Targeted Induction of Endogenous VDUP1 by Small Activating RNA Inhibits the Growth of Lung Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Induction of VDUP1 Expression by dsVDUP1-834 in A549 Cells
2.2. dsVDUP1-834 Inhibits Cell Growth and Induces Cell-Cycle Arrest in A549 Cells
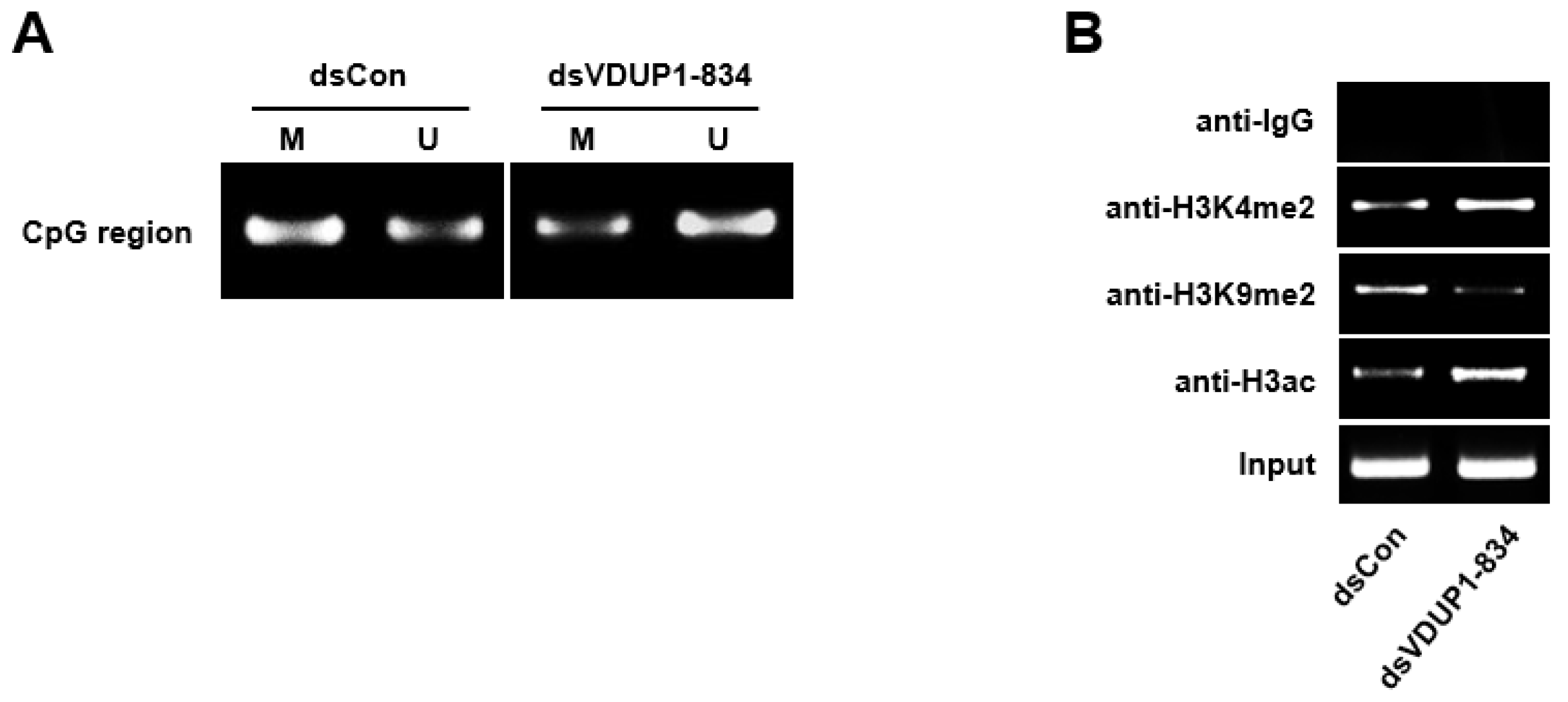
2.3. dsVDUP1-834 Induces DNA Demethylation and Histone Modification of VDUP1 Promoter
2.4. The Effects of dsVDUP1-834 on Cell Proliferation and the Expression of Cell-Cycle Regulators Were Attenuated by VDUP1 Knockdown in A549 Cells
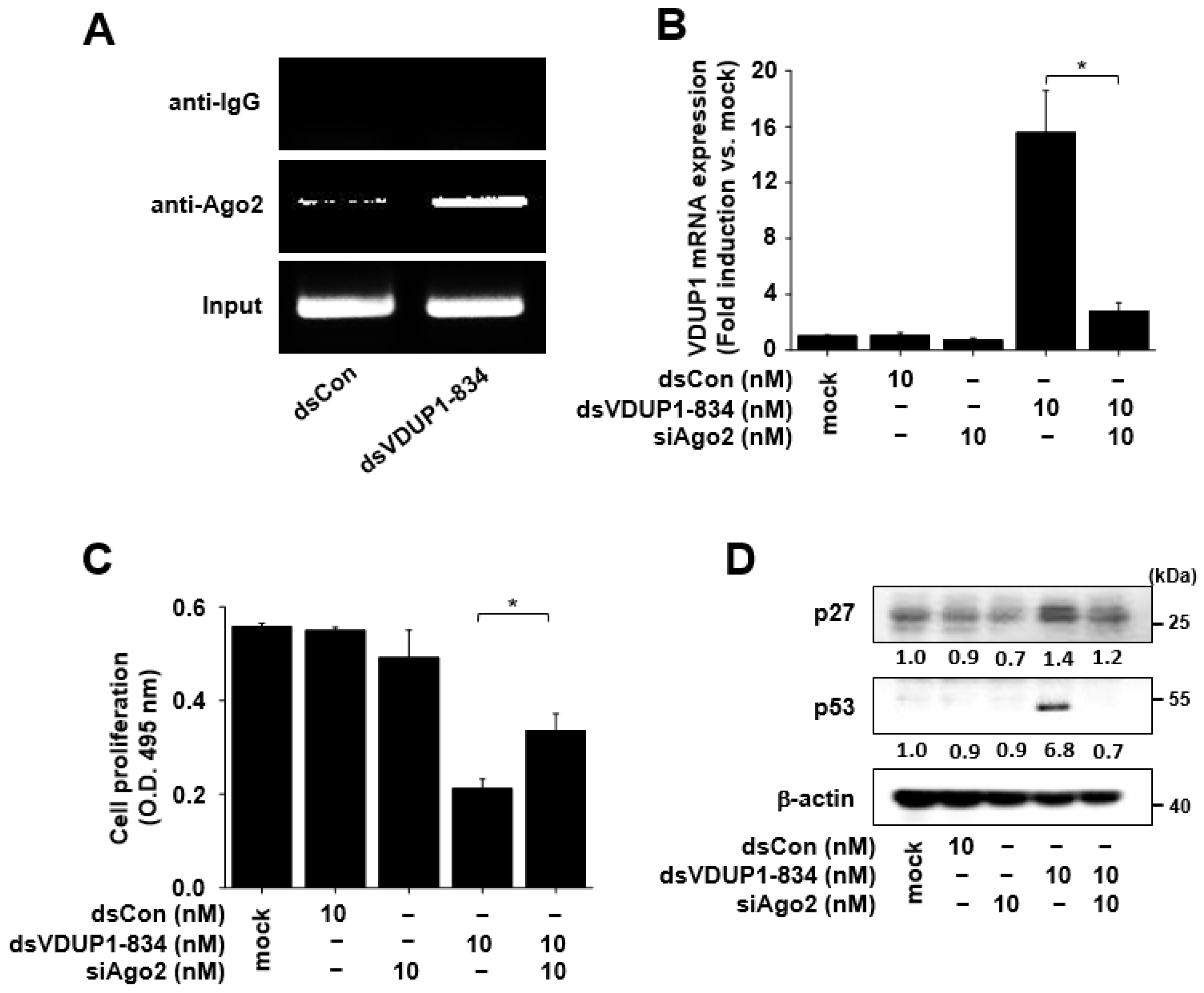
2.5. Induction of VDUP1 Expression by dsVDUP1-834 Is Ago2-Dependent, and Ago2 Knockdown Alleviates the Effects of dsVDUP1-834 on Cell Proliferation and the Expression of Cell-Cycle Regulators in A549 Cells
2.6. dsVDUP1-834 Suppressed Tumor Growth in A549 Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Preparation of dsRNAs
4.2. Cell Culture and Transfection
4.3. RNA Isolation and Quantification of mRNA Expression
4.4. Cell Proliferation Assay
4.5. Western Immunoblot Analysis
4.6. Methylation Analysis
4.7. Chromatin Immunoprecipitation (ChIP) Assay
4.8. Cell-Cycle Analysis
4.9. Human Tumor Xenograft Model
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Okino, S.T.; Zhao, H.; Pookot, D.; Place, R.F.; Urakami, S.; Enokida, H.; Dahiya, R. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. USA 2006, 103, 17337–17342. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Younger, S.T.; Hardy, D.B.; Ram, R.; Huffman, K.E.; Corey, D.R. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat. Chem. Biol. 2007, 3, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Place, R.F.; Noonan, E.J.; Foldes-Papp, Z.; Li, L.C. Defining features and exploring chemical modifications to manipulate RNAa activity. Curr. Pharm. Biotechnol. 