The Chaperone System in Breast Cancer: Roles and Therapeutic Prospects of the Molecular Chaperones Hsp27, Hsp60, Hsp70, and Hsp90
Abstract
:1. Introduction
2. The Biology and Heterogeneity of BC
3. Quantitative Levels and Functions of Hsp27, Hsp60, Hsp70, and Hsp90 in BC
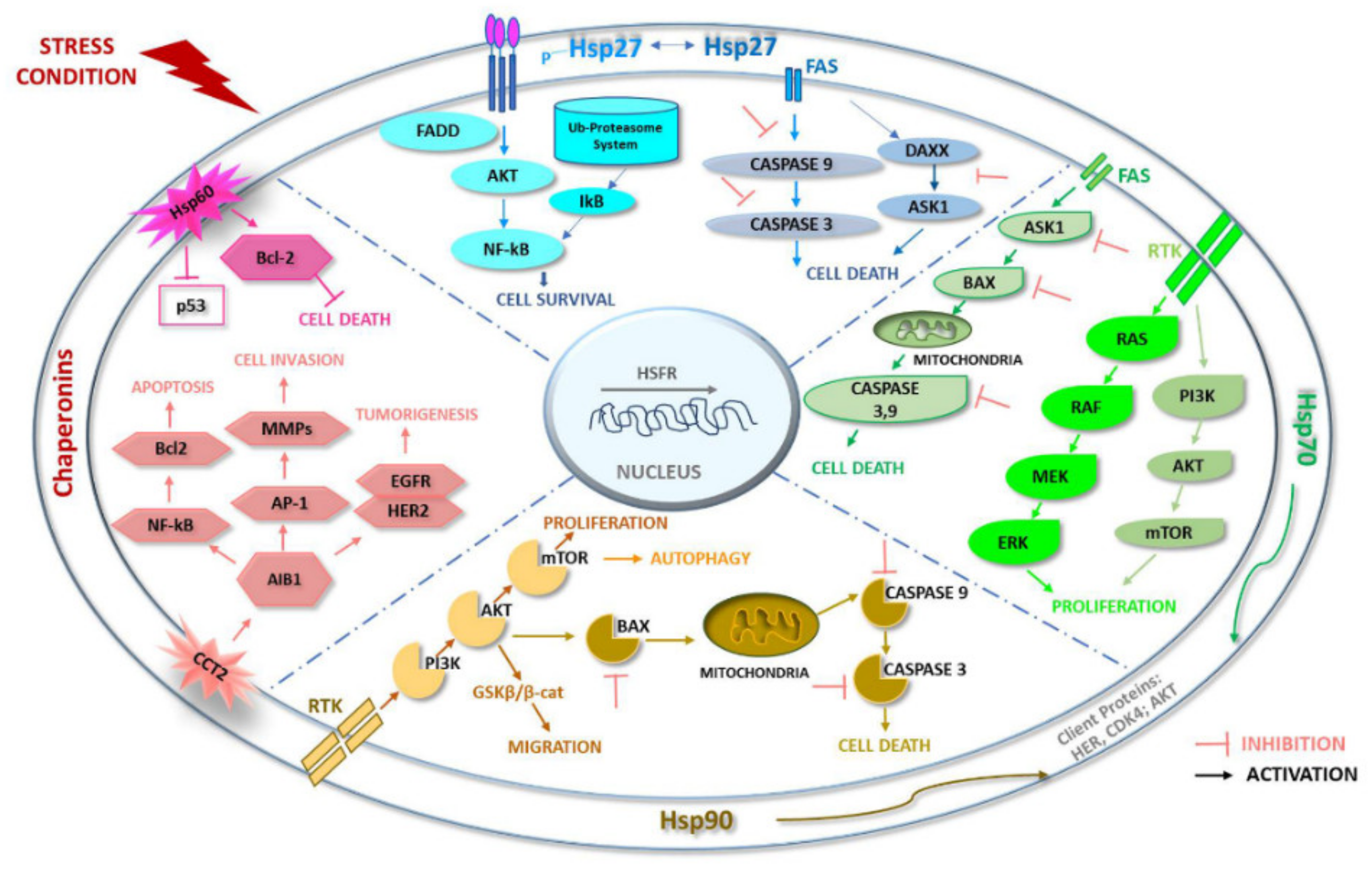
3.1. Hsp27
3.2. Hsp60
3.3. Hsp70
3.4. Hsp90
4. Hsp27, Hsp60, Hsp70, and Hsp90 in BC Therapeutics: Negative Chaperonotherapy
4.1. EVs
4.2. Hsp Inhibitors
5. Hsps in BC Immunotherapy
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | atypical ductal hyperplasia |
| BC | breast cancer |
| BCSCs | breast cancer stem cells |
| BT-474 | HER2-positive cell lines |
| CCT | Chaperonin-containing TCP1 |
| CDK | cyclin-dependent kinase |
| CLCSI | classical LCIS |
| CMA | chaperone-mediated autophagy |
| CS | chaperone system |
| CTCs | circulating tumor cells |
| DCIS | ductal carcinoma in situ |
| EMT | endothelial-to-mesenchymal transition |
| ER | estrogen receptor |
| EVs | extracellular vesicles |
| FLCSI | florid FLCIS |
| gold (III) complex | Au (TPP)Cl |
| 17-DMAG | Alvespimycin |
| IPI-504 | Retaspimycin |
| Ganetespib | 5-(2,4-dihydroxy-5-(1-methylethyl)phenyl)-4-(1-methyl-1H-indol-5-yl)-2,4-dihydro-(1,2,4)triazol-3-one |
| HER2 | human epidermal growth factor 2 receptor |
| HSPPCs | Hsp-peptide complexes |
| HSP70.PC-F | Hsp70.peptide complexes from fusion cells |
| HSF1 | heat-shock factors 1 |
| Hsps | Heat shock proteins |
| IDC | invasive ductal carcinoma |
| ILC | invasive lobular carcinoma |
| LCIS | lobular carcinoma in situ |
| MCF-7 | ER-positive cell line |
| MDA-MB-231 | triple negative human breast cancer cells |
| miRNAs | microRNA |
| NVP-AUY922 | resorcinol derivative |
| PLCIS | pleomorphic LCIS |
| PR | progesterone receptor |
| PU-H71 | purine derivative |
| SK-BR-3 | HER2-positive cell lines |
| UPS | Ubiquitin-proteasome system |
| TNBC | Triple negative human breast cancer cells |
| TSAs | tumor-specific antigens |
| TZMB | Trastuzumab |
| 17AAG | 17-N-allylamino-17-demethoxygeldanamycin or tanespimycin |
References
- Macario, A.J.L.; Conway de Macario, E. Chaperone Proteins and Chaperonopathies. In Stress: Physiology, Biochemistry, and Pathology Handbook of Stress Series; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 3, Chapter 12; pp. 135–152. [Google Scholar]
- Alberti, G.; Campanella, C.; Paladino, L.; Porcasi, R.; Bavisotto, C.C.; Pitruzzella, A.; Graziano, F.; Florena, A.M.; Argo, A.; Conway de Macario, E.; et al. The chaperone system in glioblastoma multiforme and derived cell lines: Diagnostic and mechanistic implications. Front. Biosci. 2022, 27, 97. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Unti, E.; Baiamonte, P.; Cappello, F.; Scibetta, N. Different Immunohistochemical Levels of Hsp60 and Hsp70 in a Subset of Brain Tumors and Putative Role of Hsp60 in Neuroepithelial Tumorigenesis. Eur. J. Histochem. 2013, 57, 124–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roulot, A.; Héquet, D.; Guinebretière, J.M.; Vincent-Salomon, A.; Lerebours, F.; Dubot, C.; Rouzier, R. Tumoral Heterogeneity of Breast Cancer. Ann. Biol. Clin. 2016, 74, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.M.; Henson, D.E.; Chen, D.; Rajamarthandan, S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: A study of 161 708 cases of breast cancer from the SEER Program. Arch. Pathol. Lab. Med. 2014, 138, 1048–1052. [Google Scholar] [CrossRef] [Green Version]
- Vohra, P.; Buelow, B.; Chen, Y.Y.; Serrano, M.; Vohra, M.S.; Berry, A.; Ljung, B.M. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression in breast cancer FNA cell blocks and paired histologic specimens: A large retrospective study. Cancer Cytopathol. 2016, 124, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Macario, A.J.L.; Conway de Macario, E.; Cappello, F. The Chaperonopathies. Diseases with Defective Molecular Chaperones; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2013. [Google Scholar]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the Nomenclature of the Human Heat Shock Proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Macario, A.J.L.; Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005, 353, 1489–1501. [Google Scholar] [CrossRef]
- Wawrzynow, B.; Zylicz, A.; Zylicz, M. Chaperoning the Guardian of the Genome. The Two-Faced Role of Molecular Chaperones in P53 Tumor Suppressor Action. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 161–174. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Buttacavoli, M.; di Cara, G.; D’amico, C.; Geraci, F.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches. Biology 2021, 10, 247. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; and Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Byler, S.; Goldgar, S.; Heerboth, S.; Leary, M.; Housman, G.; Moulton, K.; Sarkar, S. Genetic and Epigenetic Aspects of Breast Cancer Progression and Therapy. Anticancer Res. 2014, 34, 1071–1077. [Google Scholar]
- Kuncman, W.; Orzechowska, M.; Kuncman, Ł.; Kordek, R.; Taran, K. Intertumoral Heterogeneity of Primary Breast Tumors and Synchronous Axillary Lymph Node Metastases Reflected in IHC-Assessed Expression of Routine and Nonstandard Biomarkers. Front. Oncol. 2021, 11, 660318. [Google Scholar] [CrossRef]
- IARC Publications Website. WHO Classification of Tumours of the Breast. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-The-Breast-2012 (accessed on 13 April 2022).
