Molecular Effects of Chronic Exposure to Palmitate in Intestinal Organoids: A New Model to Study Obesity and Diabetes
Abstract
:1. Introduction
2. Results
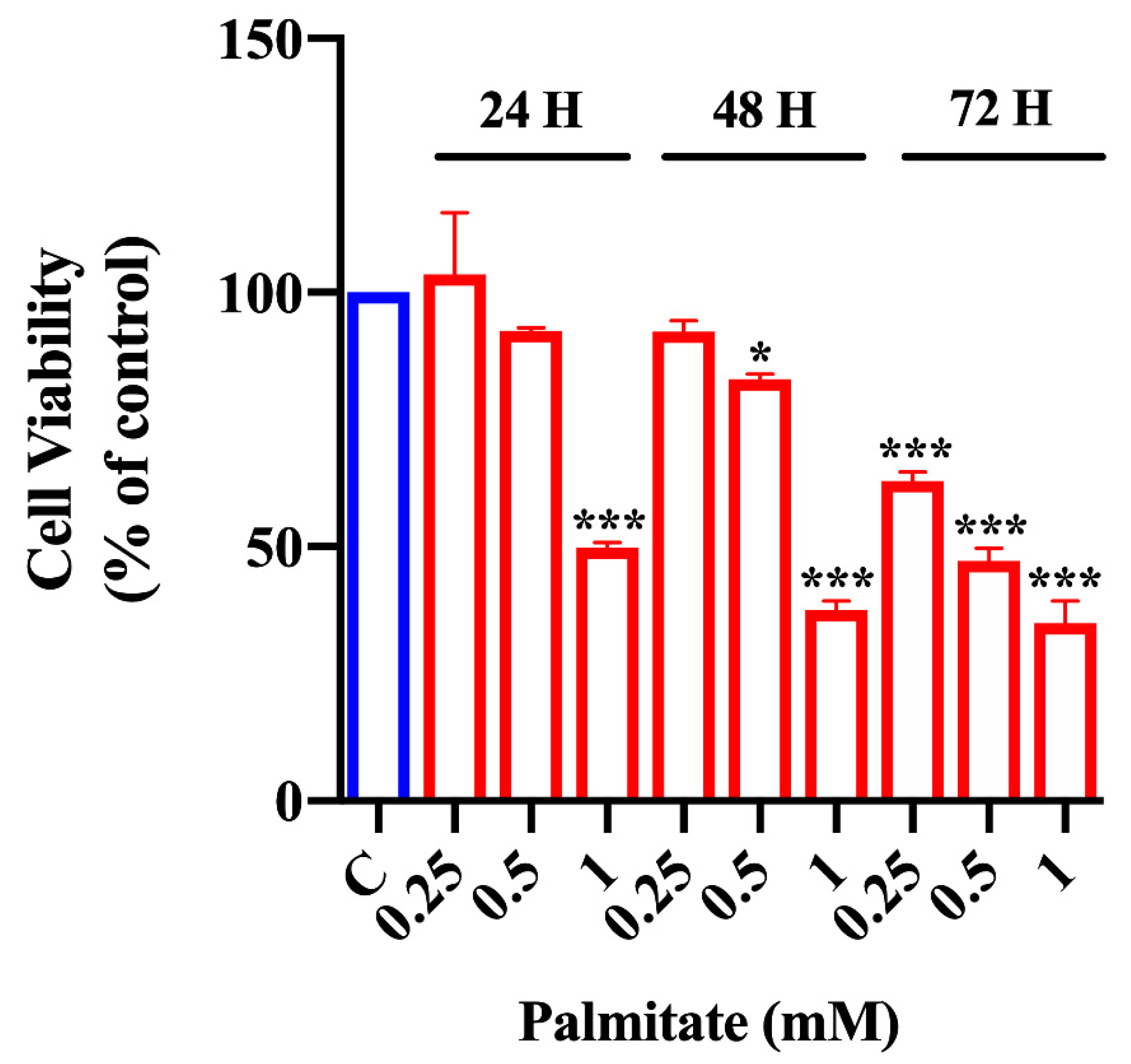
2.1. Effect of Palmitate Treatment on Organoid Viability
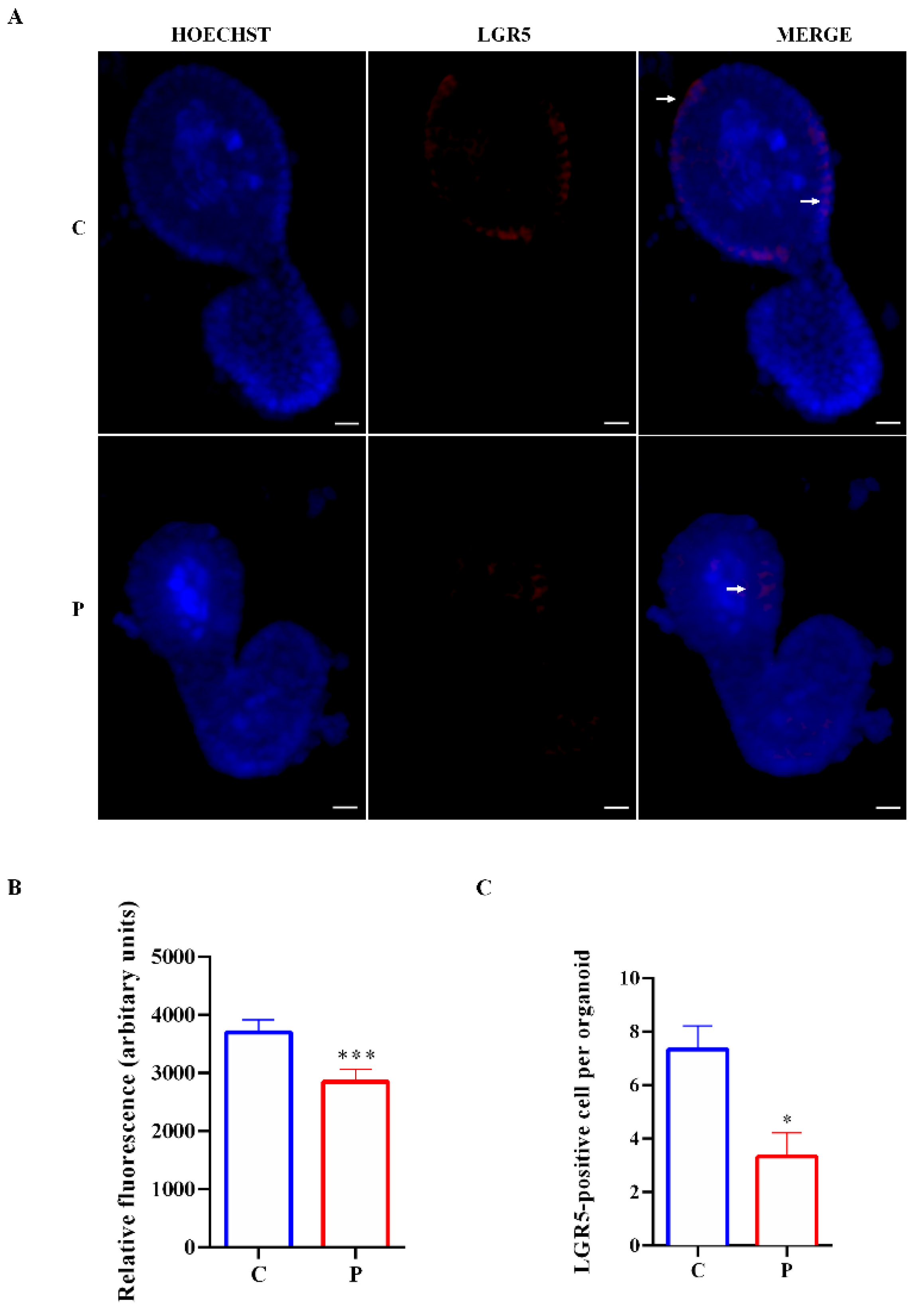
2.2. Effect of Palmitate Exposure on Morphology and Stemness Features of Organoids
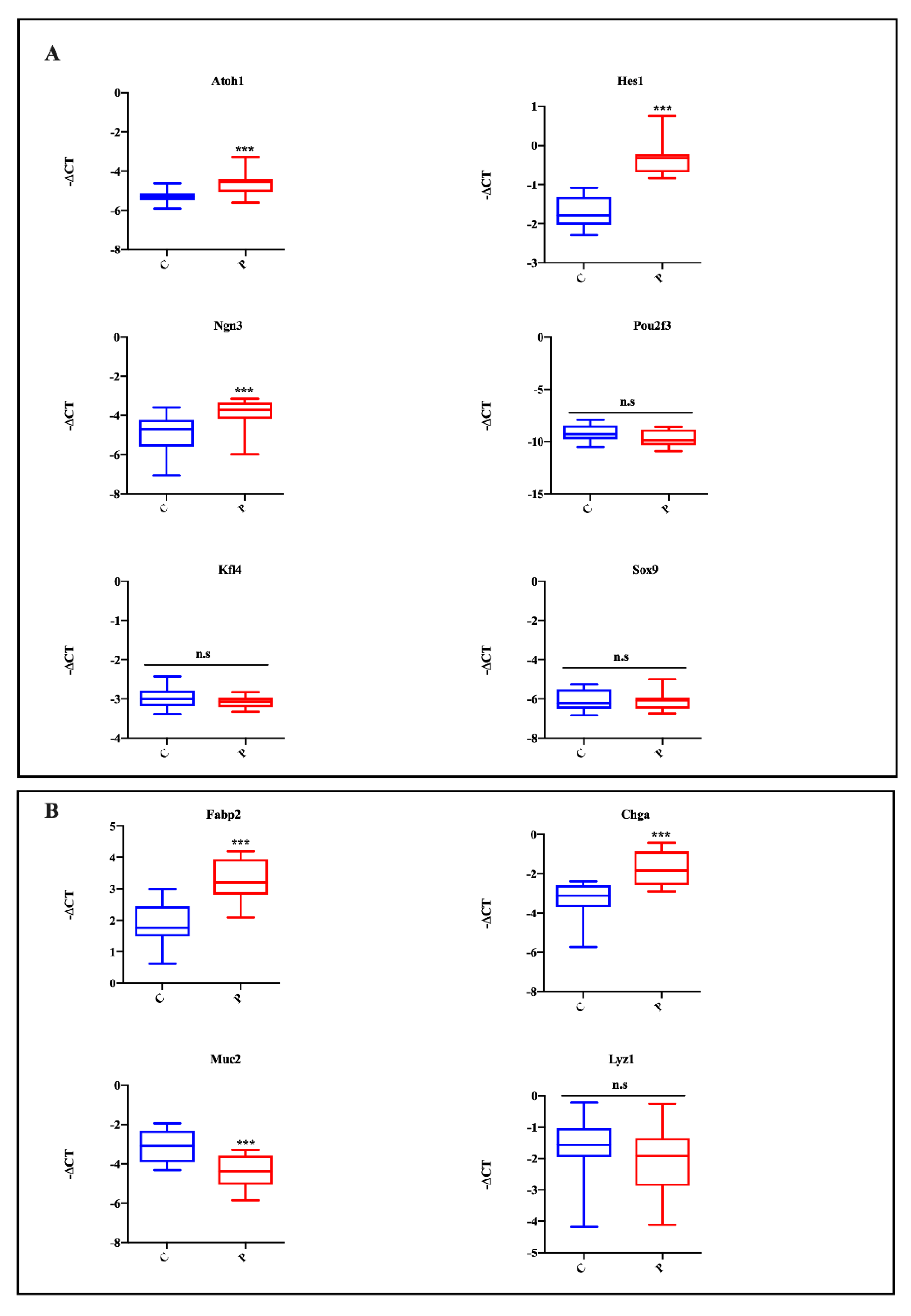
2.3. Effect of Lipotoxicity on Intestinal Cell Differentiation
2.4. Palmitate Treatment Impairs Intestinal Cell Fate in Organoids
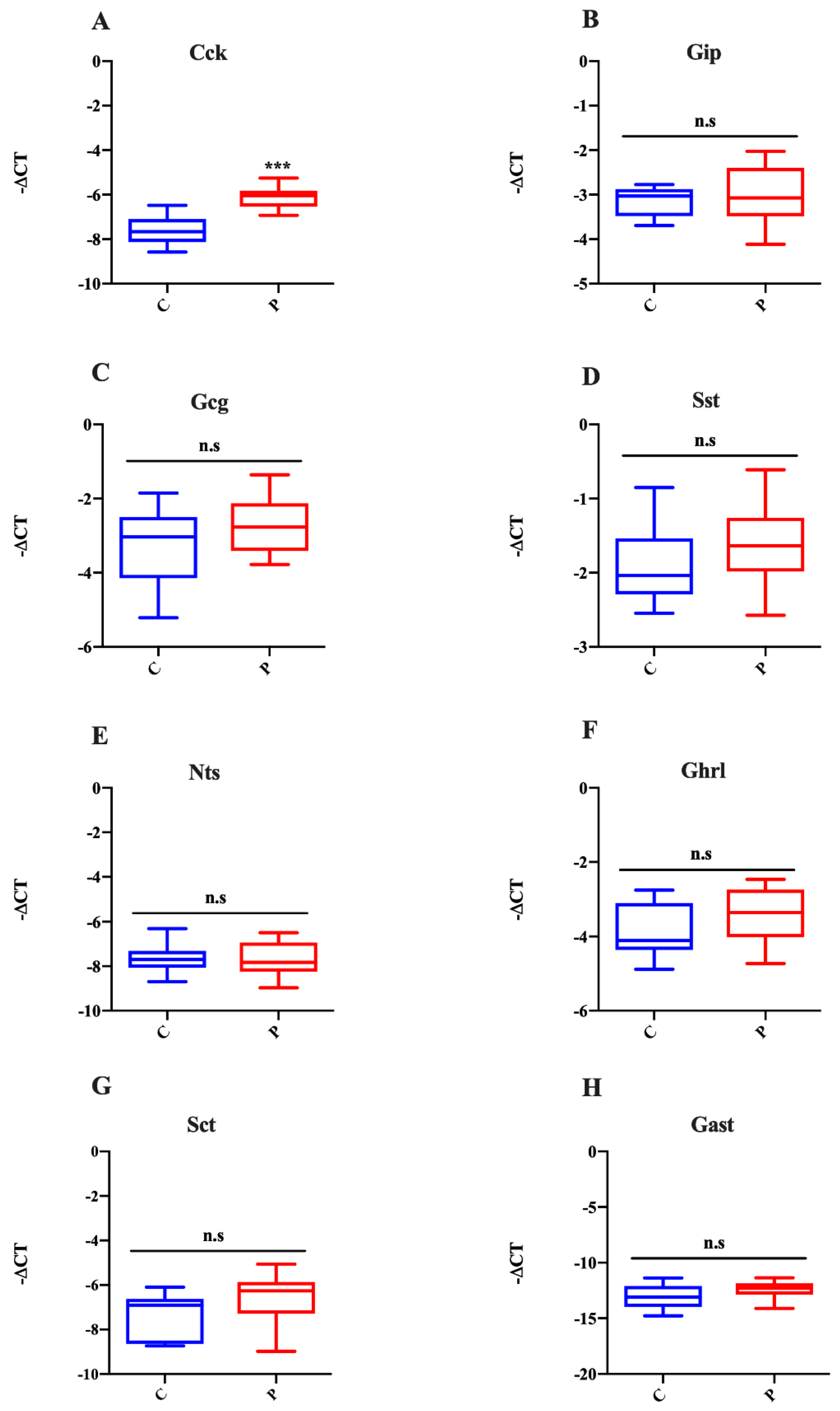
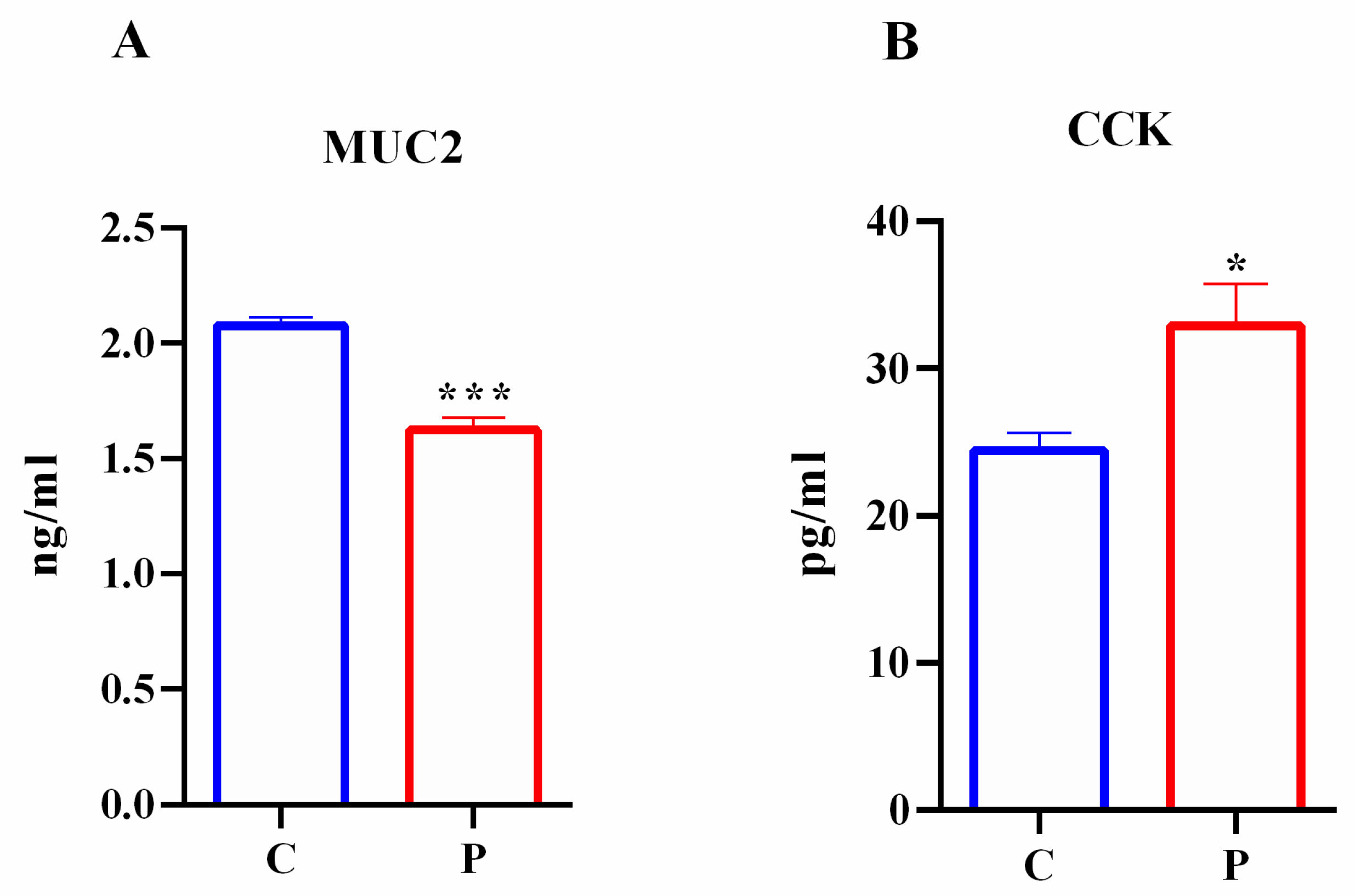
2.5. Effects of Palmitate Treatment on MUC2 and CCK Production in Intestinal Organoids
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intestinal Organoid Isolation and Culture
4.3. Chronic Exposure to Palmitate
4.4. MTT Assay
4.5. Analysis of Mouse Small Intestinal Organoid Growth Treated with Palmitate
4.6. Total RNA Extraction, Reverse Transcription and Quantitative PCR
4.7. Immunofluorescence
4.8. Elisa Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Escoula, Q.; Bellenger, S.; Narce, M.; Bellenger, J. Docosahexaenoic and Eicosapentaenoic Acids Prevent Altered-Muc2 Secretion Induced by Palmitic Acid by Alleviating Endoplasmic Reticulum Stress in LS174T Goblet Cells. Nutrients 2019, 11, 2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliluev, A.; Tritschler, S.; Sterr, M.; Oppenländer, L.; Hinterdobler, J.; Greisle, T.; Irmler, M.; Beckers, J.; Sun, N.; Walch, A.; et al. Diet-Induced Alteration of Intestinal Stem Cell Function Underlies Obesity and Prediabetes in Mice. Nat. Metab. 2021, 3, 1202–1216. [Google Scholar] [CrossRef]
- Shi, C.; Li, H.; Qu, X.; Huang, L.; Kong, C.; Qin, H.; Sun, Z.; Yan, X. High Fat Diet Exacerbates Intestinal Barrier Dysfunction and Changes Gut Microbiota in Intestinal-Specific ACF7 Knockout Mice. Biomed. Pharmacother. 2019, 110, 537–545. [Google Scholar] [CrossRef]
- Holst, J.J. Enteroendocrine Secretion of Gut Hormones in Diabetes, Obesity and after Bariatric Surgery. Curr. Opin. Pharmacol. 2013, 13, 983–988. [Google Scholar] [CrossRef]
