficient Mice. J. Nutr. 2005, 135, 729–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moiianen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-κB Activation along with Their Inhibitory Effect on INOS Expression and NO Production in Activated Macrophages. Mediators Inflamm. 2007, 2007, 11. [Google Scholar]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [Green Version]
- Abo-Salem, O.M. Kaempferol Attenuates the Development of Diabetic Neuropathic Pain in Mice: Possible Anti-Inflammatory and Anti-Oxidant Mechanisms. Open Access Maced. J. Med. Sci. 2014, 2, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Araujo, C.M.; de Lúcio, K.P.; Silva, M.E.; Isoldi, M.C.; de Souza, G.H.B.; Brandão, G.C.; Schulz, R.; Costa, D.C. Morus Nigra Leaf Extract Improves Glycemic Response and Redox Profile in the Liver of Diabetic Rats. Food Funct. 2015, 6, 3490–3499. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Gao, J.L.; Lee, W.-C.; Ryu, K.S.; Lee, K.R.; Kim, Y.C. Antioxidative Flavonoids from the Leaves of Morus Alba. Arch. Pharm. Res. 1999, 22, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Shen, W.; Wang, Y.; Cao, Y.; Nuerbulati, N.; Chen, W.; Lu, G.; Xiao, W.; Qi, R. Grape Seed Polyphenols Ameliorated Dextran Sulfate Sodium-Induced Colitis via Suppression of Inflammation and Apoptosis. Pharmacology 2020, 105, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape Seed Proanthocyanidin Extract Ameliorates Dextran Sulfate Sodium-Induced Colitis through Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokines and Gut Microbiota Modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Xiong, W.; Li, J.; An, Y.; Ren, J.; Xie, Y.; Xue, H.; Yan, D.; Li, M.; et al. Saikosaponin-d Ameliorates Dextran Sulfate Sodium-Induced Colitis by Suppressing NF-κB Activation and Modulating the Gut Microbiota in Mice. Int. Immunopharmacol. 2020, 81, 106288. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Wu, N.; Yao, Y.; Xu, M.; Du, H.; Tu, Y. The Anti-Inflammatory Activity of Peptides from Simulated Gastrointestinal Digestion of Preserved Egg White in DSS-Induced Mouse Colitis. Food Funct. 2018, 9, 6444–6454. [Google Scholar] [CrossRef]
- Dodda, D.; Chhajed, R.; Mishra, J. Protective Effect of Quercetin against Acetic Acid Induced Inflammatory Bowel Disease (IBD) like Symptoms in Rats: Possible Morphological and Biochemical Alterations. Pharmacol. Rep. 2014, 66, 169–173. [Google Scholar] [CrossRef]
- Hong, Z.; Piao, M. Effect of Quercetin Monoglycosides on Oxidative Stress and Gut Microbiota Diversity in Mice with Dextran Sodium Sulphate-Induced Colitis. BioMed Res. Int. 2018, 2018, 8343052. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and Colitis-Associated Cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, C.; Abdullah; Tian, W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S. Protective Effect of Naringenin on Acetic Acid-Induced Ulcerative Colitis in Rats. World J. Gastroenterol. 2013, 19, 5633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Wang, L.; Yang, D.; Bu, W.; Gou, L.; Huang, J.; Duan, X.; Pan, Y.; Cao, S.; et al. Troxerutin Improves Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2021, 69, 2729–2744. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter Rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zhang, S.; Wang, J.; Yang, X.; Wang, R.; Yan, T.; Wu, B.; Du, Y.; Jia, Y. Wei-Tong-Xin Ameliorates Functional Dyspepsia via Inactivating TLR4/MyD88 by Regulating Gut Microbial Structure and Metabolites. Phytomedicine 2022, 102, 154180. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients Mediate Intestinal Bacteria–Mucosal Immune Crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Luo, Y.; Zhang, J.; Wang, X.; Sun, K.; Zeng, L. Prebiotic Properties of Green and Dark Tea Contribute to Protective Effects in Chemical-Induced Colitis in Mice: A Fecal Microbiota Transplantation Study. J. Agric. Food Chem. 2020, 68, 6368–6380. [Google Scholar] [CrossRef]