2010, 11, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; DeLuca, H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta 1994, 1219, 26–32. [Google Scholar] [CrossRef]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Q.; Chng, W.J. TXNIP (VDUP-1, TBP-2): A major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2011, 43, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Won, Y.S.; Suh, H.W.; Jeon, J.H.; Shao, Y.; Yoon, S.R.; Chung, J.W.; Kim, T.D.; Kim, H.M.; Nam, K.H.; et al. Vitamin D3 upregulated protein 1 suppresses TNF-α-induced NF-κB activation in hepatocarcinogenesis. J. Immunol. 2010, 185, 3980–3989. [Google Scholar] [CrossRef]
- Nishizawa, K.; Nishiyama, H.; Matsui, Y.; Kobayashi, T.; Saito, R.; Kotani, H.; Masutani, H.; Oishi, S.; Toda, Y.; Fujii, N.; et al. Thioredoxin-interacting protein suppresses bladder carcinogenesis. Carcinogenesis 2011, 32, 1459–1466. [Google Scholar] [CrossRef]
- Kwon, H.J.; Won, Y.S.; Nam, K.T.; Yoon, Y.D.; Jee, H.; Yoon, W.K.; Nam, K.H.; Kang, J.S.; Han, S.U.; Choi, I.P.; et al. Vitamin D3 upregulated protein 1 deficiency promotes N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric carcinogenesis in mice. Gut 2012, 61, 53–63. [Google Scholar] [CrossRef]
- Frullanti, E.; Colombo, F.; Falvella, F.S.; Galvan, A.; Noci, S.; De Cecco, L.; Incarbone, M.; Alloisio, M.; Santambrogio, L.; Nosotti, M.; et al. Association of lung adenocarcinoma clinical stage with gene expression pattern in noninvolved lung tissue. Int. J. Cancer 2012, 131, E643–E648. [Google Scholar] [CrossRef]
- Jin, X.; Wu, N.; Dai, J.; Li, Q.; Xiao, X. TXNIP mediates the differential responses of A549 cells to sodium butyrate and sodium 4-phenylbutyrate treatment. Cancer Med. 2017, 6, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.P.; Lehtola, T.; Heinonen, S.E.; Assefa, G.S.; Korpisalo, P.; Girnary, R.; Glass, C.K.; Vaisanen, S.; Yla-Herttuala, S. Efficient regulation of VEGF expression by promoter-targeted lentiviral shRNAs based on epigenetic mechanism: A novel example of epigenetherapy. Circ. Res. 2009, 105, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.R.; Park, K.H.; Yang, J.O.; Lee, C.W.; Oh, S.J.; Yun, J.; Lee, M.Y.; Han, S.B.; Kang, J.S. miR-6734 Up-Regulates p21 Gene Expression and Induces Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. PLoS ONE 2016, 11, e0160961. