Extracellular Vesicles Inhibit the Response of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine and TRAIL Treatment
Abstract
:1. Introduction
2. Results
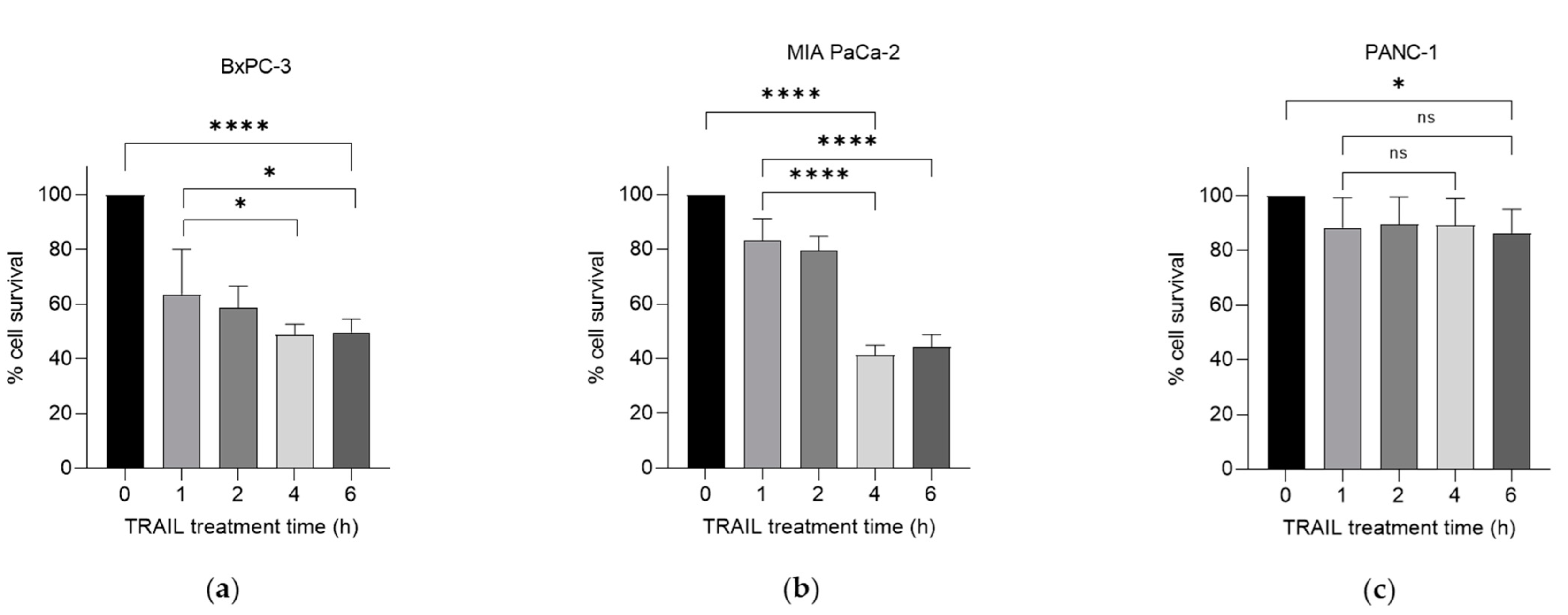
2.1. TRAIL Enhances the Cytotoxic Effects of Gemcitabine Treatment on Human PC Cells
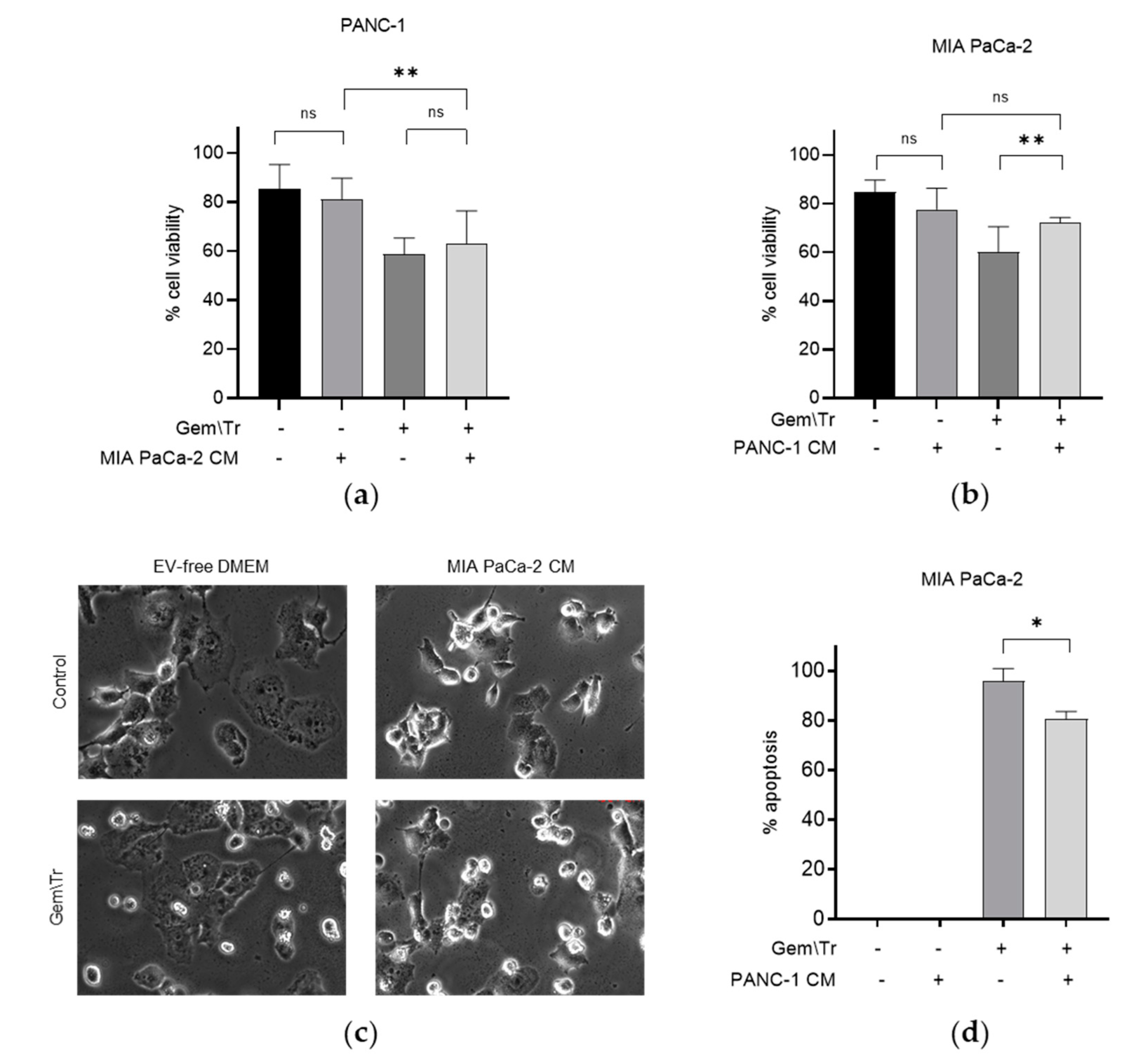
2.2. PDAC Cell-Conditioned Medium Alters the Response of Other PDAC Cells Lines to Treatment with Gemcitabine and TRAIL
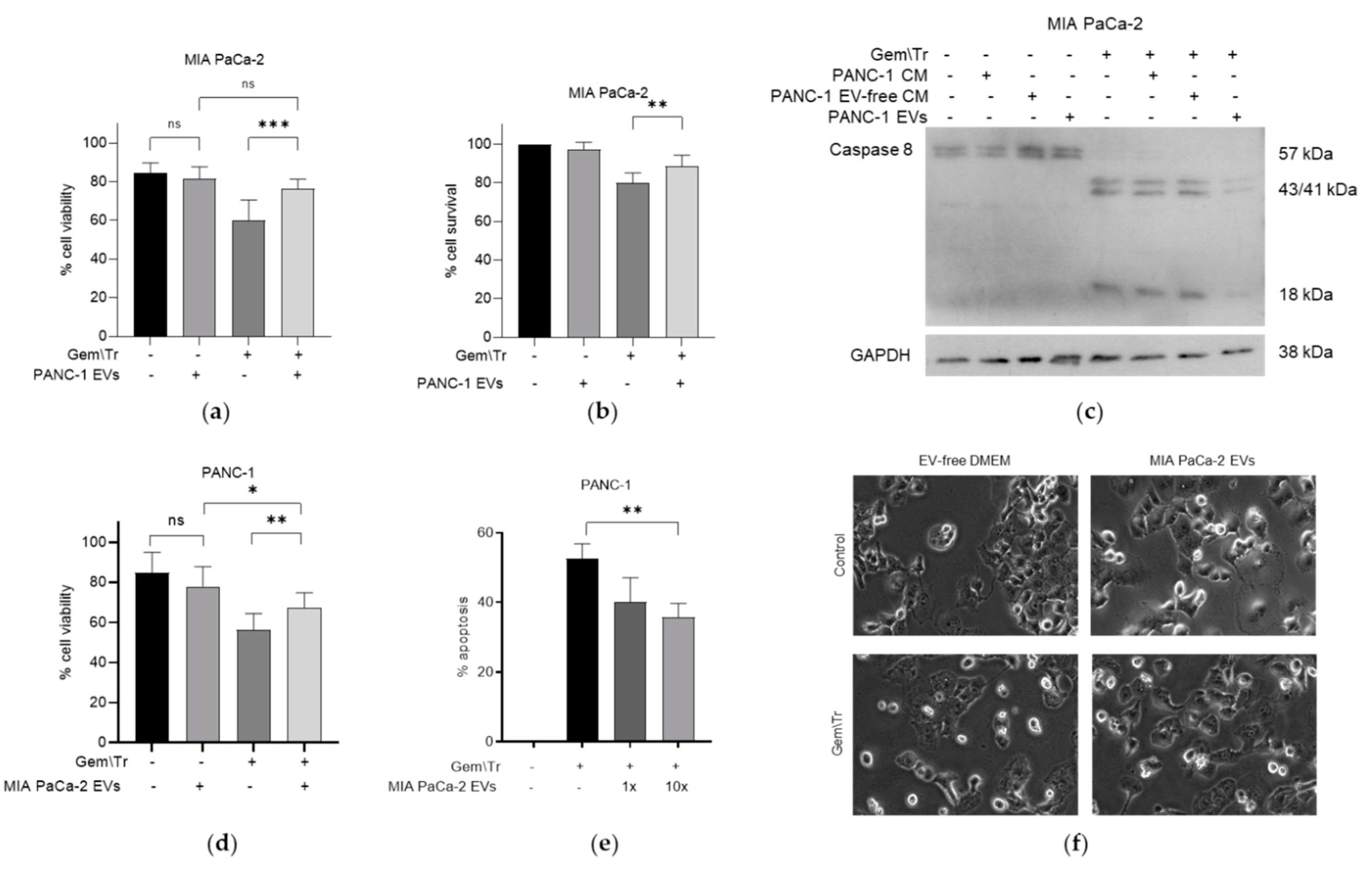
2.3. PDAC-Cell-Derived EVs Confer Resistance to Treatment with Gemcitabine and TRAIL
2.4. Small EVs Decrease the Response of PDAC Cells to Gemcitabine and TRAIL Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Maintenance
4.3. Media Preparation
4.4. EV Isolation
4.5. Tetrazolium Reduction (MTT) Assay
4.6. Trypan Blue Exclusion Assay
4.7. Time-Lapse Microscopy
4.8. Western Blotting
4.9. Nanoparticle Tracking Analysis
4.10. Transmission Electron Microscopy
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín, A.M.; Hidalgo, M.; Alvarez, R.; Arrazubi, V.; Martínez-Galán, J.; Salgado, M.; Macarulla, T.; Carrato, A. From first line to sequential treatment in the management of metastatic pancreatic cancer. J. Cancer 2018, 9, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, V.; Sperduti, I.; Vari, S.; Bria, E.; Melisi, D.; Garufi, C.; Nuzzo, C.; Scarpa, A.; Tortora, G.; Cognetti, F.; et al. Metastatic pancreatic cancer: Is there a light at the end of the tunnel? World J. Gastroenterol. 2015, 21, 4788–4801. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. World Cancer Report: Cancer Research for Cancer Prevention. In World Cancer Reports; Wild, C.P., Weiderpass, E., Stewart, B.W., Eds.