Identification of Multiple Domains of Entamoeba histolytica Intermediate Subunit Lectin-1 with Hemolytic and Cytotoxic Activities
Abstract
1. Introduction
2. Results
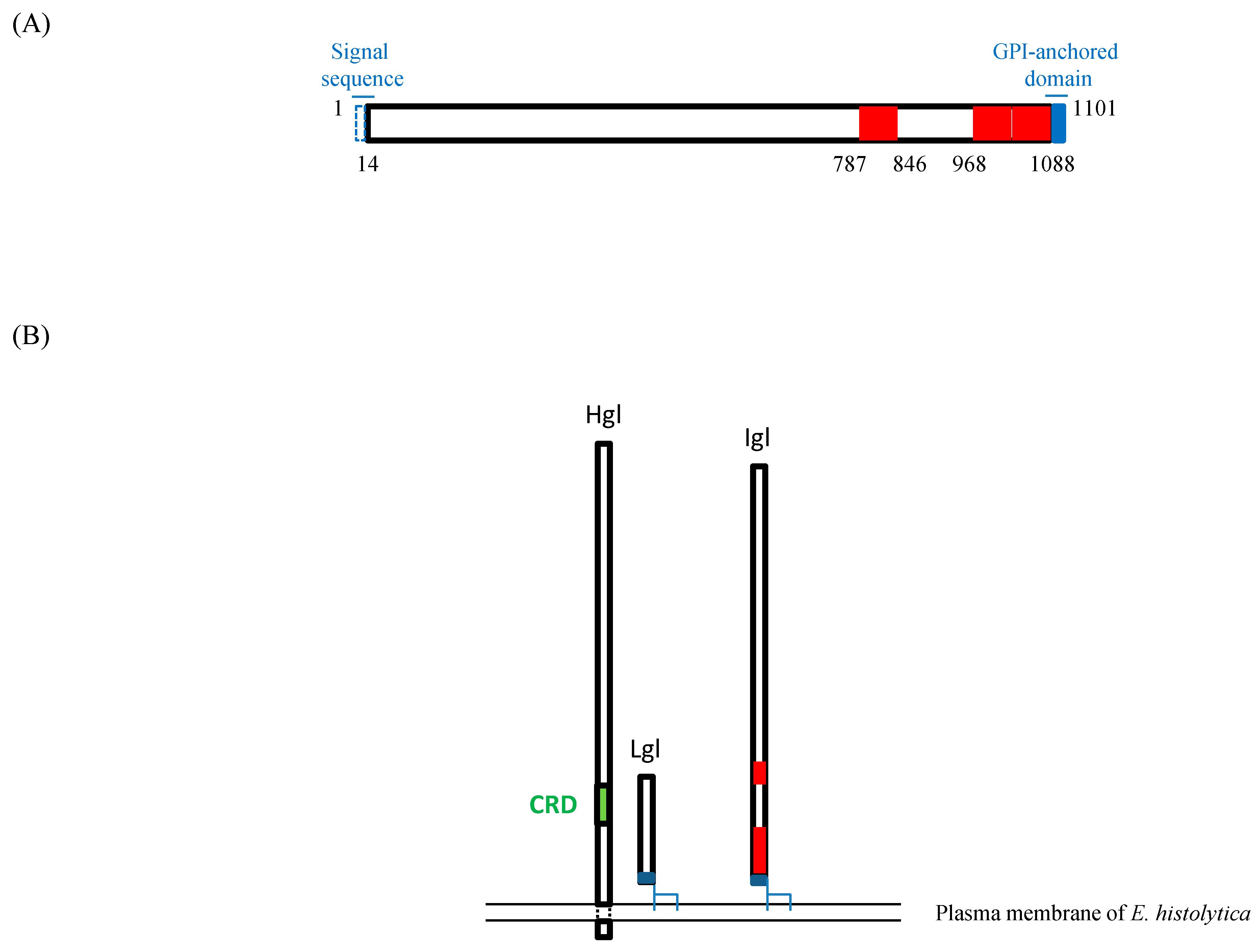
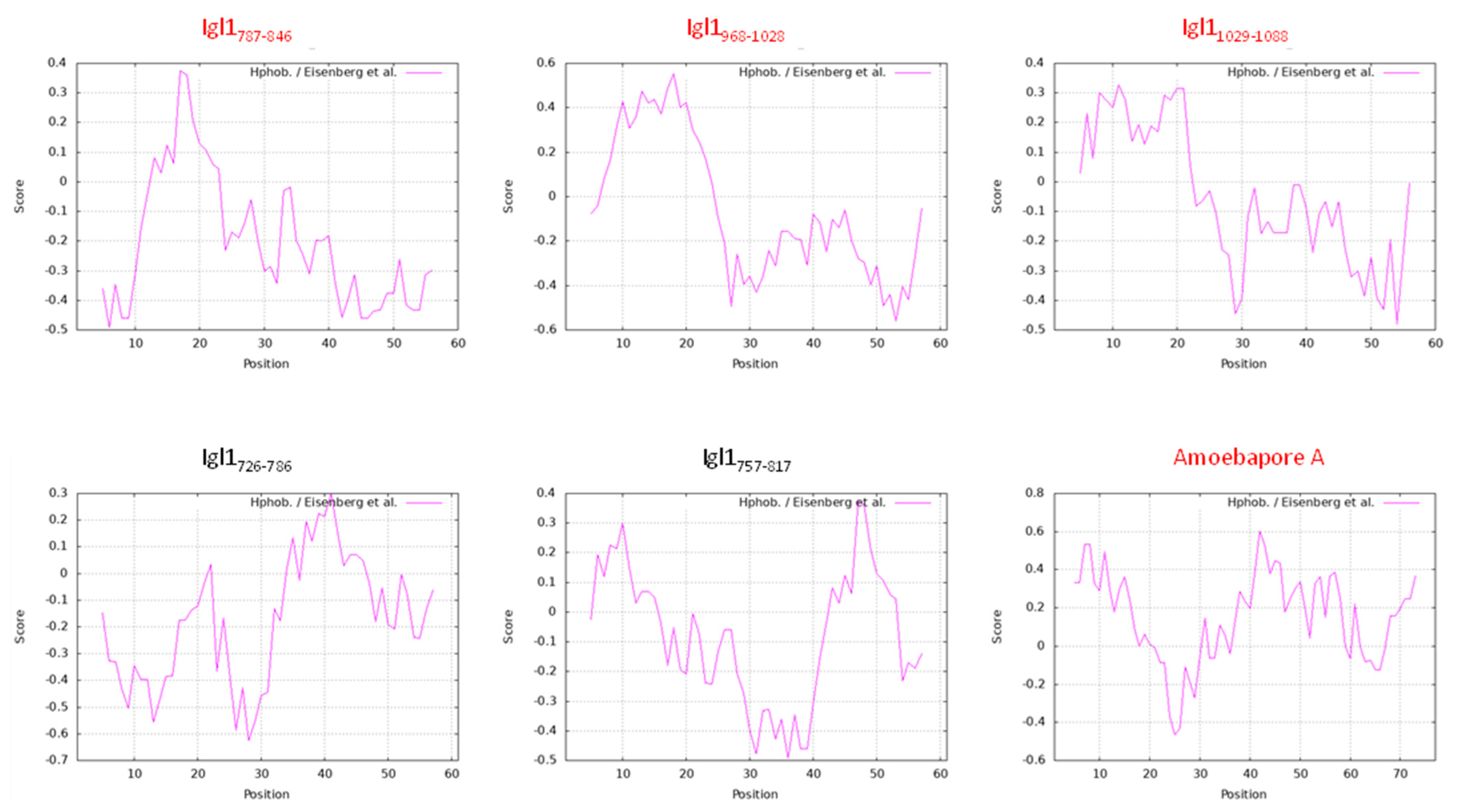
2.1. E. histolytica Igl1 Has Hemolytic Activity between Amino Acids 787 and 846
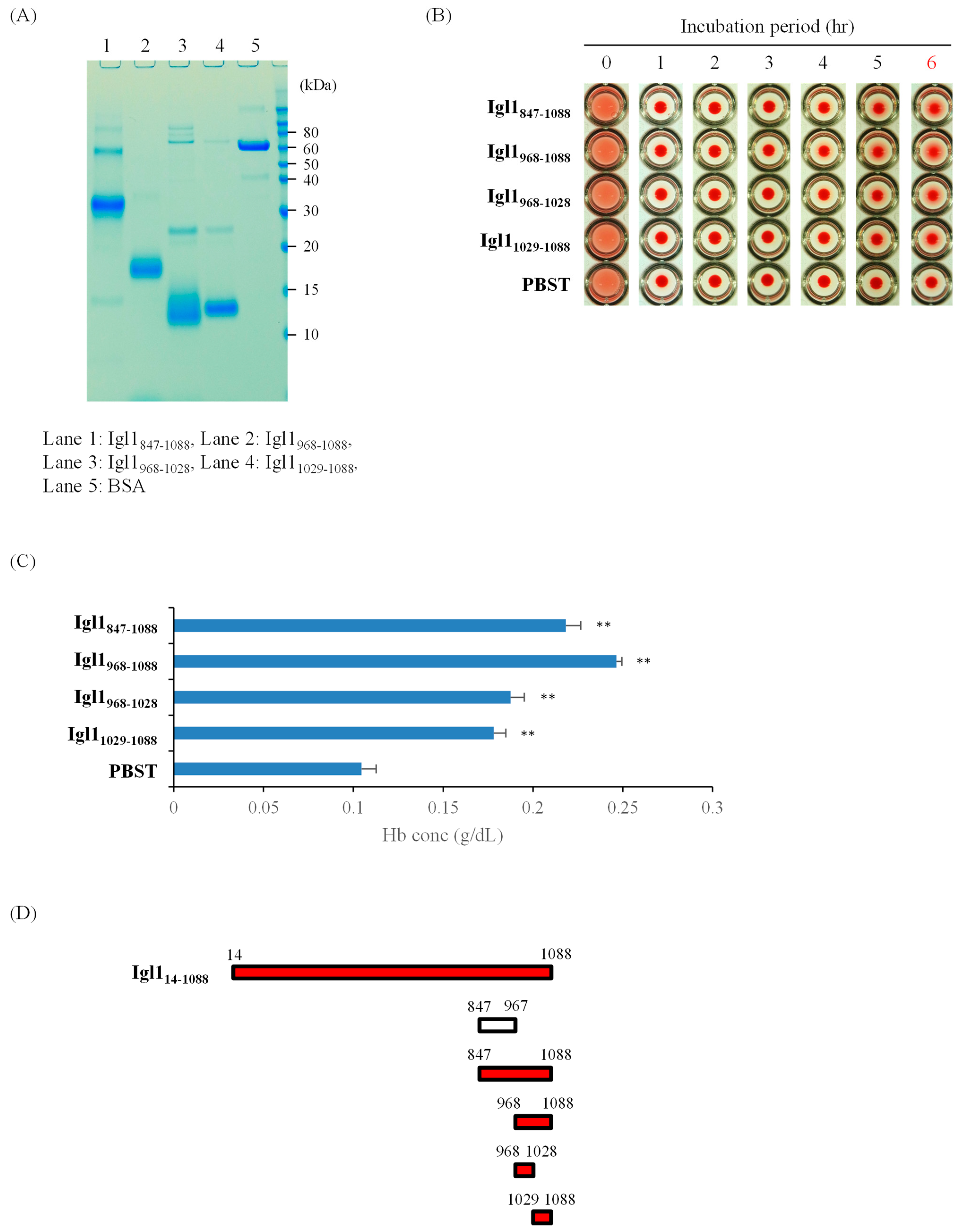
2.2. E. histolytica Igl1 Has Other Regions with Hemolytic Activity
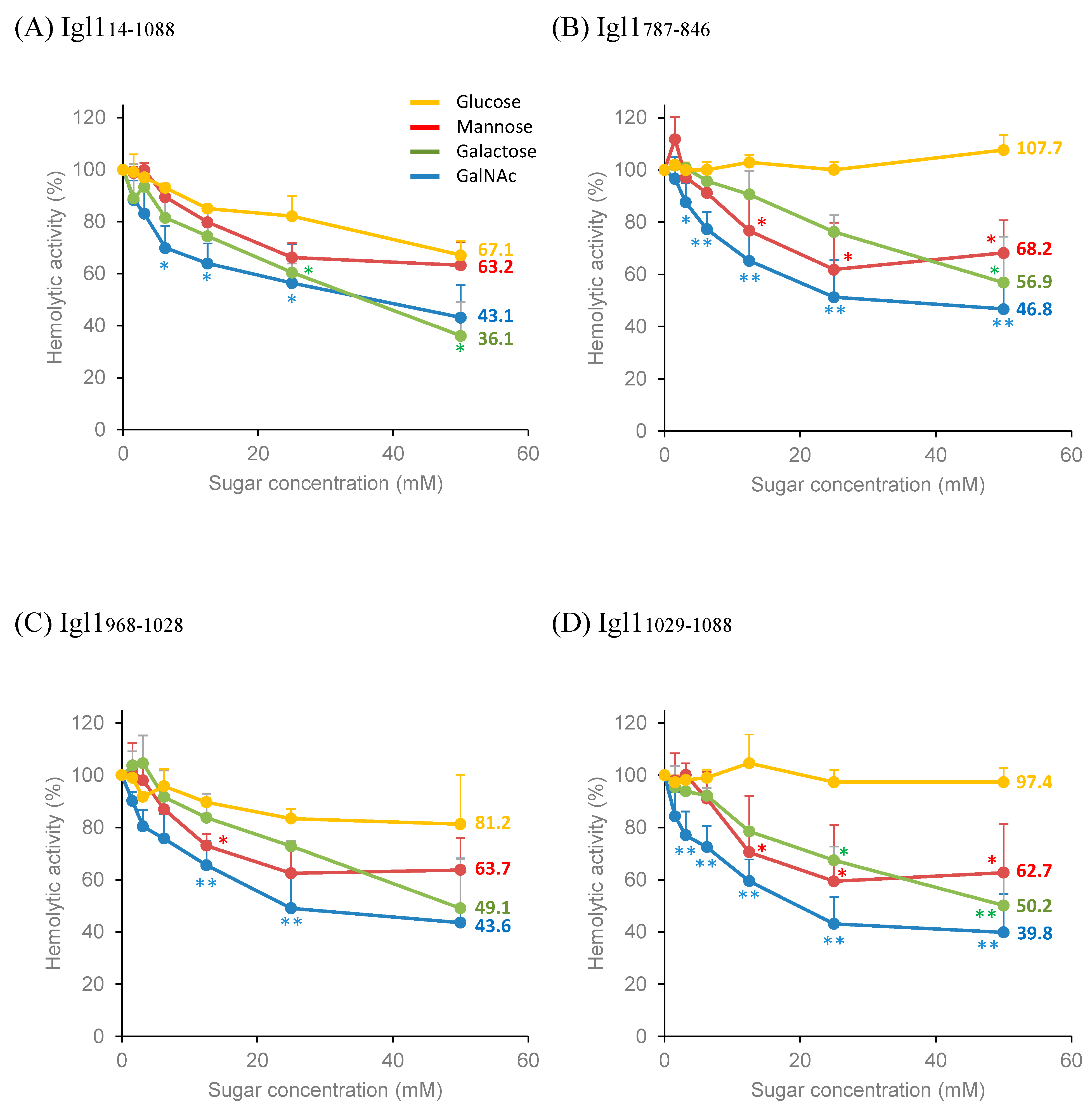
2.3. Partial Inhibition of Hemolytic Activity of Igl1 Fragment Proteins by Monosaccharides
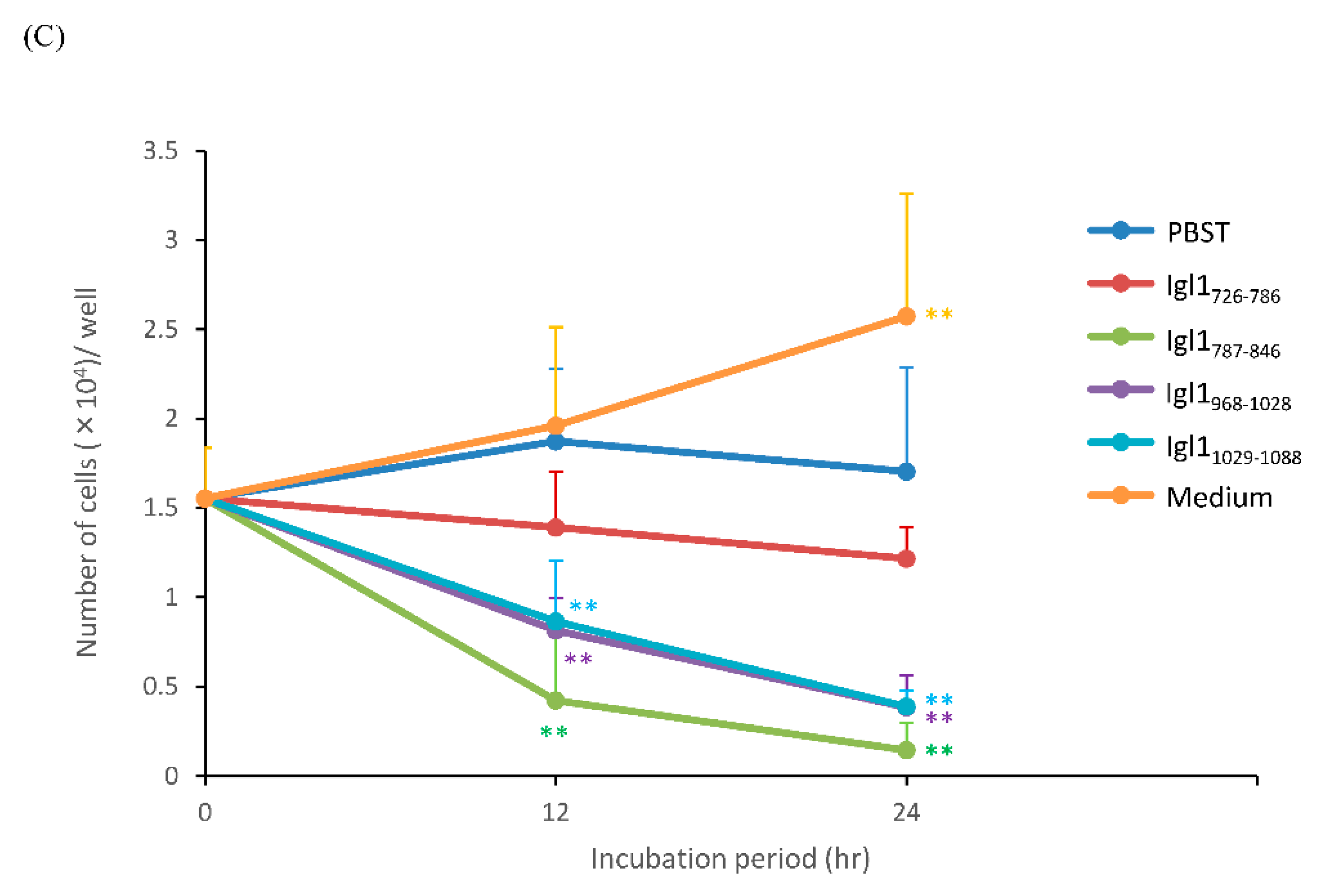
2.4. Cytotoxicity of Recombinant Fragment Proteins of Igl1 against Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Expression and Refolding of Recombinant Igl1 Proteins and Ni Column Purification
4.2. SDS-PAGE and Coomassie Brilliant Blue Staining of Purified Recombinant Proteins
4.3. Hemolytic Assays Using Recombinant Lectins and Measurement of Released Hemoglobin
4.4. Cytotoxicity Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanley, S.L., Jr. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar] [CrossRef]