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/Neu Oncogene. Science 1987, 235, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.T.; Oltra, S.S.; Peña-Chilet, M.; Alonso, E.; Hernando, C.; Burgues, O.; Chirivella, I.; Bermejo, B.; Lluch, A.; Ribas, G. Breast Cancer in Very Young Patients in a Spanish Cohort: Age as an Independent Bad Prognostic Indicator. Breast Cancer 2019, 13, 1178223419828766. [Google Scholar] [CrossRef] [Green Version]
- Coyne, J.D. DCIS and LCIS with Multinucleated Giant Cells-a Report of 4 Cases. Histopathology 2007, 50, 669–671. [Google Scholar] [CrossRef]
- Gorringe, K.L.; Fox, S.B. Ductal Carcinoma In Situ Biology, Biomarkers, and Diagnosis. Front. Oncol. 2017, 7, 248. [Google Scholar] [CrossRef] [Green Version]
- Strehl, J.D.; Wachter, D.L.; Fasching, P.A.; Beckmann, M.W.; Hartmann, A. Invasive Breast Cancer: Recognition of Molecular Subtypes. Breast Care 2011, 6, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Fulga, V.; Rudico, L.; Balica, A.R.; Cimpean, A.M.; Saptefrati, L.; Raica, M. Invasive ductal carcinoma of no special type and its corresponding lymph node metastasis: Do they have the same immunophenotypic profile? Pol. J. Pathol. 2015, 66, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Li, C.I.; Anderson, B.O.; Daling, J.R.; Moe, R.E. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003, 289, 1421–1424. [Google Scholar] [CrossRef] [Green Version]
- Rakha, E.A.; Gill, M.S.; El-Sayed, M.E.; Khan, M.M.; Hodi, Z.; Blamey, R.W.; Evans, A.J.; Lee, A.H.; Ellis, I.O. The biological and clinical characteristics of breast carcinoma with mixed ductal and lobular morphology. Breast Cancer Res. Treat. 2009, 114, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Pipa, J.; Shin, S.J. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am. J. Surg. Pathol. 2014, 38, 434–435. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and omics. Breast Cancer Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Teo, K.; Gómez-Cuadrado, L.; Tenhagen, M.; Byron, A.; Rätze, M.; van Amersfoort, M.; Renes, J.; Strengman, E.; Mandoli, A.; Singh, A.A.; et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci. Rep. 2018, 8, 15454. [Google Scholar] [CrossRef] [Green Version]
- Nagle, A.M.; Levine, K.M.; Tasdemir, N.; Scott, J.A.; Burlbaugh, K.; Kehm, J.; Katz, T.A.; Boone, D.N.; Jacobsen, B.M.; Atkinson, J.M.; et al. Loss of E-cadherin Enhances IGF1-IGF1R Pathway Activation and Sensitizes Breast Cancers to Anti-IGF1R/InsR Inhibitors. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 2018, 24, 5165–5177. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. WHO Classification of Tumours Editorial Board. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive Lobular Carcinoma of the Breast: The Increasing Importance of This Special Subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef]
- Logan, G.J.; Dabbs, D.J.; Lucas, P.C.; Jankowitz, R.C.; Brown, D.D.; Clark, B.Z.; Oesterreich, S.; McAuliffe, P.F. Molecular Drivers of Lobular Carcinoma in Situ. Breast Cancer Res. 2015, 17, 76. [Google Scholar] [CrossRef] [Green Version]
- Ginter, P.S.; D’Alfonso, T.M. Current Concepts in Diagnosis, Molecular Features, and Management of Lobular Carcinoma In Situ of the Breast With a Discussion of Morphologic Variants. Arch. Pathol. Lab. Med. 2017, 141, 1668–1678. [Google Scholar] [CrossRef] [Green Version]
- Mymrikov, E.V.; Daake, M.; Richter, B.; Haslbeck, M.; Buchner, J. The Chaperone Activity and Substrate Spectrum of Human Small Heat Shock Proteins. J. Biol. Chem. 2017, 292, 672–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudnitsyna, M.V.; Sluchanko, N.N.; Gusev, N.B. The Big Book on Small Heat Shock Proteins; Springer: Berlin/Heidelberg, Germany, 2015; Volume 8. [Google Scholar]
- Nosareva, O.L.; Ryazantseva, N.V.; Stepovaya, E.A.; Shakhristova, E.V.; Stepanova, E.A.; Gulaya, V.S. The Role of Heat Shock Proteins 27 and 70 in Redox-Dependent Regulation of Apoptosis in Jurkat Tumor Cells. Biomed. Khim. 2016, 62, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Barna, J.; Csermely, P.; Vellai, T. Roles of Heat Shock Factor 1 beyond the Heat Shock Response. Cell Mol. Life Sci. 2018, 75, 2897–2916. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Mammalian HspB1 (Hsp27) Is a Molecular Sensor Linked to the Physiology and Environment of the Cell. Cell Stress Chaperones 2017, 22, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drexler, R.; Wagner, K.C.; Küchler, M.; Feyerabend, B.; Kleine, M.; Oldhafer, K.J. Significance of Unphosphorylated and Phosphorylated Heat Shock Protein 27 as a Prognostic Biomarker in Pancreatic Ductal Adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 1125–1137. [Google Scholar] [CrossRef] [Green Version]
- Kaigorodova, E.V.; Zavyalova, M.V.; Bogatyuk, M.V.; Tarabanovskaya, N.A.; Slonimskaya, E.M.; Perelmuter, V.M. Relationship between the Expression of Phosphorylated Heat Shock Protein Beta-1 with Lymph Node Metastases of Breast Cancer. Cancer Biomark. 2015, 15, 143–150. [Google Scholar] [CrossRef]