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Patki, M.; Saraswat, A.; Bhutkar, S.; Dukhande, V.; Patel, K. In vitro assessment of a synergistic combination of gemcitabine and zebularine in pancreatic cancer cells. Exp. Cell Res. 2021, 405, 112660. [Google Scholar] [CrossRef] [PubMed]
- Dosch, A.R.; Dai, X.; Reyzer, M.L.; Mehra, S.; Srinivasan, S.; Willobee, B.A.; Kwon, D.; Kashikar, N.; Caprioli, R.; Merchant, N.B.; et al. Combined Src/EGFR Inhibition Targets STAT3 Signaling and Induces Stromal Remodeling to Improve Survival in Pancreatic Cancer. Mol. Cancer Res. 2020, 18, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.L.; Garcia, P.L.; Yoon, K.J. Developing effective combination therapy for pancreatic cancer: An overview. Pharmacol. Res. 2020, 155, 104740. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Cui, K.; Wang, C.-L.; Wang, A.-L.; Sun, Z.-Q.; Zhang, B.; Zhou, W.-Y.; Niu, Z.-X.; Tian, H.; et al. Mechanisms of TRAIL and gemcitabine induction of pancreatic cancer cell apoptosis. Asian Pac. J. Cancer Prev. 2011, 12, 2675–2678. [Google Scholar]
- Elia, A.; Henry-Grant, R.; Adiseshiah, C.; Marboeuf, C.; Buckley, R.J.; Clemens, M.J.; Mudan, S.; Pyronnet, S. Implication of 4E-BP1 protein dephosphorylation and accumulation in pancreatic cancer cell death induced by combined gemcitabine and TRAIL. Cell Death Dis. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Cui, Y.; Zhong, N.; Han, J. Proteomic characterisation of pancreatic islet b-cells stimulated with pancreatic carcinoma cell conditioned medium. J. Clin. Pathol. 2009, 62, 802–807. [Google Scholar] [CrossRef]
