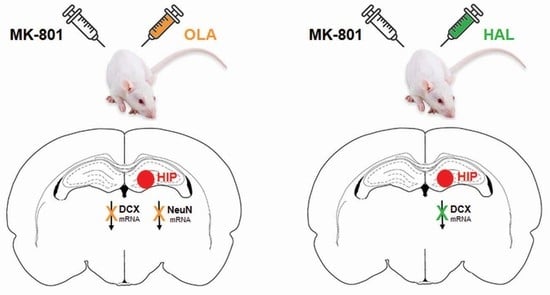
Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia
Abstract
:1. Introduction
2. Results
2.1. In Vitro Studies
2.1.1. Proliferation and Neuronal Viability
2.1.2. Projection Length
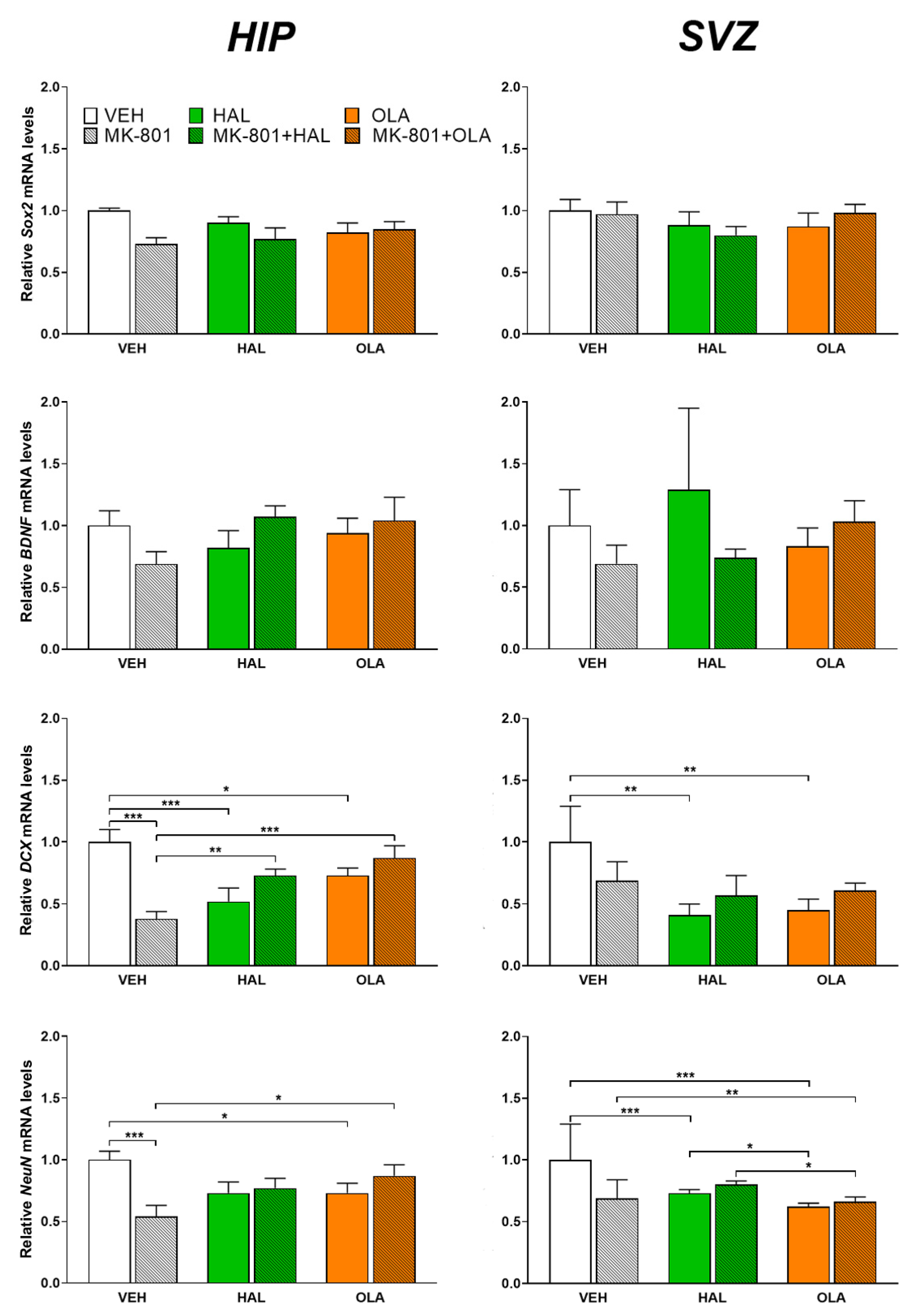
2.2. Genes’ Expression
2.2.1. DCX mRNA
2.2.2. NeuN mRNA
2.2.3. Sox2 mRNA
2.2.4. BDNF mRNA
2.3. Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. In Vitro Experiments
4.1.1. Cell Culture
4.1.2. Cell Counting
4.1.3. Projection Length
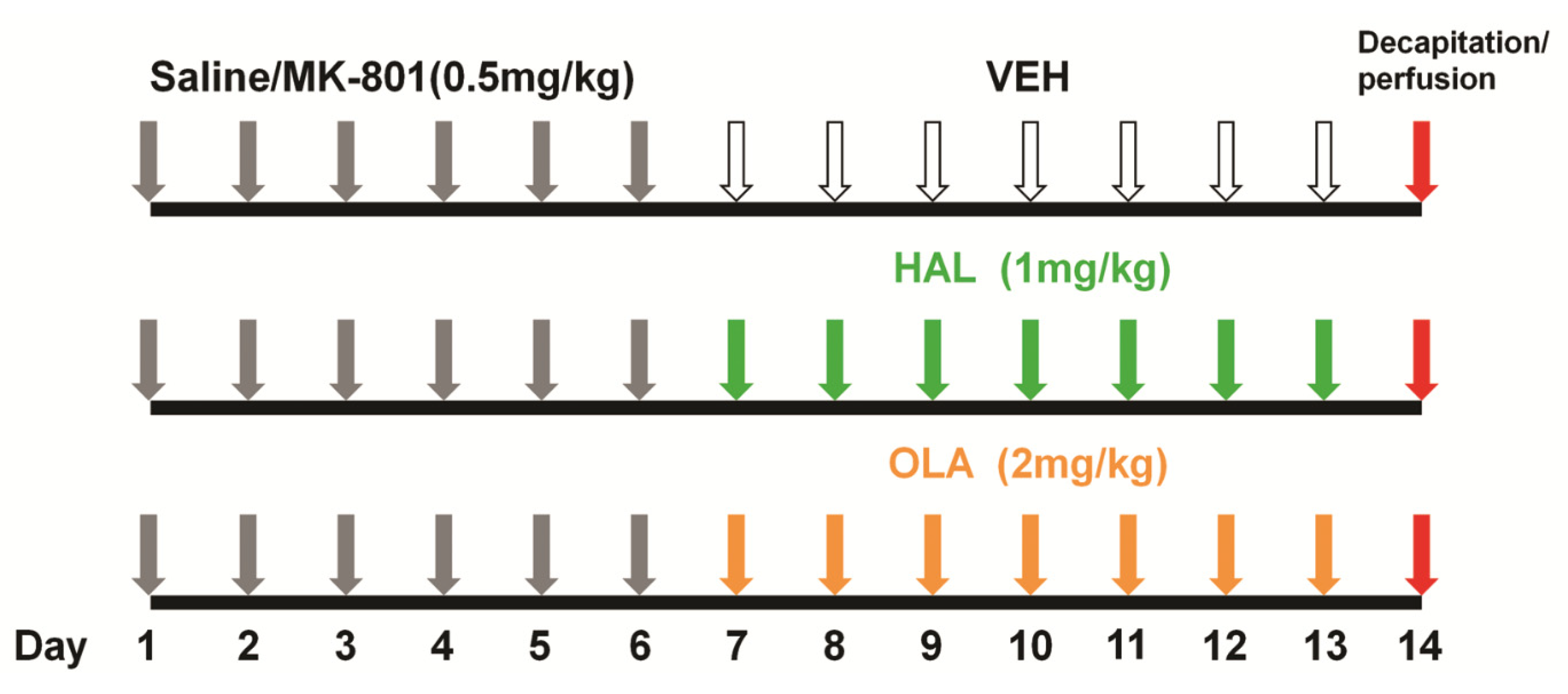
4.2. In Vivo Experiments
4.2.1. Experimental Design
4.2.2. Euthanasia of Animals, Tissue Processing
4.2.3. Microdissection of Brain Area
4.2.4. RNA Isolation and Real-Time PCR
4.2.5. Immunohistochemistry
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weissleder, C.; North, H.F.; Shannon Weickert, C. Important unanswered questions about adult neurogenesis in schizophrenia. Curr. Opin. Psychiatry 2019, 32, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Cameron, H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology 2015, 40, 113–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnbaum, R.; Weinberger, D. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Chikama, K.; Yamada, H.; Tsukamoto, T.; Kajitani, K.; Nakabeppu, Y.; Uchimura, N. Chronic atypical antipsychotics, but not haloperidol, increase neurogenesis in the hippocampus of adult mouse. Brain Res. 2017, 1676, 77–82. [Google Scholar] [CrossRef]
- Rojczyk-Gołębiewska, E.; Pałasz, A.; Wiaderkiewicz, R. Hypothalamic subependymal niche: A novel site of the adult neurogenesis. Cell. Mol. Neurobiol. 2014, 34, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Migaud, M.; Butrille, L.; Batailler, M. Seasonal regulation of structural plasticity and neurogenesis in the adult mammalian brain: Focus on the sheep hypothalamus. Front. Neuroendocrinol. 2015, 37, 146–157. [Google Scholar] [CrossRef]
- Niwa, A.; Nishibori, M.; Hamasaki, S.; Kobori, T.; Liu, K.; Wake, H.; Mori, S.; Yoshino, T.; Takahashi, H. Voluntary exercise induces neurogenesis in the hypothalamus and ependymal lining of the third ventricle. Brain Struct. Funct. 2016, 221, 1653–1666. [Google Scholar] [CrossRef]
- Klein, C.; Jonas, W.; Wiedmer, P.; Schreyer, S.; Akyüz, L.; Spranger, J.; Hellweg, R.; Steiner, B. High-fat Diet and Physical Exercise Differentially Modulate Adult Neurogenesis in the Mouse Hypothalamus. Neuroscience 2019, 400, 146–156. [Google Scholar] [CrossRef]