- Koliaraki, V.; Chalkidi, N.; Henriques, A.; Tzaferis, C.; Polykratis, A.; Waisman, A.; Muller, W.; Hackam, D.J.; Pasparakis, M.; Kollias, G. Innate Sensing through Mesenchymal TLR4/MyD88 Signals Promotes Spontaneous Intestinal Tumorigenesis. Cell Rep. 2019, 26, 536–545.e4. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ma, B.; Shang, Y.; Yin, Q.; Hong, Y.; Xu, S.; Shen, C.; Hou, X.; Liu, X. Flavonoid-Rich Ethanol Extract from the Leaves of Diospyros Kaki Attenuates Cognitive Deficits, Amyloid-Beta Production, Oxidative Stress, and Neuroinflammation in APP/PS1 Transgenic Mice. Brain Res. 2018, 1678, 85–93. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Yu, L.; Zhang, S.-D.; Ping, K.; Ni, H.-Y.; Qin, X.-Y.; Zhao, C.-J.; Wang, W.; Efferth, T.; Fu, Y.-J. Cryptochlorogenic Acid Attenuates LPS-Induced Inflammatory Response and Oxidative Stress via Upregulation of the Nrf2/HO-1 Signaling Pathway in RAW 264.7 Macrophages. Int. Immunopharmacol. 2020, 83, 106436. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Citrus Lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of Antioxidant and Antiproliferative Acidic Polysaccharides from Chinese Wolfberry Fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, J.; Cao, F.; Chen, F. Anti-Inflammatory Properties of Extracts from Chimonanthus Nitens Oliv. Leaf. PLoS ONE 2017, 12, e0181094. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium Pseudocatenulatum Ameliorates DSS-Induced Colitis by Maintaining Intestinal Mechanical Barrier, Blocking Proinflammatory Cytokines, Inhibiting TLR4/NF-κB Signaling, and Altering Gut Microbiota. J. Agric. Food Chem. 2021, 69, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Fukuda, T.; Zhang, H.; Sakurai, T.; Taniguchi, Y.; Watanabe, H.; Mitsuzumi, H.; Matsui, T.; Mine, Y. Intervention of Isomaltodextrin Mitigates Intestinal Inflammation in a Dextran Sodium Sulfate-Induced Mouse Model of Colitis via Inhibition of Toll-like Receptor-4. J. Agric. Food Chem. 2017, 65, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The Preventive Effect and Underlying Mechanism of Rhus Chinensis Mill. Fruits on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef]




| MLF | IC501 (μg/mL) | Reducing Power (%) | |
|---|---|---|---|
| DPPH Scavenge | Chelating Activity | ||
| 30%MLF | 12.57 ± 0.90 b | 310.56 ± 9.72 c | 41.69 ± 2.41 b |
| 50%MLF | 12.35 ± 0.55 b | 389.83 ± 14.14 b | 38.95 ± 0.45 b |
| 75%MLF | 19.53 ± 1.16 a | 482.19 ± 34.74 a | 31.11 ± 0.39 c |
| Vitamin C | 6.01 ± 0.50 c | 100.00 ± 0.06 a | |
| EDTA | 1.72 ± 0.27 d | ||
| MLF | TFC (%) |
|---|---|
| 30%MLF | 72.89 ± 0.18 a |
| 50%MLF | 61.84 ± 0.04 b |
| 75%MLF | 55.12 ± 0.50 c |
| Compound Name | Retention Time/min | Detected Mass (ESI+) | Ion Types | MS/MS Fragments |
|---|---|---|---|---|
| Butin | 21.76 | 273.0755 | M + H | 137, 81 |
| Loureirin B | 20.88 | 317.1382 | M + H | 299, 167, 149, 121 |
| Kaempferol a | 22.32 | 287.0548 | M + H | 287, 153, 135, 107 |