- Vaes, R.D.; van Dijk, D.P.; Farshadi, E.A.; Damink, S.W.O.; Rensen, S.S.; Langen, R.C. Human pancreatic tumour organoid-derived factors enhance myogenic differentiation. J. Cachex. Sarcopenia Muscle 2022, 13, 1302–1313. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, the origin, composition, purpose and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dong, C.; Jiang, K.; Sun, R.; Zhou, Y.; Yin, Z.; Lv, J.; Zhang, J.; Wang, Q.; Wang, L. Rab27B enhances drug resistance in hepatocellular carcinoma by promoting exosome-mediated drug efflux. Carcinogenesis 2020, 41, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, X.; Du, L.; Wang, Y.; Liu, X.; Tian, H.; Wang, L.; Li, P.; Zhao, Y.; Duan, W.; et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol. Cancer 2019, 18, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Wei, Q.; Koay, E.J.; Liu, Y.; Ning, B.; Bernard, P.W.; Zhang, N.; Han, H.; Katz, M.H.; Zhao, Z.; et al. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics 2018, 8, 5986–5994. [Google Scholar] [CrossRef]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Ou, C.; Shu, Y.; Luo, R.; Li, X.; Chen, S.; Wu, Q.; Gong, C.; Liu, L. A self-sustaining nanoplatform overcomes TRAIL-resistance of pancreatic cnacer by a source-broadening and expenditure-reducing apoptosis strategy. Biomaterials 2021, 211, 110137. [Google Scholar]