- Boztepe, T.; Gulec, S. Investigation of the Influence of High Glucose on Molecular and Genetic Responses: An in Vitro Study Using a Human Intestine Model. Genes Nutr. 2018, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, H.; Zheng, M.; Yang, Y.; Ren, H.; Kong, Y.; Wang, S.; Wang, J.; Jiang, Y.; Yang, J.; et al. Berberine Slows the Progression of Prediabetes to Diabetes in Zucker Diabetic Fatty Rats by Enhancing Intestinal Secretion of Glucagon-Like Peptide-2 and Improving the Gut Microbiota. Front. Endocrinol. 2021, 12, 609134. [Google Scholar] [CrossRef]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine Cells-Sensory Sentinels of the Intestinal Environment and Orchestrators of Mucosal Immunity. Mucosal. Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Cell Fate Specification and Differentiation in the Adult Mammalian Intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef]
- Huang, C.-Z.; Xu, J.-H.; Zhong, W.; Xia, Z.-S.; Wang, S.-Y.; Cheng, D.; Li, J.-Y.; Wu, T.-F.; Chen, Q.-K.; Yu, T. Sox9 Transcriptionally Regulates Wnt Signaling in Intestinal Epithelial Stem Cells in Hypomethylated Crypts in the Diabetic State. Stem Cell Res. Ther. 2017, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wölnerhanssen, B.K.; Moran, A.W.; Burdyga, G.; Meyer-Gerspach, A.C.; Peterli, R.; Manz, M.; Thumshirn, M.; Daly, K.; Beglinger, C.; Shirazi-Beechey, S.P. Deregulation of Transcription Factors Controlling Intestinal Epithelial Cell Differentiation; a Predisposing Factor for Reduced Enteroendocrine Cell Number in Morbidly Obese Individuals. Sci. Rep. 2017, 7, 8174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, G.P.; Larraufie, P.; Richards, P.; Kay, R.G.; Galvin, S.G.; Miedzybrodzka, E.L.; Leiter, A.; Li, H.J.; Glass, L.L.; Ma, M.K.L.; et al. Comparison of Human and Murine Enteroendocrine Cells by Transcriptomic and Peptidomic Profiling. Diabetes 2019, 68, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Date, S.; Sato, T. Mini-Gut Organoids: Reconstitution of the Stem Cell Niche. Annu. Rev. Cell Dev. Biol. 2015, 31, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Tsakmaki, A.; Fonseca Pedro, P.; Bewick, G.A. 3D Intestinal Organoids in Metabolic Research: Virtual Reality in a Dish. Curr. Opin. Pharmacol. 