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Sakurai, F.; Elbashir, S.; Foster, D.J.; Manoharan, M.; Corey, D.R. Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter. Chem. Biol. 2010, 17, 1344–1355. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Novina, C.D.; Sharp, P.A. Killing the messenger: Short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 2003, 4, 457–467. [Google Scholar] [CrossRef]
- Chen, Y.; Ning, J.; Cao, W.; Wang, S.; Du, T.; Jiang, J.; Feng, X.; Zhang, B. Research Progress of TXNIP as a Tumor Suppressor Gene Participating in the Metabolic Reprogramming and Oxidative Stress of Cancer Cells in Various Cancers. Front. Oncol. 2020, 10, 568574. [Google Scholar] [CrossRef]
- Li, J.; Vervoorts, J.; Carloni, P.; Rossetti, G.; Luscher, B. Structural prediction of the interaction of the tumor suppressor p27KIP1 with cyclin A/CDK2 identifies a novel catalytically relevant determinant. BMC Bioinform. 2017, 18, 15. [Google Scholar] [CrossRef]
- Hoffmann, T.K.; Trellakis, S.; Okulicz, K.; Schuler, P.; Greve, J.; Arnolds, J.; Bergmann, C.; Ras, M.; Lang, S.; Lehnerdt, G.; et al. Cyclin B1 expression and p53 status in suqamous cell carcinomas of the head and neck. Anticancer. Res. 2011, 31, 10, 3141–3157. [Google Scholar]
- Jeon, J.H.; Lee, K.N.; Hwang, C.Y.; Kwon, K.S.; You, K.H.; Choi, I. Tumor suppressor VDUP1 increases p27kip1 stability by inhibiting JAB1. Cancer Res. 2005, 65, 4485–4489. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Takata, M.; Kamitori, K.; Nonaka, M.; Dong, Y.; Sui, L.; Tokuda, M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int. J. Oncol. 