- McDonald, E.T.; Bortolus, M.; Koteiche, H.A.; McHaourab, H.S. Sequence, Structure, and Dynamic Determinants of Hsp27 (HspB1) Equilibrium Dissociation Are Encoded by the N-Terminal Domain. Biochemistry 2012, 51, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Konda, J.D.; Olivero, M.; Musiani, D.; Lamba, S.; di Renzo, M.F. Heat-Shock Protein 27 (HSP27, HSPB1) Is Synthetic Lethal to Cells with Oncogenic Activation of MET, EGFR and BRAF. Mol. Oncol. 2017, 11, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Mojtahedi, Z.; Safaei, A.; Yousefi, Z.; Ghaderi, A. Immunoproteomics of HER2-Positive and HER2-Negative Breast Cancer Patients with Positive Lymph Nodes. OMICS 2011, 15, 409–418. [Google Scholar] [CrossRef]
- Díaz-Chávez, J.; Fonseca-Sánchez, M.A.; Arechaga-Ocampo, E.; Flores-Pérez, A.; Palacios-Rodríguez, Y.; Domínguez-Gómez, G.; Marchat, L.A.; Fuentes-Mera, L.; Mendoza-Hernández, G.; Gariglio, P.; et al. Proteomic Profiling Reveals That Resveratrol Inhibits HSP27 Expression and Sensitizes Breast Cancer Cells to Doxorubicin Therapy. PLoS ONE 2013, 8, e64378. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.Y.; Choi, S.K.; Seo, S.H.; Jo, H.; Shin, J.H.; Na, Y.; Lee, Y.S.; Kwon, Y. Specific Roles of HSP27 S15 Phosphorylation Augmenting the Nuclear Function of HER2 to Promote Trastuzumab Resistance. Cancers 2020, 12, 1540. [Google Scholar] [CrossRef]
- Zhang, D.; Wong, L.; Koay, E.S.C. Phosphorylation of Ser78 of Hsp27 Correlated with HER-2/Neu Status and Lymph Node Positivity in Breast Cancer. Mol. Cancer 2007, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, M.A.; Cuello Carrión, F.D.; Dekker, J.; Schoemaker, J.; Ciocca, D.R. Serological Detection of Heat Shock Protein Hsp27 in Normal and Breast Cancer Patients. Cancer Epidemiol. Biomark. Prev. 1998, 7, 791–795. [Google Scholar]
- Zhao, R.; Ji, J.G.; Tong, Y.P.; Pu, H.; Ru, B.G. Use of Serological Proteomic Methods to Find Biomarkers Associated with Breast Cancer. Proteomics 2003, 3, 433–439. [Google Scholar] [CrossRef]
- Gibert, B.; Eckel, B.; Gonin, V.; Goldschneider, D.; Fombonne, J.; Deux, B.; Mehlen, P.; Arrigo, A.P.; Clézardin, P.; Diaz-Latoud, C. Targeting Heat Shock Protein 27 (HspB1) InterfeRes. with Bone Metastasis and Tumour Formation in Vivo. Br. J. Cancer 2012, 107, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Idowu, M.O.; Kmieciak, M.; Dumur, C.; Burton, R.S.; Grimes, M.M.; Powers, C.N.; Manjili, M.H. CD44(+)/CD24(-/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum. Pathol. 2012, 43, 364–373. [Google Scholar] [CrossRef]
- Wei, L.; Liu, T.T.; Wang, H.H.; Hong, H.M.; Yu, A.L.; Feng, H.P.; Chang, W.W. Hsp27 Participates in the Maintenance of Breast Cancer Stem Cells through Regulation of Epithelial-Mesenchymal Transition and Nuclear Factor-ΚB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, X.; Wang, H.; Wang, Y.; Chen, P.; Wang, L. Heat Shock Protein 27 Enhances SUMOylation of Heat Shock Protein B8 to Accelerate the Progression of Breast Cancer. Am. J. Pathol. 2020, 190, 2464–2477. [Google Scholar] [CrossRef]
- Liu, X.; Feng, C.; Liu, J.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Jiao, J.; Niu, Y. Androgen Receptor and Heat Shock Protein 27 Co-Regulate the Malignant Potential of Molecular Apocrine Breast Cancer. J. Exp. Clin. Cancer Res. 2018, 37, 90. [Google Scholar] [CrossRef]
- Straume, O.; Shimamura, T.; Lampa, M.J.G.; Carretero, J.; Jia, D.; Borgman, C.L.; Soucheray, M.; Downing, S.R.; Short, S.M.; Kang, S.Y.; et al. Suppression of Heat Shock Protein 27 Induces Long-Term Dormancy in Human Breast Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 8699–8704. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Wu, Y.T.; Hsieh, H.C.; Yu, Y.; Yu, A.L.; Chang, W.W. Epidermal Growth Factor/Heat Shock Protein 27 Pathway Regulates Vasculogenic Mimicry Activity of Breast Cancer Stem/Progenitor Cells. Biochimie 2014, 104, 117–126. [Google Scholar] [CrossRef]
- de Azevedo-Santos, A.P.S.; Rocha, M.C.B.; Guimarães, S.J.A.; Vale, A.A.M.; Laginha, F.M.; Nascimento, F.R.F.; Nagai, M.A.; Bergami-Santos, P.C.; Barbuto, J.A.M. Could Increased Expression of Hsp27, an “Anti-Inflammatory” Chaperone, Contribute to the Monocyte-Derived Dendritic Cell Bias towards Tolerance Induction in Breast Cancer Patients? Mediat. Inflamm. 2019, 2019, 8346930. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Conway de Macario, E.; Macario, A.J.L.; Brocchieri, L. Chaperonin genes on the rise: New divergent classes and intense duplication in human and other vertebrate genomes. BMC Evol. Biol. 2010, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Macario, A.J.L.; Conway de Macario, E. Chaperonins in Cancer: Expression, Function, and Migration in Extracellular Vesicles. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Liu, Q.; Qi, Y.; Kong, X.; Wang, X.; Zhang, W.; Zhai, J.; Yang, Y.; Fang, Y.; Wang, J. Molecular and Clinical Characterization of CCT2 Expression and Prognosis via Large-Scale Transcriptome Profile of Breast Cancer. Front. Oncol. 2021, 11, 614497. [Google Scholar] [CrossRef]
- Bini, L.; Magi, B.; Marzocchi, B.; Arcuri, F.; Tripodi, S.; Cintorino, M.; Sanchez, J.C.; Frutiger, S.; Hughes, G.; Pallini, V.; et al. Protein Expression Profiles in Human Breast Ductal Carcinoma and Histologically Normal Tissue. Electrophoresis 1997, 18, 2832–2841. [Google Scholar] [CrossRef]
- Bodoor, K.; Abu-Sheikha, A.; Matalka, I.; Alzou’bi, H.; Batiha, O.; Abu-Awad, A.; Jalboush, S.A.; Fayyad, L.M.; Qadiri, E.; Jarun, Y.; et al. Immunohistochemical Analysis of Heat Shock Proteins in Triple Negative Breast Cancer: HSP60 Expression Is a Marker of Poor Prognosis. Eur. J. Gynaecol. Oncol. 2018, 39, 926–934. [Google Scholar] [CrossRef]