- Grinchii, D.; Dremencov, E. Mechanism of Action of Atypical Antipsychotic Drugs in Mood Disorders. Int. J. Mol. Sci. 2020, 21, 9532. [Google Scholar] [CrossRef]
- Gasso, P.; Mas, S.; Molina, O.; Bernardo, M.; Lafuente, A.; Parellada, E. Neurotoxic/neuroprotective activity of haloperidol, risperidone and paliperidone in neuroblastoma cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 71–77. [Google Scholar] [CrossRef]
- Kusumi, I.; Boku, S.; Takahashi, Y. Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis. Psychiatry Clin. Neurosci. 2015, 69, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.T.; Nasrallah, H.A. Neuroprotective effects of the second generation antipsychotics. Schizophr. Res. 2019, 208, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ukai, W.; Ozawa, H.; Tateno, M.; Hashimoto, E.; Saito, T. Neurotoxic potential of haloperidol in comparison with risperidone: Implication of Akt-mediated signal changes by haloperidol. J. Neural Transm. 2004, 111, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Zhuravliova, E.; Barbakadze, T.; Natsvlishvili, N.; Mikeladze, D. Haloperidol induces neurotoxicity by the NMDA receptor downstream signaling pathway, alternative from glutamate excitotoxicity. Neurochem. Int. 2007, 50, 976–982. [Google Scholar] [CrossRef]
- Zhang, J.P.; Gallego, J.A.; Robinson, D.G.; Malhotra, A.K.; Kane, J.M.; Correll, C.U. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: A systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2013, 16, 1205–1218. [Google Scholar] [CrossRef] [Green Version]
- Wakade, C.G.; Mahadik, S.P.; Waller, J.L.; Chiu, F.-C. Atypical neuroleptics stimulate neurogenesis in adult rat brain. J. Neurosci. Res. 2002, 69, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.C.; Lung, F.W. Neuroprotection of paliperidone on SH-SY5Y cells against beta-amyloid peptide(25–35), N-methyl-4-phenylpyridinium ion, and hydrogen peroxide-induced cell death. Psychopharmacology 2011, 217, 397–410. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011, 345, 1–19. [Google Scholar] [CrossRef]