2008, 32, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Saito, M.; Tsukamoto, I.; Yamaguchi, F.; Sui, L.; Kamitori, K.; Dong, Y.; Uehara, E.; Konishi, R.; Janjua, N.; et al. Analysis of the inhibitory mechanism of D-allose on MOLT-4F leukemia cell proliferation. J. Biosci. Bioeng. 2009, 107, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Maleszewska, M.; Vanchin, B.; Harmsen, M.C.; Krenning, G. The decrease in histone methyltransferase EZH2 in response to fluid shear stress alters endothelial gene expression and promotes quiescence. Angiogenesis 2016, 19, 1, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, V.; Place, R.F.; Portnoy, V.; Wang, J.; Qi, Z.; Jia, Z.; Yu, A.; Shuman, M.; Yu, J.; Li, L.C. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2012, 40, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shen, J.; Xie, Y.Q.; Lin, Y.W.; Qin, J.; Mao, Q.Q.; Zheng, X.Y.; Xie, L.P. Promoter-targeted double-stranded small RNAs activate PAWR gene expression in human cancer cells. Int. J. Biochem. Cell Biol. 2013, 45, 1338–1346. [Google Scholar] [CrossRef]
- Holland, W.S.; Tepper, C.G.; Pietri, J.E.; Chinn, D.C.; Gandara, D.R.; Mack, P.C.; Lara, P.N., Jr. Evaluating rational non-cross-resistant combination therapy in advanced clear cell renal cell carcinoma: Combined mTOR and AKT inhibitor therapy. Cancer Chemother. Pharm. 2012, 69, 185–194. [Google Scholar] [CrossRef][Green Version]
- Su, R.; Fan, L.H.; Cao, C.; Wang, L.; Du, Z.; Cai, Z.; Ouyang, Y.C.; Wang, Y.; Zhou, Q.; Wu, L.; et al. Global profiling of RNA-binding protein target sites by LACE-seq. Nat. Cell Biol. 2021, 23, 664–675. [Google Scholar] [CrossRef]
- Portnoy, V.; Huang, V.; Place, R.F.; Li, L.C. Small RNA and transcriptional upregulation. Wiley Interdiscip. Rev. RNA 2011, 2, 748–760. [Google Scholar] [CrossRef]
- Kang, M.R.; Park, K.H.; Lee, C.W.; Lee, M.Y.; Han, S.B.; Li, L.C.; Kang, J.S. Small activating RNA induced expression of VHL gene in renal cell carcinoma. Int. J. Biochem. Cell Biol. 2018, 97, 36–42. [Google Scholar] [CrossRef]