2017, 37, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippello, A.; Di Mauro, S.; Scamporrino, A.; Malaguarnera, R.; Torrisi, S.A.; Leggio, G.M.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. High Glucose Exposure Impairs L-Cell Differentiation in Intestinal Organoids: Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 6660. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, J.-Y.; Park, K.-Y.; Lee, J. Lipotoxicity of Palmitic Acid on Neural Progenitor Cells and Hippocampal Neurogenesis. Toxicol. Res. 2011, 27, 103–110. [Google Scholar] [CrossRef]
- Cheon, H.G.; Cho, Y.S. Protection of Palmitic Acid-Mediated Lipotoxicity by Arachidonic Acid via Channeling of Palmitic Acid into Triglycerides in C2C12. J. Biomed. Sci. 2014, 21, 13. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, S.; Ragusa, M.; Urbano, F.; Filippello, A.; Di Pino, A.; Scamporrino, A.; Pulvirenti, A.; Ferro, A.; Rabuazzo, A.M.; Purrello, M.; et al. Intracellular and Extracellular MiRNome Deregulation in Cellular Models of NAFLD or NASH: Clinical Implications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1129–1139. [Google Scholar] [CrossRef]
- Filippello, A.; Urbano, F.; Di Mauro, S.; Scamporrino, A.; Di Pino, A.; Scicali, R.; Rabuazzo, A.M.; Purrello, F.; Piro, S. Chronic Exposure to Palmitate Impairs Insulin Signaling in an Intestinal L-Cell Line: A Possible Shift from GLP-1 to Glucagon Production. Int. J. Mol. Sci. 2018, 19, 3791. [Google Scholar] [CrossRef] [Green Version]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, L.; Petersen, R.K.; Kristiansen, K. Regulation of Adipocyte Differentiation and Function by Polyunsaturated Fatty Acids. Biochim. Biophys. Acta 2005, 1740, 266–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachtsis, B.; Whitfield, J.; Hawley, J.A.; Hoffman, N.J. Omega-3 Polyunsaturated Fatty Acids Mitigate Palmitate-Induced Impairments in Skeletal Muscle Cell Viability and Differentiation. Front. Physiol. 2020, 11, 563. [Google Scholar] [CrossRef]