- Petri, W.A., Jr.; Haque, R.; Mann, B.J. The bittersweet interface of parasite and host: Lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 2002, 56, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Huston, C.D.; Hughes, M.; Houpt, E.; Petri, W.A., Jr. Amebiasis. N. Engl. J. Med. 2003, 348, 1565–1573. [Google Scholar] [CrossRef]
- Laughlin, R.C.; McGugan, G.C.; Powell, R.R.; Welter, B.H.; Temesvari, L.A. Involvement of Raft-Like Plasma Membrane Domains of Entamoeba histolytica in Pinocytosis and Adhesion. Infect. Immun. 2004, 72, 5349–5357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Welter, B.H.; Goldston, A.M.; Temesvari, L.A. Localisation to lipid rafts correlates with increased function of the Gal/GalNAc lectin in the human protozoan parasite, Entamoeba histolytica. Int. J. Parasitol. 2011, 41, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Takekoshi, M.; Cheng, X.J.; Maeda, F.; Aotsuka, S.; Ihara, S. Bacterial expression of a neutralizing mouse monoclonal antibody Fab fragment to a 150-kilodalton surface antigen of Entamoeba histolytica. Am. J. Trop. Med. Hyg. 1999, 60, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Tsukamoto, H.; Kaneda, Y.; Tachibana, H. Identification of the 150-kDa surface antigen of Entamoeba histolytica as a galactose- and N-acetyl-D-galactosamine-inhibitable lectin. Parasitol. Res. 1998, 84, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Hughes, M.A.; Huston, C.D.; Loftus, B.; Gilchrist, C.A.; Lockhart, L.A.; Ghosh, S.; Miller-Sims, V.; Mann, B.J.; Petri, W.A., Jr.; et al. Intermediate subunit of the Gal/GalNAc lectin of Entamoeba histolytica is a member of a gene family containing multiple CXXC sequence motifs. Infect. Immun. 2001, 69, 5892–5898. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Cheng, X.J.; Tsukamoto, H.; Itoh, J. Characterization of Entamoeba histolytica intermediate subunit lectin-specific human monoclonal antibodies generated in transgenic mice expressing human immunoglobulin loci. Infect. Immun. 2009, 77, 549–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kato, K.; Yahata, K.; Dhoubhadel, B.G.; Fujii, Y.; Tachibana, H. Novel hemagglutinating, hemolytic and cytotoxic activities of the intermediate subunit of Entamoeba histolytica lectin. Sci. Rep. 2015, 5, e13901. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Makiuchi, T.; Cheng, X.; Tachibana, H. Comparison of hemolytic activity of the intermediate subunit of Entamoeba histolytica and Entamoeba dispar lectins. PLoS ONE 2017, 12, e0181864. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Kamine, T.; Konishi, Y.; Kuwahara, H.; Niidome, T.; Aoyagi, H. Carbohydrate-dependent hemolytic activity of the conjugate composed of a C-type lectin, CEL-I, and an amphiphilic alpha-helical peptide, 4(3)-beta Ala2. Biosci. Biotechnol. Biochem. 1999, 63, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- McCoy, J.J.; Mann, B.J. Proteomic analysis of Gal/GalNAc lectin-associated proteins in Entamoeba histolytica. Exp. Parasitol. 2005, 110, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Cheng, X.J.; Masuda, G.; Horiki, N.; Takeuchi, T. Evaluation of recombinant fragments of Entamoeba histolytica Gal/GalNAc lectin intermediate subunit for serodiagnosis of amebiasis. J. Clin. Microbiol. 2004, 42, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Feng, M.; Guan, Y.; Man, S.; Fu, Y.; Cheng, X.; Tachibana, H. Evaluation of the C-terminal fragment of Entamoeba histolytica Gal/GalNAc lectin intermediate subunit as a vaccine candidate against amebic liver abscess. PLoS. Negl. Trop. Dis. 2016, 10, e0004419. [Google Scholar] [CrossRef] [PubMed]
- Timmons, P.B.; Hewage, C.M. HAPPENN is a novel tool for hemolytic activity prediction for therapeutic peptides which employs neural networks. Sci. Rep. 2020, 10, 10869. [Google Scholar] [CrossRef] [PubMed]
- Powthongchin, B.; Angsuthanasombat, C. Effects on haemolytic activity of single proline substitutions in the Bordetella pertussis CyaA pore-forming fragment. Arch. Microbiol. 2009, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Kaduk, C.; Tachikawa, E.; Melzig, M.F.; Wenschuh, H.; Bienert, M. Proline at position 14 of alamethicin is essential for hemolytic activity, catecholamine secretion from chromaffin cells and enhanced metabolic activity in endothelial cells. Biochim. Biophys. Acta. 1998, 1370, 175–183. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Schwarz, E.; Komaromy, M.; Wall, R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 1984, 179, 125–142. [Google Scholar] [CrossRef]



| Primer | Position a | Sequence (5’ to 3’) b |
|---|---|---|
| EhIgl-S14 | 40–59 | CCCTCGAGGATTATACTGCTGATAAGCT |
| EhIgl-S726 | 2176–2195 | CCCTCGAGCCATGTCCTGCAAAATGTAA |
| EhIgl-S757 | 2269–2289 | CCCTCGAGAAAGCACCAGAATGTGCTTGT |
| EhIgl-S787 | 2359–2378 | CCCTCGAGTGTAAAAAAACTGATTCATG |
| EhIgl-S847 | 2539–2558 | CCCTCGAGACATGTTCAGATAAAGACAC |
| EhIgl-S968 | 2902–2921 | CCCTCGAGTCAACAAAAGATCATATTGC |
| EhIgl-S1029 | 3085–3105 | CCCTCGAGGAAGCAAAAGGAGGAGAATGT |
| EhIgl-AS786 | 2341–2358 | CCCTCGAGTTATCCTGGATATTTTGAAAG |
| EhIgl-AS817 | 2434–2451 | CCCTCGAGTTAATAAGGACTACGTCCACT |
| EhIgl-AS846 | 2521–2538 | CCCTCGAGTTATGCACATTTATCATCACA |
| EhIgl-AS906 | 2701–2718 | CCCTCGAGTTACTTGTATGTATCACTCTC |
| EhIgl-AS967 | 2884–2901 | CCCTCGAGTTAACATTTAGTACATTCTTC |
| EhIgl-AS1028 | 3066–3084 | CCCTCGAGTTATAAATATTCAGCATTGCAT |