- Koprivica, V.; Regardie, K.; Wolff, C.; Fernalld, R.; Murphy, J.J.; Kambayashi, J.; Kikuchi, T.; Jordan, S. Aripiprazole protects cortical neurons from glutamate toxicity. Eur. J. Pharmacol. 2011, 651, 73–76. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, J.; Wu, J.; Li, G.; Yao, W.; Hao, J.; Sun, J. Paliperidone Protects SH-SY5Y Cells against MK-801-Induced Neuronal Damage through Inhibition of Ca2+ Influx and Regulation of SIRT1/miR-134 Signal Pathway. Mol. Neurobiol. 2016, 53, 2498–2509. [Google Scholar] [CrossRef]
- Vasefi, M.S.; Yang, K.; Li, J.; Kruk, J.S.; Heikkila, J.J.; Jackson, M.F.; MacDonald, J.F.; Beazely, M.A. Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol. Brain 2013, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vales, K.; Holubova, K. Minireview: Animal model of schizophrenia from the perspective of behavioral pharmacology: Effect of treatment on cognitive functions. Neurosci. Lett. 2021, 761, 136098. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, L. Adult neural stem cells and schizophrenia. World J. Stem Cells 2022, 14, 219–230. [Google Scholar] [CrossRef]
- Toro, C.T.; Deakin, J.F. Adult neurogenesis and schizophrenia: A window on abnormal early brain development? Schizophr. Res. 2007, 90, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Yoo, K.-Y.; Lee, C.H.; Yi, S.S.; Yoo, D.Y.; Seong, J.K.; Yoon, Y.S.; Hwang, I.K.; Won, M.-H. Effects of treadmill exercise combined with MK 801 treatment on neuroblast differentiation in the dentate gyrus in rats. Cell. Mol. Neurobiol. 2011, 31, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kawade, H.M.; Borkar, C.D.; Shambharkar, A.S.; Singh, O.; Singru, P.S.; Subhedar, N.K.; Kokare, D.M. Intracellular mechanisms and behavioral changes in mouse model of attention deficit hyperactivity disorder: Importance of age-specific NMDA receptor blockade. Pharmacol. Biochem. Behav. 2020, 188, 172830. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Jacob, R.S.; Omkumar, R.V. Differential expression of miR-148b, miR-129-2 and miR-296 in animal models of schizophrenia-Relevance to NMDA receptor hypofunction. Neuropharmacology 2022, 210, 109024. [Google Scholar] [CrossRef]
- Amellem, I.; Yovianto, G.; Chong, H.T.; Nair, R.R.; Cnops, V.; Thanawalla, A.; Tashiro, A. Role of NMDA Receptors in Adult Neurogenesis and Normal Development of the Dentate Gyrus. eNeuro 2021, 8, ENEURO.0566-20.2021. [Google Scholar] [CrossRef]