- Glenske, K.; Schäpe, K.; Wieck, A.; Failing, K.; Werner, J.; Rohnke, M.; Wenisch, S.; Mazurek, S. Effect of Long Term Palmitate Treatment on Osteogenic Differentiation of Human Mesenchymal Stromal Cells-Impact of Albumin. Bone Rep. 2020, 13, 100707. [Google Scholar] [CrossRef] [PubMed]
- Piro, S.; Anello, M.; Di Pietro, C.; Lizzio, M.N.; Patan, G.; Rabuazzo, A.M.; Vigneri, R.; Purrello, M.; Purrello, F. Chronic Exposure to Free Fatty Acids or High Glucose Induces Apoptosis in Rat Pancreatic Islets: Possible Role of Oxidative Stress. Metabolism 2002, 51, 1340–1347. [Google Scholar] [CrossRef]
- Pang, J.; Xi, C.; Jin, J.; Han, Y.; Zhang, T. Relative Quantitative Comparison between Lipotoxicity and Glucotoxicity Affecting the PARP-NAD-SIRT1 Pathway in Hepatocytes. Cell Physiol. Biochem. 2013, 32, 719–727. [Google Scholar] [CrossRef]
- Vasu, S.; Moffett, R.C.; McClenaghan, N.H.; Flatt, P.R. Differential Molecular and Cellular Responses of GLP-1 Secreting L-Cells and Pancreatic Alpha Cells to Glucotoxicity and Lipotoxicity. Exp. Cell Res. 2015, 336, 100–108. [Google Scholar] [CrossRef]
- Tsakmaki, A.; Fonseca Pedro, P.; Bewick, G.A. Diabetes through a 3D Lens: Organoid Models. Diabetologia 2020, 63, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Kasendra, M.; Troutt, M.; Broda, T.; Bacon, W.C.; Wang, T.C.; Niland, J.C.; Helmrath, M.A. Intestinal Organoids: Roadmap to the Clinic. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G1–G10. [Google Scholar] [CrossRef]
- Efiana, N.A.; Dizdarević, A.; Huck, C.W.; Bernkop-Schnürch, A. Improved Intestinal Mucus Permeation of Vancomycin via Incorporation Into Nanocarrier Containing Papain-Palmitate. J. Pharm. Sci. 2019, 108, 3329–3339. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty Acid-Binding Proteins: Role in Metabolic Diseases and Potential as Drug Targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, G.; Han, L.; Zhao, K.; Qu, Y.; Xu, A.; Huang, Q. Association of the FABP2 Ala54Thr Polymorphism with Type 2 Diabetes, Obesity, and Metabolic Syndrome: A Population-Based Case-Control Study and a Systematic Meta-Analysis. Genet. Mol. Res. 2015, 14, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Han, T.