- Feng, H.; Zeng, Y.; Graner, M.W.; Katsanis, E. Stressed Apoptotic Tumor Cells Stimulate Dendritic Cells and Induce Specific Cytotoxic T Cells. Blood 2002, 100, 4108–4115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterloh, A.; Meier-Stiegen, F.; Veit, A.; Fleischer, B.; von Bonin, A.; Breloer, M. Lipopolysaccharide-Free Heat Shock Protein 60 Activates T Cells. J. Biol. Chem. 2004, 279, 47906–47911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappello, F.; Conway de Macario, E.; Marasà, L.; Zummo, G.; Macario, A.J.L. Hsp60 Expression, New Locations, Functions and Perspectives for Cancer Diagnosis and Therapy. Cancer Biol. Ther. 2008, 7, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Brocchieri, L.; Conway de Macario, E.; Macario, A.J.L. hsp-70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 2008, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Zuiderweg, E.R.P.; Hightower, L.E.; Gestwicki, J.E. The Remarkable Multivalency of the Hsp70 Chaperones. Cell Stress Chaperones 2017, 22, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Fernández, M.R.; Valpuesta, J.M. Hsp70 Chaperone: A Master Player in Protein Homeostasis. F1000 Res. 2018, 7, 1497. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Fernández, M.R.; Gragera, M.; Ochoa-Ibarrola, L.; Quintana-Gallardo, L.; Valpuesta, J.M. Hsp70—A Master Regulator in Protein Degradation. FEBS Lett. 2017, 591, 2648–2660. [Google Scholar] [CrossRef] [Green Version]
- Rappa, F.; Farina, F.; Zummo, G.; David, S.; Campanella, C.; Carini, F.; Tomasello, G.; Damiani, P.; Cappello, F.; Conway de Macario, E.; et al. HSP-Molecular Chaperones in Cancer Biogenesis and Tumor Therapy: An Overview. Anticancer Res. 2012, 32, 5139–5150. [Google Scholar]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- Nylandsted, J.; Wick, W.; Hirt, U.A.; Brand, K.; Rohde, M.; Leist, M.; Weller, M.; Jäättelä, M. Eradication of Glioblastoma, and Breast and Colon Carcinoma Xenografts by Hsp70 Depletion. Cancer Res. 2002, 62, 7139–7142. [Google Scholar]
- Sims, J.D.; McCready, J.; Jay, D.G. Extracellular Heat Shock Protein (Hsp)70 and Hsp90α Assist in Matrix Metalloproteinase-2 Activation and Breast Cancer Cell Migration and Invasion. PLoS ONE 2011, 6, e18848. [Google Scholar] [CrossRef] [Green Version]
- Jagadish, N.; Agarwal, S.; Gupta, N.; Fatima, R.; Devi, S.; Kumar, V.; Suri, V.; Kumar, R.; Suri, V.; Sadasukhi, T.C.; et al. Heat Shock Protein 70-2 (HSP70-2) Overexpression in Breast Cancer. J. Exp. Clin. Cancer Res. 2016, 35, 150. [Google Scholar] [CrossRef] [Green Version]
- Gabai, V.L.; Sherman, M.Y.; Yaglom, J.A. HSP72 Depletion Suppresses GammaH2AX Activation by Genotoxic Stresses via P53/P21 Signaling. Oncogene 2010, 29, 1952–1962. [Google Scholar] [CrossRef] [Green Version]
- Conner, C.; Lager, T.W.; Guldner, I.H.; Wu, M.Z.; Hishida, Y.; Hishida, T.; Ruiz, S.; Yamasaki, A.E.; Gilson, R.C.; Belmonte, J.C.I.; et al. Cell Surface GRP78 Promotes Stemness in Normal and Neoplastic Cells. Sci. Rep. 2020, 10, 3474. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.Y.; Jiang, H.S.; Li, K.; Zheng, Y.Z.; Liu, Y.R.; Qiao, F.; Li, S.; Hu, X.; Shao, Z.M. The Phosphorylation-Specific Association of STMN1 with GRP78 Promotes Breast Cancer Metastasis. Cancer Lett. 2016, 377, 87–96. [Google Scholar] [CrossRef]
- Dong, D.; Stapleton, C.; Luo, B.; Xiong, S.; Ye, W.; Zhang, Y.; Jhaveri, N.; Zhu, G.; Ye, R.; Liu, Z.; et al. A Critical Role for GRP78/BiP in the Tumor Microenvironment for Neovascularization during Tumor Growth and Metastasis. Cancer Res. 2011, 71, 2848–2857. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liu, H.; Zhang, X.; Zhang, L.; Li, X.; Wang, C.; Sun, S. Cell Surface GRP78 Accelerated Breast Cancer Cell Proliferation and Migration by Activating STAT3. PLoS ONE 2015, 10, e0125634. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, Z.; Wu, X.; Zhang, M.; Zhang, S.; Jin, T. Mortalin Promotes Breast Cancer Malignancy. Exp. Mol. Pathol. 2021, 118, 104593. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. HSP70s in Breast Cancer: Promoters of Tumorigenesis and Potential Targets/Tools for Therapy. Cells 2021, 10, 3446. [Google Scholar] [CrossRef]
- Huang, M.B.; Gonzalez, R.R.; Lillard, J.; Bond, V.C. Secretion Modification Region-Derived Peptide Blocks Exosome Release and Mediates Cell Cycle Arrest in Breast Cancer Cells. Oncotarget 2017, 8, 11302–11315. [Google Scholar] [CrossRef] [Green Version]
- Jubran, R.; Saar, M.R.; Wawruszak, A.; Ziporen, L.; Donin, N.; Bairey, O.; Fishelson, Z. Mortalin Peptides Exert Antitumor Activities and Act as Adjuvants to Antibody-Mediated Complement-Dependent Cytotoxicity. Int. J. Oncol. 2020, 57, 1013–1026. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative Genomics and Evolution of the HSP90 Family of Genes across All Kingdoms of Organisms. BMC Genom. 2006, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [Green Version]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef] [PubMed]