- Huang, V.; Qin, Y.; Wang, J.; Wang, X.; Place, R.F.; Lin, G.; Lue, T.F.; Li, L.C. RNAa is conserved in mammalian cells. PLoS ONE 2010, 5, e8848. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Place, R.F.; Portnoy, V.; Huang, V.; Kang, M.R.; Kosaka, M.; Ho, M.K.; Li, L.C. Inducing gene expression by targeting promoter sequences using small activating RNAs. J. Biol. Methods 2015, 2, e14. [Google Scholar] [CrossRef] [PubMed]
- Huang, V.; Zheng, J.; Qi, Z.; Wang, J.; Place, R.F.; Yu, J.; Li, H.; Li, L.C. Ago1 Interacts with RNA polymerase II and binds to the promoters of actively transcribed genes in human cancer cells. PLoS Genet. 2013, 9, e1003821. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Yang, J.-W.; Kwon, J.-H.; Lee, H.; Yoon, Y.D.; Choi, B.J.; Lee, M.Y.; Lee, C.W.; Han, S.-B.; Kang, J.S. Targeted Induction of Endogenous VDUP1 by Small Activating RNA Inhibits the Growth of Lung Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7743. https://doi.org/10.3390/ijms23147743
Park KH, Yang J-W, Kwon J-H, Lee H, Yoon YD, Choi BJ, Lee MY, Lee CW, Han S-B, Kang JS. Targeted Induction of Endogenous VDUP1 by Small Activating RNA Inhibits the Growth of Lung Cancer Cells. International Journal of Molecular Sciences. 2022; 23(14):7743. https://doi.org/10.3390/ijms23147743
Chicago/Turabian StylePark, Ki Hwan, Jeong-Wook Yang, Joo-Hee Kwon, Hyunju Lee, Yeo Dae Yoon, Byeong Jo Choi, Myeong Youl Lee, Chang Woo Lee, Sang-Bae Han, and Jong Soon Kang. 2022. "Targeted Induction of Endogenous VDUP1 by Small Activating RNA Inhibits the Growth of Lung Cancer Cells" International Journal of Molecular Sciences 23, no. 14: 7743. https://doi.org/10.3390/ijms23147743
APA StylePark, K. H., Yang, J.-W., Kwon, J.-H., Lee, H., Yoon, Y. D., Choi, B. J., Lee, M. Y., Lee, C. W., Han, S.-B., & Kang, J. S. (2022). Targeted Induction of Endogenous VDUP1 by Small Activating RNA Inhibits the Growth of Lung Cancer Cells. International Journal of Molecular Sciences, 23(14), 7743. https://doi.org/10.3390/ijms23147743