-K.; So, W.-Y. Effects of FABP2 Ala54Thr Gene Polymorphism on Obesity and Metabolic Syndrome in Middle-Aged Korean Women with Abdominal Obesity. Cent. Eur. J. Public Health 2019, 27, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.J.; Liu, Y.P.; Qin, H.K.; Xing, T.; Li, S.S.; Bao, Y.Y. Effects of Polymorphism in FABP2 Ala54Thr on Serum Lipids and Glycemic Control in Low Glycemic Index Diets Are Associated with Gender among Han Chinese with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2019, 12, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Gajda, A.M.; Zhou, Y.X.; Agellon, L.B.; Fried, S.K.; Kodukula, S.; Fortson, W.; Patel, K.; Storch, J. Direct Comparison of Mice Null for Liver or Intestinal Fatty Acid-Binding Proteins Reveals Highly Divergent Phenotypic Responses to High Fat Feeding. J. Biol. Chem. 2013, 288, 30330–30344. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, S.; Arnold, M.; Diaz, A.A.; Neubauer, H.; Wolfrum, S.; Köfeler, H.; Langhans, W.; Krieger, J.-P. Roux-En-Y Gastric Bypass Surgery Reprograms Enterocyte Triglyceride Metabolism and Postprandial Secretion in Rats. Mol. Metab. 2019, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. The Biology of Mucus: Composition, Synthesis and Organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal. Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Heazlewood, C.K.; Cook, M.C.; Eri, R.; Price, G.R.; Tauro, S.B.; Taupin, D.; Thornton, D.J.; Png, C.W.; Crockford, T.L.; Cornall, R.J.; et al. Aberrant Mucin Assembly in Mice Causes Endoplasmic Reticulum Stress and Spontaneous Inflammation Resembling Ulcerative Colitis. PLoS Med. 2008, 5, e54. [Google Scholar] [CrossRef] [Green Version]
- Giraldez, M.D.; Carneros, D.; Garbers, C.; Rose-John, S.; Bustos, M. New Insights into IL-6 Family Cytokines in Metabolism, Hepatology and Gastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Koop, I.; Schindler, M.; Bosshammer, A.; Scheibner, J.; Stange, E.; Koop, H. Physiological Control of Cholecystokinin Release and Pancreatic Enzyme Secretion by Intraduodenal Bile Acids. Gut 