- Verba, K.A.; Wang, R.Y.R.; Arakawa, A.; Liu, Y.; Shirouzu, M.; Yokoyama, S.; Agard, D.A. Atomic Structure of Hsp90-Cdc37-Cdk4 Reveals That Hsp90 Traps and Stabilizes an Unfolded Kinase. Science 2016, 352, 1542–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderwood, S.K. Cdc37 as a Co-Chaperone to Hsp90. Subcell. Biochem. 2015, 78, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, C. The “active Life” of Hsp90 Complexes. Biochim. Biophys. Acta 2012, 1823, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.R.; de Billy, E.; Hobbs, S.; Powers, M.; Prodromou, C.; Pearl, L.; Clarke, P.A.; Workman, P. Restricting Direct Interaction of CDC37 with HSP90 Does Not Compromise Chaperoning of Client Proteins. Oncogene 2015, 34, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the Dynamic HSP90 Complex in Cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Zagouri, F.; Bournakis, E.; Koutsoukos, K.; Papadimitriou, C.A. Heat shock protein 90 (hsp90) expression and breast cancer. Pharmaceuticals 2012, 5, 1008–1020. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Chang, J.T.; Geradts, J.; Neckers, L.M.; Haystead, T.; Spector, N.L.; Lyerly, H.K. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012, 14, R62. [Google Scholar] [CrossRef] [Green Version]
- Gökmen-Polar, Y.; Badve, S. Upregulation of HSF1 in Estrogen Receptor Positive Breast Cancer. Oncotarget 2016, 7, 84239–84245. [Google Scholar] [CrossRef] [Green Version]
- Zagouri, F.; Nonni, A.; Sergentanis, T.N.; Papadimitriou, C.A.; Michalopoulos, N.V.; Lazaris, A.C.; Patsouris, E.; Zografos, G.C. Heat Shock Protein90 in Lobular Neoplasia of the Breast. BMC Cancer 2008, 8, 312. [Google Scholar] [CrossRef] [Green Version]
- Zagouri, F.; Sergentanis, T.N.; Nonni, A.; Papadimitriou, C.A.; Michalopoulos, N.V.; Domeyer, P.; Theodoropoulos, G.; Lazaris, A.; Patsouris, E.; Zogafos, E.; et al. Hsp90 in the continuum of breast ductal carcinogenesis: Evaluation in precursors, preinvasive and ductal carcinoma lesions. BMC Cancer 2010, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Kotwal, A.; Suran, S.; Amere Subbarao, S. Hsp90 chaperone facilitates E2F1/2-dependent gene transcription in human breast cancer cells. Eur. J. Cell Biol. 2021, 100, 151148. [Google Scholar] [CrossRef]
- Dong, H.; Zou, M.; Bhatia, A.; Jayaprakash, P.; Hofman, F.; Ying, Q.; Chen, M.; Woodley, D.T.; Li, W. Breast Cancer MDA-MB-231 Cells Use Secreted Heat Shock Protein-90alpha (Hsp90α) to Survive a Hostile Hypoxic Environment. Sci. Rep. 2016, 6, 20605. [Google Scholar] [CrossRef] [Green Version]
- Zagouri, F.; Sergentanis, T.; Nonni, A.; Papadimitriou, C.; Pazaiti, A.; Michalopoulos, N.V.; Safioleas, P.; Lazaris, A.; Theodoropoulos, G.; Patsouris, E.; et al. Decreased Hsp90 expression in infiltrative lobular carcinoma: An immunohistochemical study. BMC Cancer 2010, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.; Streller, F.; Scheel, A.H.; Rüschoff, J.; Reinert, M.C.; Dobbelstein, M.; Marchenko, N.D.; Moll, U.M. HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer. Cell Death Dis. 2014, 5, e980. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Iwanowycz, S.; Ngoi, S.; Hill, M.; Zhao, Q.; Liu, B. Molecular Chaperone GRP94/GP96 in Cancers: Oncogenesis and Therapeutic Target. Front. Oncol. 2021, 11, 629846. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Hou, J.; Gui, M.; Ying, J.; Zhao, H.; Lv, N.; Meng, S. Cell membrane gp96 facilitates HER2 dimerization and serves as a novel target in breast cancer. Int. J. Cancer 2015, 137, 512–524. [Google Scholar] [CrossRef]
- Liu, K.; Chen, J.; Yang, F.; Zhou, Z.; Liu, Y.; Guo, Y.; Hu, H.; Gao, H.; Li, H. BJ-B11, an Hsp90 Inhibitor, Constrains the Proliferation and Invasion of Breast Cancer Cells. Front. Oncol. 2019, 9, 1447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Qi, X.; Du, F.; Zhang, G.; Li, D.; Li, J. PNSA, a Novel C-Terminal Inhibitor of HSP90, Reverses Epithelial-Mesenchymal Transition and Suppresses Metastasis of Breast Cancer Cells In Vitro. Mar. Drugs 2021, 19, 117. [Google Scholar] [CrossRef]
- Shien, T.; Iwata, H. Adjuvant and Neoadjuvant Therapy for Breast Cancer. Jpn. J. Clin. Oncol. 2020, 50, 225–229. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Conway de Macario, E. Chaperonopathies and Chaperonotherapy. FEBS Lett. 2007, 581, 3681–3688. [Google Scholar] [CrossRef] [Green Version]
- Macario, A.J.L.; Conway de Macario, E. Chaperonopathies by defect, excess, or mistake. Ann. N. Y. Acad. Sci. 2007, 1113, 178–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Campanella, C.; Caruso Bavisotto, C.; Logozzi, M.; Marino Gammazza, A.; Mizzoni, D.; Cappello, F.; Fais, S. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int. J. Mol. Sci. 2019, 20, 236. [Google Scholar] [CrossRef] [Green Version]
- Alberti, G.; Sánchez-López, C.M.; Andres, A.; Santonocito, R.; Campanella, C.; Cappello, F.; Marcilla, A. Molecular Profile Study of Extracellular Vesicles for the Identification of Useful Small “Hit” in Cancer Diagnosis. Appl. Sci. 2021, 11, 10787. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Wang, Q.; Jayasinghe, U.; Luo, X.; Wei, Q.; Wang, J.; Xiong, H.; Chen, C.; Xu, B.; et al. Exosome: Emerging Biomarker in Breast Cancer. Oncotarget 2017, 8, 41717–41733. [Google Scholar] [CrossRef] [Green Version]
- Caruso Bavisotto, C.; Marino Gammazza, A.; Campanella, C.; Bucchieri, F.; Cappello, F. Extracellular Heat Shock Proteins in Cancer: From Early Diagnosis to New Therapeutic Approach. Semin. Cancer Biol. 2021, in press. [Google Scholar] [CrossRef]