- Lehrich, B.M.; Liang, Y.; Fiandaca, M.S. Foetal bovine serum influence on in vitro extracellular vesicle analysis. J. Extracell. Vesicles 2021, 10, e12061. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, W.; Rong, Y.; Kuang, T.; Xu, X.; Wu, W.; Wang, D.; Lou, W. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2019, 383, 111543. [Google Scholar] [CrossRef]
- Urbanova, M.; Buocikova, V.; Trnkova, L.; Strapcova, S.; Kajabova, V.H.; Melian, E.B.; Novisedlakova, M.; Tomas, M.; Dubovan, P.; Earl, J.; et al. DNA Methylation Mediates EMT Gene Expression in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 2117. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br. J. Cancer 2017, 116, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Wu, X.-H.; Wang, D.; Luo, C.-L.; Chen, L.-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol. Med. Rep. 2013, 8, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, K.; Li, M.; Lin, X.; Mei, Y.; Huang, X.; Yang, H. Chemoresistance Transmission via Exosome-Transferred MMP14 in Pancreatic Cancer. Front. Oncol. 2022, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K.; et al. MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef]
- Hekmatirad, S.; Moloudizargari, M.; Moghadamnia, A.A.; Kazemi, S.; Mohammadnia-Afrouzi, M.; Baeeri, M.; Moradkhani, F.; Asghari, M.H. Inhibition of Exosome Release Sensitizes U937 Cells to PEGylated Liposomal Doxorubicin. Front. Immunol. 2021, 12, 692654. [Google Scholar] [CrossRef]
- BKreger, T.; Johansen, E.R.; Cerione, R.A.; Antonyak, M.A. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell sruvival and chemoresistance. Cancers 2016, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Bandari, S.K.; Purushothaman, A.; Ramani, V.C.; Brinkley, G.J.; Chandrashekar, D.S.; Varambally, S.; Mobley, J.A.; Zhang, Y.; Brown, E.E.; Vlodavsky, I.; et al. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2017, 65, 104–118. [Google Scholar] [CrossRef]