1996, 39, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, V.; Flatt, P.R.; Irwin, N. Cholecystokinin (CCK) and Related Adjunct Peptide Therapies for the Treatment of Obesity and Type 2 Diabetes. Peptides 2018, 100, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, B.; Radzikowska, M.; Wasilewska-Dziubinska, E.; Roguski, K.; Borowiec, M. Disturbed Release of Gastrointestinal Peptides in Anorexia Nervosa and in Obesity. Diabetes Obes. Metab. 2000, 2, 99–103. [Google Scholar] [CrossRef]
- Brennan, I.M.; Luscombe-Marsh, N.D.; Seimon, R.V.; Otto, B.; Horowitz, M.; Wishart, J.M.; Feinle-Bisset, C. Effects of Fat, Protein, and Carbohydrate and Protein Load on Appetite, Plasma Cholecystokinin, Peptide YY, and Ghrelin, and Energy Intake in Lean and Obese Men. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G129–G140. [Google Scholar] [CrossRef]
- Sirchak, Y.S.; Stan, M.P.; Brych, V.V. Changes in Cholecystokinin Level in Patients with Gastroesophageal Reflux Disease on the Background of Type II Diabetes. Wiad. Lek. 2018, 71, 333–336. [Google Scholar]
- Chearskul, S.; Delbridge, E.; Shulkes, A.; Proietto, J.; Kriketos, A. Effect of Weight Loss and Ketosis on Postprandial Cholecystokinin and Free Fatty Acid Concentrations. Am. J. Clin. Nutr. 2008, 87, 1238–1246. [Google Scholar] [CrossRef] [Green Version]
- Weickert, M.O.; Möhlig, M.; Spranger, J.; Schöfl, C.; Loeffelholz, C.V.; Riepl, R.L.; Otto, B.; Pfeiffer, A.F.H. Effects of Euglycemic Hyperinsulinemia and Lipid Infusion on Circulating Cholecystokinin. J. Clin. Endocrinol. Metab. 2008, 93, 2328–2333. [Google Scholar] [CrossRef] [Green Version]
- Koop, I. Regulation of CCK Release by Bile Acids. In Cholecystokinin Antagonists in Gastroenterology; Adler, G., Beglinger, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 152–158. ISBN 978-3-642-76364-9. [Google Scholar]
- Lund, M.L.; Sorrentino, G.; Egerod, K.L.; Kroone, C.; Mortensen, B.; Knop, F.K.; Reimann, F.; Gribble, F.M.; Drucker, D.J.; de Koning, E.J.P.; et al. L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling. Diabetes 2020, 69, 614–623. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef]