- Albakova, Z.; Siam, M.K.S.; Sacitharan, P.K.; Ziganshin, R.H.; Ryazantsev, D.Y.; Sapozhnikov, A.M. Extracellular Heat Shock Proteins and Cancer: New Perspectives. Transl. Oncol. 2021, 14, 100995. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Graner, M.W.; Cumming, R.I.; Bigner, D.D. The Heat Shock Response and Chaperones/Heat Shock Proteins in Brain Tumors: Surface Expression, Release, and Possible Immune Consequences. J. Neurosci. 2007, 27, 11214–11227. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Cappello, F.; Macario, A.J.L.; Conway de Macario, E.; Logozzi, M.; Fais, S.; Campanella, C. Exosomal HSP60: A Potentially Useful Biomarker for Diagnosis, Assessing Prognosis, and Monitoring Response to Treatment. Expert Rev. Mol. Diagn. 2017, 17, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Grzegrzółka, J.; Kurnol, K.; Piotrów, P.; Puła, B.; Kobierzycki, C.; Piotrowska, A.; Jabłońska, K.; Wojnar, A.; Ryś, J.; Dzięgiel, P.; et al. Hsp-27 Expression in Invasive Ductal Breast Carcinoma. Folia Histochem. Cytobiol. 2012, 50, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 Exosomes in Cancer Patients’ follow up: A Clinical Prospective Pilot Study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Vecchione, A. MicroRNA: Diagnostic Perspective. Front. Med. 2015, 2, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi, S.; Hughes, T.A.; Priya, S. Exosomes and exosomal RNAs in breast cancer: A status update. Eur. J. Cancer. 2021, 144, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.B.; Ma, S.X.; Chen, Z.H.; Huang, Q.Y.; Wu, L.Y.; Wang, Y.; Zhao, R.C.; Xiong, L.X. Exosomal microRNAs: Pleiotropic Impacts on Breast Cancer Metastasis and Their Clinical Perspectives. Biology 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Elmageed, Z.Y.A.; Hawke, D.H.; Wörner, P.M.; Jansen, D.A.; Abdel-Mageed, A.B.; Alt, E.U.; Izadpanah, R. Molecular Characterization of Exosome-like Vesicles from Breast Cancer Cells. BMC Cancer 2014, 14, 44. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [Green Version]
- Ozgur, A.; Tutar, L.; Tutar, Y. Regulation of Heat Shock Proteins by miRNAs in human breast cancer. MicroRNA 2014, 3, 118–135. [Google Scholar] [CrossRef]
- Choghaei, E.; Khamisipour, G.; Falahati, M.; Naeimi, B.; Mossahebi-Mohammadi, M.; Tahmasebi, R.; Hasanpour, M.; Shamsian, S.; Hashemi, Z.S. Knockdown of MicroRNA-29a Changes the Expression of Heat Shock Proteins in Breast Carcinoma MCF-7 Cells. Oncol. Res. 2016, 23, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.; Bolaender, A.; Patel, H.; Taldone, T. Heat Shock Protein (HSP) Drug Discovery and Development: Targeting Heat Shock Proteins in Disease. Curr. Top. Med. Chem. 2016, 16, 2753–2764. [Google Scholar] [CrossRef] [Green Version]
- Chi, K.N.; Yu, E.Y.; Jacobs, C.; Bazov, J.; Kollmannsberger, C.; Higano, C.S.; Mukherjee, S.D.; Gleave, M.E.; Stewart, P.S.; Hotte, S.J. A Phase I Dose-Escalation Study of Apatorsen (OGX-427), an Antisense Inhibitor Targeting Heat Shock Protein 27 (Hsp27), in Patients with Castration-Resistant Prostate Cancer and Other Advanced Cancers. Ann. Oncol. 2016, 27, 1116–1122. [Google Scholar] [CrossRef]
- Choi, S.K.; Kam, H.; Kim, K.Y.; Park, S.I.; Lee, Y.S. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef] [Green Version]
- Nagumo, Y.; Kakeya, H.; Shoji, M.; Hayashi, Y.; Dohmae, N.; Osada, H. Epolactaene Binds Human Hsp60 Cys442 Resulting in the Inhibition of Chaperone Activity. Biochem. J. 2005, 387 Pt 3, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Yang, H.; Zhang, X.; Li, H. Visualizing and Quantifying the Effect of the Inhibition of HSP70 on Breast Cancer Cells Based on Laser Scanning Microscopy. Technol. Cancer Res. Treat. 2018, 17, 1533033818785274. [Google Scholar] [CrossRef] [Green Version]
- Rodina, A.; Patel, P.D.; Kang, Y.; Patel, Y.; Baaklini, I.; Wong, M.J.H.; Taldone, T.; Yan, P.; Yang, C.; Maharaj, R.; et al. Identification of an Allosteric Pocket on Human Hsp70 Reveals a Mode of Inhibition of This Therapeutically Important Protein. Chem. Biol. 2013, 20, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Rodina, A.; Taldone, T.; Kang, Y.; Patel, P.D.; Koren, J.; Yan, P.; DaGama Gomes, E.M.; Yang, C.; Patel, M.R.; Shrestha, L.; et al. Affinity purification probes of potential use to investigate the endogenous Hsp70 interactome in cancer. ACS Chem. Biol. 2014, 9, 1698–1705. [Google Scholar] [CrossRef]
- Howe, M.K.; Bodoor, K.; Carlson, D.A.; Hughes, P.F.; Alwarawrah, Y.; Loiselle, D.R.; Jaeger, A.M.; Darr, D.B.; Jordan, J.L.; Hunter, L.M.; et al. Identification of an Allosteric Small-Molecule Inhibitor Selective for the Inducible Form of Heat Shock Protein 70. Chem. Biol. 2014, 21, 1648–1659. [Google Scholar] [CrossRef] [Green Version]
- Huryn, D.M.; Brodsky, J.L.; Brummond, K.M.; Chambers, P.G.; Eyer, B.; Ireland, A.W.; Kawasumi, M.; LaPorte, M.G.; Lloyd, K.; Manteau, B.; et al. Chemical Methodology as a Source of Small-Molecule CheckpoInt. Inhibitors and Heat Shock Protein 70 (Hsp70) Modulators. Proc. Natl. Acad. Sci. USA 2011, 108, 6757–6762. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, Z.; Peng, C.; You, J.; Shen, J.; Han, S.; Chen, J. Dietary Compound Isoliquiritigenin Targets GRP78 to Chemosensitize Breast Cancer Stem Cells via β-Catenin/ABCG2 Signaling. Carcinogenesis 2014, 35, 2544–2554. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Stopeck, A.T.; Gordon, M.S.; Mendelson, D.; Solit, D.B.; Bagatell, R.; Ma, W.; Wheler, J.; Rosen, N.; Norton, L.; et al. Combination of Trastuzumab and Tanespimycin (17-AAG, KOS-953) Is Safe and Active in Trastuzumab-Refractory HER-2 Overexpressing Breast Cancer: A Phase I Dose-Escalation Study. J. Clin. Oncol. 