- Thery, C.; Clayton, A.; Amigorena, S.; Raposo, G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Dash, P.R.; McCormick, J.; Thomson, M.J.; Johnstone, A.P.; Cartwright, J.E.; Whitley, G.S. Fas ligand-induced apoptosis is regulated by nitric oxide through the inhibition of fas receptor clustering and the nitrosylation of protein kinase Cε. Exp. Cell Res. 2007, 313, 3421–3431. [Google Scholar] [CrossRef]
- Elia, A.; Constantinou, C.; Clemens, M.J. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene 2007, 27, 811–822. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimmer, E.; Rashid, S.; Kraev, I.; Miralles, F.; Elia, A. Extracellular Vesicles Inhibit the Response of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine and TRAIL Treatment. Int. J. Mol. Sci. 2022, 23, 7810. https://doi.org/10.3390/ijms23147810
Rimmer E, Rashid S, Kraev I, Miralles F, Elia A. Extracellular Vesicles Inhibit the Response of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine and TRAIL Treatment. International Journal of Molecular Sciences. 2022; 23(14):7810. https://doi.org/10.3390/ijms23147810
Chicago/Turabian StyleRimmer, Ella, Sadaf Rashid, Igor Kraev, Francesc Miralles, and Androulla Elia. 2022. "Extracellular Vesicles Inhibit the Response of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine and TRAIL Treatment" International Journal of Molecular Sciences 23, no. 14: 7810. https://doi.org/10.3390/ijms23147810