- Yilmaz, Ö.H.; Katajisto, P.; Lamming, D.W.; Gültekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birsoy, K.; Dursun, A.; Yilmaz, V.O.; Selig, M.; et al. MTORC1 in the Paneth Cell Niche Couples Intestinal Stem-Cell Function to Calorie Intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jatko, J.T.; Darling, C.L.; Kellett, M.P.; Bain, L.J. Arsenic Exposure in Drinking Water Reduces Lgr5 and Secretory Cell Marker Gene Expression in Mouse Intestines. Toxicol. Appl. Pharmacol. 2021, 422, 115561. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Singh, M.; Wu, Q.; Gentillon, C.; Preininger, M.K.; Xu, C. Downregulation of LGR5 Expression Inhibits Cardiomyocyte Differentiation and Potentiates Endothelial Differentiation from Human Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 513–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez Vallone, V.; Leprovots, M.; Ribatallada-Soriano, D.; Gerbier, R.; Lefort, A.; Libert, F.; Vassart, G.; Garcia, M.-I. LGR5 Controls Extracellular Matrix Production by Stem Cells in the Developing Intestine. EMBO Rep. 2020, 21, e49224. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Salomone, S.; Geraci, F.; Caraci, F.; Bucolo, C.; Drago, F.; Leggio, G.M. Buspirone Counteracts MK-801-Induced Schizophrenia-Like Phenotypes through Dopamine D3 Receptor Blockade. Front. Pharmacol. 2017, 8, 710. [Google Scholar] [CrossRef]
- Leggio, G.M.; Di Marco, R.; Gulisano, W.; D’Ascenzo, M.; Torrisi, S.A.; Geraci, F.; Lavanco, G.; Dahl, K.; Giurdanella, G.; Castorina, A.; et al. Dopaminergic-GABAergic Interplay and Alcohol Binge Drinking. Pharmacol. Res. 2019, 141, 384–391. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Filippello, A.; Scamporrino, A.; Di Mauro, S.; Malaguarnera, R.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules 2020, 10, 1404. [Google Scholar] [CrossRef]