2007, 25, 5410–5417. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, S.; Kang, J.; Han, K.C.; Kim, Y.H.; Bae, J.W.; Park, K.H. HSP90 Inhibitor, 17-DMAG, Alone and in Combination with Lapatinib Attenuates Acquired Lapatinib-Resistance in ER-Positive, HER2-Overexpressing Breast Cancer Cell Line. Cancers 2020, 12, 2630. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Henderson, C.; Lin, N.U.; Mahtani, R.; Goddard, J.; Rodenas, E.; Hudis, C.; O’Shaughnessy, J.; Baselga, J. A Multicenter Trial Evaluating Retaspimycin HCL (IPI-504) plus Trastuzumab in Patients with Advanced or Metastatic HER2-Positive Breast Cancer. Breast Cancer Res. Treat. 2013, 139, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Friedland, J.C.; Smith, D.L.; Sang, J.; Acquaviva, J.; He, S.; Zhang, C.; Proia, D.A. Targeted Inhibition of Hsp90 by Ganetespib Is Effective across a Broad Spectrum of Breast Cancer Subtypes. Investig. New Drugs 2014, 32, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, K.; Wang, R.; Teplinsky, E.; Chandarlapaty, S.; Solit, D.; Cadoo, K.; Speyer, J.; D’Andrea, G.; Adams, S.; Patil, S.; et al. A Phase I Trial of Ganetespib in Combination with Paclitaxel and Trastuzumab in Patients with Human Epidermal Growth Factor Receptor-2 (HER2)-Positive Metastatic Breast Cancer. Breast Cancer Res. 2017, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Marino Gammazza, A.; Macaluso, F.; Di Felice, V.; Cappello, F.; Barone, R. Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology. Cells 2018, 7, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Li, B.X.; Xiao, X. Toward Developing Chemical Modulators of Hsp60 as Potential Therapeutics. Front. Mol. Biosci. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, H.S.; Shimizu, K.; Minegishi, H.; Nakamura, H. Identification of HSP60 as a Primary Target of O-Carboranylphenoxyacetanilide, an HIF-1alpha Inhibitor. J. Am. Chem. Soc. 2010, 132, 11870–11871. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, Y.; Lai, Y.T.; Tong, K.C.; Fung, Y.M.; Lok, C.N.; Che, C.M. Anticancer Gold(III) Porphyrins Target Mitochondrial Chaperone Hsp60. Angew. Chem. Int. 2016, 55, 1387–1391. [Google Scholar] [CrossRef]
- Nigam, N.; Grover, A.; Goyal, S.; Katiyar, S.P.; Bhargava, P.; Wang, P.C.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Targeting Mortalin by Embelin Causes Activation of Tumor Suppressor P53 and Deactivation of Metastatic Signaling in Human Breast Cancer Cells. PLoS ONE 2015, 10, e0138192. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Mohammadian, M.; Feizollahzadeh, S.; Mahmoudi, R.; Milani, A.T.; Firouzi, S.R.; Douna, B.K. Hsp90 Inhibitor; NVP-AUY922 in Combination with Doxorubicin Induces Apoptosis and Downregulates VEGF in MCF-7 Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2020, 21, 1773–1778. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; dos Anjos, C.H.; Taldone, T.; Wang, R.; Comen, E.; Fornier, M.; Bromberg, J.F.; Ma, W.; Patil, S.; Rodina, A.; et al. Measuring Tumor Epichaperome Expression Using [124 I] PU-H71 Positron Emission Tomography as a Biomarker of Response for PU-H71 Plus Nab-Paclitaxel in HER2-Negative Metastatic Breast Cancer. JCO Precis. Oncol. 2020, 4, 1414–1424. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Steven, A.; Seliger, B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care 2018, 13, 16–21. [Google Scholar] [CrossRef]
- Alberti, G.; Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway de Macario, E.; Campanella, C.; Macario, A.J.L.; Marino Gammazza, A. Functions and Therapeutic Potential of Extracellular Hsp60, Hsp70, and Hsp90 in Neuroinflammatory Disorders. Appl. Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Banerjee, S.; Lin, C.F.L.; Skinner, K.A.; Schiffhauer, L.M.; Peacock, J.; Hicks, D.G.; Redmond, E.M.; Morrow, D.; Huston, A.; Shayne, M.; et al. Heat Shock Protein 27 Differentiates Tolerogenic Macrophages That May Support Human Breast Cancer Progression. Cancer Res. 2011, 71, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef] [Green Version]
- Conroy, S.E.; Sasieni, P.D.; Amin, V.; Wang, D.Y.; Smith, P.; Fentiman, I.S.; Latchman, D.S. Antibodies to Heat-Shock Protein 27 Are Associated with Improved Survival in Patients with Breast Cancer. Br. J. Cancer 1998, 77, 1875–1879. [Google Scholar] [CrossRef] [Green Version]
- Desmetz, C.; Bibeau, F.; Boissière, F.; Bellet, V.; Rouanet, P.; Maudelonde, T.; Mangé, A.; Solassol, J. Proteomics-Based Identification of HSP60 as a Tumor-Associated Antigen in Early Stage Breast Cancer and Ductal Carcinoma in Situ. J. Proteome Res. 2008, 7, 3830–3837. [Google Scholar] [CrossRef]
- Cheng, Y.; Bao, D.; Chen, X.; Wu, Y.; Wei, Y.; Wu, Z.; Li, F.; Piao, J.G. Microwave-Triggered/HSP-Targeted Gold Nano-System for Triple-Negative Breast Cancer Photothermal Therapy. Int. J. Pharm. 2021, 593, 120162. [Google Scholar] [CrossRef]
- Conroy, S.E.; Gibson, S.L.; Brunström, G.; Isenberg, D.; Luqmani, Y.; Latchman, D.S. Autoantibodies to 90 KD Heat-Shock Protein in Sera of Breast Cancer Patients. Lancet 1995, 345, 126. [Google Scholar] [CrossRef]
- Zagouri, F.; Sergentanis, T.N.; Provatopoulou, X.; Kalogera, E.; Chrysikos, D.; Lymperi, M.; Papadimitriou, C.A.; Zografos, E.; Bletsa, G.; Kalles, V.S.; et al. Serum Levels of HSP90 in the Continuum of Breast Ductal and Lobular Lesions. In Vivo 2011, 25, 669–672. [Google Scholar] [CrossRef]