- Halim, N.D.; Weickert, C.S.; McClintock, B.W.; Weinberger, D.R.; Lipska, B.K. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology 2004, 29, 1063–1069. [Google Scholar] [CrossRef]
- Kodama, M.; Fujioka, T.; Duman, R.S. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol. Psychiatry 2004, 56, 570–580. [Google Scholar] [CrossRef]
- Lasut, B.; Palasz, A.; Filipczyk, L.; Arias-Carrion, O.; Rojczyk, E.; Savchyna, M.; Bogus, K.; Worthington, J.J.; Krzystanek, M.; Wiaderkiewicz, R. Long-term Treatment with Olanzapine Increases the Number of Sox2 and Doublecortin Expressing Cells in the Adult Subventricular Zone. CNS Neurol. Disord. Drug Targets 2018, 17, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Rojczyk, E.; Palasz, A.; Wiaderkiewicz, R. Effects of neuroleptics administration on adult neurogenesis in the rat hypothalamus. Pharmacol. Rep. 2015, 67, 1208–1214. [Google Scholar] [CrossRef]
- Takamura, N.; Nakagawa, S.; Masuda, T.; Boku, S.; Kato, A.; Song, N.; An, Y.; Kitaichi, Y.; Inoue, T.; Koyama, T.; et al. The effect of dopamine on adult hippocampal neurogenesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Klempin, F.; Babu, H.; Tonel, D.D.P.; Alarcon, E.; Fabel, K.; Kempermann, G. Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front. Mol. Neurosci. 2010, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.K.; Kim, Y.H.; McIntyre, R.S.; Mansur, R.B.; Lee, Y.; Carmona, N.E.; Choi, A.J.; Kim, G.-M.; Lee, J.G.; Park, S.W. Effects of Antipsychotic Drugs on the Epigenetic Modification of Brain-Derived Neurotrophic Factor Gene Expression in the Hippocampi of Chronic Restraint Stress Rats. Neural Plast. 2018, 2018, 2682037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osacka, J.; Koprdova, R.; Tillinger, A.; Pirnik, Z.; Kiss, A. Haloperidol and aripiprazole impact on the BDNF and glucocorticoid receptor levels in the rat hippocampus and prefrontal cortex: Effect of the chronic mild stress. Endocr. Regul. 2021, 55, 153–162. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, Y.; Sodhi, R.K.; Saroj, P.; Medhi, B.; Kuhad, A. Modeling of antipsychotic-induced metabolic alterations in mice: An experimental approach precluding psychosis as a predisposing factor. Toxicol. Appl. Pharmacol. 2019, 378, 114643. [Google Scholar] [CrossRef]