- Grabinger, T.; Luks, L.; Kostadinova, F.; Zimberlin, C.; Medema, J.P.; Leist, M.; Brunner, T. Ex Vivo Culture of Intestinal Crypt Organoids as a Model System for Assessing Cell Death Induction in Intestinal Epithelial Cells and Enteropathy. Cell Death Dis. 2014, 5, e1228. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, S.; Scamporrino, A.; Petta, S.; Urbano, F.; Filippello, A.; Ragusa, M.; Di Martino, M.T.; Scionti, F.; Grimaudo, S.; Pipitone, R.M.; et al. Serum Coding and Non-Coding RNAs as Biomarkers of NAFLD and Fibrosis Severity. Liver Int. 2019, 39, 1742–1754. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, S.; Salomone, F.; Scamporrino, A.; Filippello, A.; Morisco, F.; Guido, M.; Lembo, V.; Cossiga, V.; Pipitone, R.M.; Grimaudo, S.; et al. Coffee Restores Expression of LncRNAs Involved in Steatosis and Fibrosis in a Mouse Model of NAFLD. Nutrients 2021, 13, 2952. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Scamporrino, A.; Fruciano, M.; Filippello, A.; Fagone, E.; Gili, E.; Scionti, F.; Purrazzo, G.; Di Pino, A.; Scicali, R.; et al. Circulating Coding and Long Non-Coding RNAs as Potential Biomarkers of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 8812. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippello, A.; Di Mauro, S.; Scamporrino, A.; Torrisi, S.A.; Leggio, G.M.; Di Pino, A.; Scicali, R.; Di Marco, M.; Malaguarnera, R.; Purrello, F.; et al. Molecular Effects of Chronic Exposure to Palmitate in Intestinal Organoids: A New Model to Study Obesity and Diabetes. Int. J. Mol. Sci. 2022, 23, 7751. https://doi.org/10.3390/ijms23147751
Filippello A, Di Mauro S, Scamporrino A, Torrisi SA, Leggio GM, Di Pino A, Scicali R, Di Marco M, Malaguarnera R, Purrello F, et al. Molecular Effects of Chronic Exposure to Palmitate in Intestinal Organoids: A New Model to Study Obesity and Diabetes. International Journal of Molecular Sciences. 2022; 23(14):7751. https://doi.org/10.3390/ijms23147751
Chicago/Turabian StyleFilippello, Agnese, Stefania Di Mauro, Alessandra Scamporrino, Sebastiano Alfio Torrisi, Gian Marco Leggio, Antonino Di Pino, Roberto Scicali, Maurizio Di Marco, Roberta Malaguarnera, Francesco Purrello, and et al. 2022. "Molecular Effects of Chronic Exposure to Palmitate in Intestinal Organoids: A New Model to Study Obesity and Diabetes" International Journal of Molecular Sciences 23, no. 14: 7751. https://doi.org/10.3390/ijms23147751