| EhIgl-AS1088 | 3247–3264 | CCCTCGAGTTAAATGCCTTTAGCTCCATT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, K.; Tachibana, H. Identification of Multiple Domains of Entamoeba histolytica Intermediate Subunit Lectin-1 with Hemolytic and Cytotoxic Activities. Int. J. Mol. Sci. 2022, 23, 7700. https://doi.org/10.3390/ijms23147700
Kato K, Tachibana H. Identification of Multiple Domains of Entamoeba histolytica Intermediate Subunit Lectin-1 with Hemolytic and Cytotoxic Activities. International Journal of Molecular Sciences. 2022; 23(14):7700. https://doi.org/10.3390/ijms23147700
Chicago/Turabian StyleKato, Kentaro, and Hiroshi Tachibana. 2022. "Identification of Multiple Domains of Entamoeba histolytica Intermediate Subunit Lectin-1 with Hemolytic and Cytotoxic Activities" International Journal of Molecular Sciences 23, no. 14: 7700. https://doi.org/10.3390/ijms23147700
APA StyleKato, K., & Tachibana, H. (2022). Identification of Multiple Domains of Entamoeba histolytica Intermediate Subunit Lectin-1 with Hemolytic and Cytotoxic Activities. International Journal of Molecular Sciences, 23(14), 7700. https://doi.org/10.3390/ijms23147700