- Song, J.C.; Seo, M.K.; Park, S.W.; Lee, J.G.; Kim, Y.H. Differential Effects of Olanzapine and Haloperidol on MK-801-induced Memory Impairment in Mice. Clin. Psychopharmacol. Neurosci. 2016, 14, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Sugino, H.; Hirose, T.; Kitagawa, H.; Nagai, T.; Mizoguchi, H.; Takuma, K.; Yamada, K. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J. Pharmacol. Sci. 2007, 103, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garrote, M.; Parga, J.A.; Labandeira, P.J.; Labandeira-Garcia, J.L.; Rodriguez-Pallares, J. Dopamine regulates adult neurogenesis in the ventricular-subventricular zone via dopamine D3 angiotensin type 2 receptor interactions. Stem Cells 2021, 39, 1778–1794. [Google Scholar] [CrossRef]
- Higley, M.J.; Sabatini, B.L. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat. Neurosci. 2010, 13, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frau, R.; Pardu, A.; Godar, S.; Bini, V.; Bortolato, M. Combined Antagonism of 5-HT2 and NMDA Receptors Reduces the Aggression of Monoamine Oxidase a Knockout Mice. Pharmaceuticals 2022, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Brenner, E.; Eyjolfsson, E.M.; Markinhuhta, K.R.; Carlsson, M.L.; Sonnewald, U. Glial-neuronal interactions are impaired in the schizophrenia model of repeated MK801 exposure. Neuropsychopharmacology 2006, 31, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press, Inc.: San Diego, CA, USA, 1997. [Google Scholar]
- Palkovits, M.; Brownstein, M.J. Maps and Guide to Microdissection of the Rat Brain; Elsevier: New York, NY, USA, 1988; pp. 1–223. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osacka, J.; Kiss, A.; Bacova, Z.; Tillinger, A. Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia. Int. J. Mol. Sci. 2022, 23, 7711. https://doi.org/10.3390/ijms23147711
Osacka J, Kiss A, Bacova Z, Tillinger A. Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia. International Journal of Molecular Sciences. 2022; 23(14):7711. https://doi.org/10.3390/ijms23147711
Chicago/Turabian StyleOsacka, Jana, Alexander Kiss, Zuzana Bacova, and Andrej Tillinger. 2022. "Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia" International Journal of Molecular Sciences 23, no. 14: 7711. https://doi.org/10.3390/ijms23147711
APA StyleOsacka, J., Kiss, A., Bacova, Z., & Tillinger, A. (2022). Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia. International Journal of Molecular Sciences, 23(14), 7711. https://doi.org/10.3390/ijms23147711