- van Eden, W.; Jansen, M.A.A.; Ludwig, I.; van Kooten, P.; van der Zee, R.; Broere, F. The Enigma of Heat Shock Proteins in Immune Tolerance. Front. Immunol. 2017, 8, 1599. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J. Molecular Chaperones in Mammary Cancer Growth and Breast Tumor Therapy. J. Cell Biochem. 2012, 113, 1096–1103. [Google Scholar] [CrossRef] [Green Version]
- Shevtsov, M.; Multhoff, G. Heat Shock Protein-Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front. Immunol. 2016, 7, 171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zheng, L. Tumor Immunotherapy Based on Tumor-Derived Heat Shock Proteins (Review). Oncol. Lett. 2013, 6, 1543–1549. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Zhang, Y.; Durfee, J.; Weng, D.; Liu, C.; Koido, S.; Song, B.; Apostolopoulos, V.; Calderwood, S.K. A Heat Shock Protein 70-Based Vaccine with Enhanced Immunogenicity for Clinical Use. J. Immunol. 2010, 184, 488–496. [Google Scholar] [CrossRef]
- Faure, O.; Graff-Dubois, S.; Bretaudeau, L.; Derré, L.; Gross, D.A.; Alves, P.M.S.; Cornet, S.; Duffour, M.T.; Chouaib, S.; Miconnet, I.; et al. Inducible Hsp70 as Target of Anticancer Immunotherapy: Identification of HLA-A*0201-Restricted Epitopes. Int. J. Cancer 2004, 108, 863–870. [Google Scholar] [CrossRef]
- Kim, J.H.; Majumder, N.; Lin, H.; Chen, J.; Falo, L.D.; You, Z. Enhanced Immunity by NeuEDhsp70 DNA Vaccine Is Needed to Combat an Aggressive Spontaneous Metastatic Breast Cancer. Mol. Ther. 2005, 11, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Bharti, A.; Khaleque, A.A.; Song, B.; Liu, C.; Apostolopoulos, V.; Xing, P.; Calderwood, S.K.; Gong, J. Enhanced Immunogenicity of Heat Shock Protein 70 Peptide Complexes from Dendritic Cell-Tumor Fusion Cells. J. Immunol. 2006, 177, 5946–5955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Luo, W.; Wang, Y.; Chen, J.; Liu, Y.; Zhang, Y. Enhanced Antitumor Immunity of Nanoliposome-Encapsulated Heat Shock Protein 70 Peptide Complex Derived from Dendritic Tumor Fusion Cells. Oncol. Rep. 2015, 33, 2695–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V.; et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes with a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 2015, 108, djv330. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials. Vaccination Against High Risk Breast Cancer Using Tumor Derived Heat Shock Protein 70—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT00027131 (accessed on 14 April 2022).

| Inhibitor’s Target | Therapeutic Agent | Experimental Design and Trial Phase | Reference |
|---|---|---|---|
| Hsp27 | OGX-427 OGX-427 + Quercetin | In vitro, Phase II In vitro | [126] [51,127] |
| Hsp60 | Epolactaene | In vitro | [128] |
| Hsp70 (Hsp70 family member not specified) | VER-155008 | In vitro | [129] |
| Hsp70 and Hsc70 | YK5 | In vitro | [130,131] |
| HSPA1A | HS-72 | In vitro and in vivo | [132] |
| Hsp70 (Hsp70 family member not specified) | DMT3132 | In vitro | [133] |
| Grp78 | Flavonoid derivated | In vitro | [134] |
| Hsp90 (isoform not specified) | Tanespimycin + TZMB | In vitro, Phase II | [135] |
| Hsp90 alpha | Alvespimycin+ lapatinib | In vitro and in vivo | [136] |
| Hsp90 (isoform not specified) | IPI-504 + TZMB | In vitro, Phase II | [137] |
| Hsp90 alpha and Hsp90beta | Ganetespib | In vitro, Phase I | [138] |
| Hsp90 (isoform not specified) | Ganetespib + Paclitaxel | In vitro | [139] |
| Hsp | Therapeutic | Trial Phase | Reference |
|---|---|---|---|
| Hsp70 (Hsp70 family member not specified) | Hsp70-peptide complexes | In vitro and in vivo, Phase I/II | [166] |
| Hsp70 (Hsp70 family member not specified) | Hsp70-peptide complex at 4T1 | In vivo, Phase I/II | [167] |
| Hsp70 (Hsp70 family member not specified) | NeuEDhsp70 DNA | In vitro | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, G.; Vergilio, G.; Paladino, L.; Barone, R.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L.; Bucchieri, F.; Rappa, F. The Chaperone System in Breast Cancer: Roles and Therapeutic Prospects of the Molecular Chaperones Hsp27, Hsp60, Hsp70, and Hsp90. Int. J. Mol. Sci. 2022, 23, 7792. https://doi.org/10.3390/ijms23147792
Alberti G, Vergilio G, Paladino L, Barone R, Cappello F, Conway de Macario E, Macario AJL, Bucchieri F, Rappa F. The Chaperone System in Breast Cancer: Roles and Therapeutic Prospects of the Molecular Chaperones Hsp27, Hsp60, Hsp70, and Hsp90. International Journal of Molecular Sciences. 2022; 23(14):7792. https://doi.org/10.3390/ijms23147792
Chicago/Turabian StyleAlberti, Giusi, Giuseppe Vergilio, Letizia Paladino, Rosario Barone, Francesco Cappello, Everly Conway de Macario, Alberto J. L. Macario, Fabio Bucchieri, and Francesca Rappa. 2022. "The Chaperone System in Breast Cancer: Roles and Therapeutic Prospects of the Molecular Chaperones Hsp27, Hsp60, Hsp70, and Hsp90" International Journal of Molecular Sciences 23, no. 14: 7792. https://doi.org/10.3390/ijms23147792
APA StyleAlberti, G., Vergilio, G., Paladino, L., Barone, R., Cappello, F., Conway de Macario, E., Macario, A. J. L., Bucchieri, F., & Rappa, F. (2022). The Chaperone System in Breast Cancer: Roles and Therapeutic Prospects of the Molecular Chaperones Hsp27, Hsp60, Hsp70, and Hsp90. International Journal of Molecular Sciences, 23(14), 7792. https://doi.org/10.3390/ijms23147792