| Kaempferol 3-(6’’-malonylglucoside) | 22.88 | 535.1085 | M + H | 287 |
| Kaempferol 3-O-diglucoside isomer 1 | 10.27 | 611.1606 | M + H | 449, 287 |
| Kaempferol 3-O-diglucoside isomer 2 | 10.84 | 611.1616 | M + H | 449, 287 |
| Kaempferol 3-O-diglucoside isomer 3 | 8.68 | 611.1622 | M + H | 449, 287 |
| Kaempferol 3-O-diglucoside isomer 4 | 13.14 | 611.1626 | M + H | 449, 287 |
| Kaempferol 3-O-diglucoside 5 | 15.42 | 633.1445 | M + Na | 633, 347 |
| Kaempferol 3-O-dirhamnosylglucoside | 17.66 | 741.2248 | M + H | 595, 449, 287 |
| Kaempferol 3-O-rhamnosyldiglucoside | 12.3 | 795.1751 | M + H | 644 |
| Kaempferol 3-O-rhamnosyldiglucoside isomer 1 | 8.644 | 757.2204 | M + H | 611, 449, 287 |
| Kaempferol 3-O-rhamnosyldiglucoside isomer 2 | 10.58 | 757.2208 | M + H | 611, 449, 287 |
| Kaempferol 3-O-rutinoside 1 | 21.82 | 595.1658 | M + H | 449, 287 |
| Kaempferol-3-O-glucoside a | 17.47 | 449.1077 | M + H | 287 |
| Quercetin a | 14.65 | 303.0496 | M + H | 257, 229, 165, 153, 137 |
| Quercetin 3-O-dirhamnosylglucoside isomer 1 | 12.28 | 757.219 | M + H | 611, 465, 303 |
| Quercetin 3-O-dirhamnosylglucoside isomer 2 | 11.76 | 757.2207 | M + H | 611, 465, 303 |
| Quercetin 3-O-rhamnosyldiglucoside 3 | 9.07 | 773.2145 | M + H | 627, 465, 303 |
| Quercetin-3-O-rutinoside isomer 1 | 12.57 | 611.1608 | M + H | 611, 465, 303 |
| Quercetin-3-O-rutinoside isomer 2 | 14.99 | 611.1614 | M + H | 465, 303 |
| Quercetin-3-O-rutinoside isomer 3 | 11.73 | 611.1621 | M + H | 465, 303 |
| Quercetin-diglucoside 4 | 8.86 | 627.1575 | M + H | 465, 303 |
| Quercetin-3-O-glucoside a | 10.84 | 465.1026 | M + H | 303, 153, 149 |
| Genes | Primer Sequences (from 5′ to 3′) | |
|---|---|---|
| TNF-α | Forward | CCACGCTCTTCTGTCTACTG |
| Reverse | ACTTGGTGGTTTGCTACGAC | |
| IL-1β | Forward | CCAACAAGTGATATTCTCCATGAG |
| Reverse | ACTCTGCAGACTCAAACTCCA | |
| IL-6 | Forward | CTCTGCAAGAGACTTCCATCC |
| Reverse | GAATTGCCATTGCACAACTC | |
| iNOS | Forward | TTTCCAGAAGCAGAATGTGACC |
| Reverse | AACACCACTTTCACCAAGACTC | |
| COX-2 | Forward | GAAATATCAGGTCATTGGTGGAG |
| Reverse | GTTTGGAATAGTTGCTCATCAC | |
| MCP-1 | Forward | AAGAAGCTGTAGTTTTTGTCACCA |
| Reverse | TGAAGACCTTAGGGCAGATGC | |
| HO-1 | Forward | ACATTGAGCTGTTTGAGGAG |
| Reverse | TACATGGCATAAATTCCCACTG | |
| GAPDH | Forward | GAGAAACCTGCCAAGTATGATGAC |
| Reverse | TAGCCGTATTCATTGTCATACCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Gan, T.; Huang, Y.; Bao, L.; Liu, S.; Cui, X.; Wang, H.; Jiao, F.; Zhang, M.; Su, C.; et al. Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 7694. https://doi.org/10.3390/ijms23147694
Lin Z, Gan T, Huang Y, Bao L, Liu S, Cui X, Wang H, Jiao F, Zhang M, Su C, et al. Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo. International Journal of Molecular Sciences. 2022; 23(14):7694. https://doi.org/10.3390/ijms23147694
Chicago/Turabian StyleLin, Ziwei, Tiantian Gan, Yanzhen Huang, Lijun Bao, Shuang Liu, Xiaopeng Cui, Hexin Wang, Feng Jiao, Minjuan Zhang, Chao Su, and et al. 2022. "Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo" International Journal of Molecular Sciences 23, no. 14: 7694. https://doi.org/10.3390/ijms23147694
APA StyleLin, Z., Gan, T., Huang, Y., Bao, L., Liu, S., Cui, X., Wang, H., Jiao, F., Zhang, M., Su, C., & Qian, Y. (2022). Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo. International Journal of Molecular Sciences, 23(14), 7694. https://doi.org/10.3390/ijms23147694






