CLIBASIA_00460 Disrupts Hypersensitive Response and Interacts with Citrus Rad23 Proteins
Abstract
:1. Introduction
2. Results
2.1. Suppression of Plant Cell Death
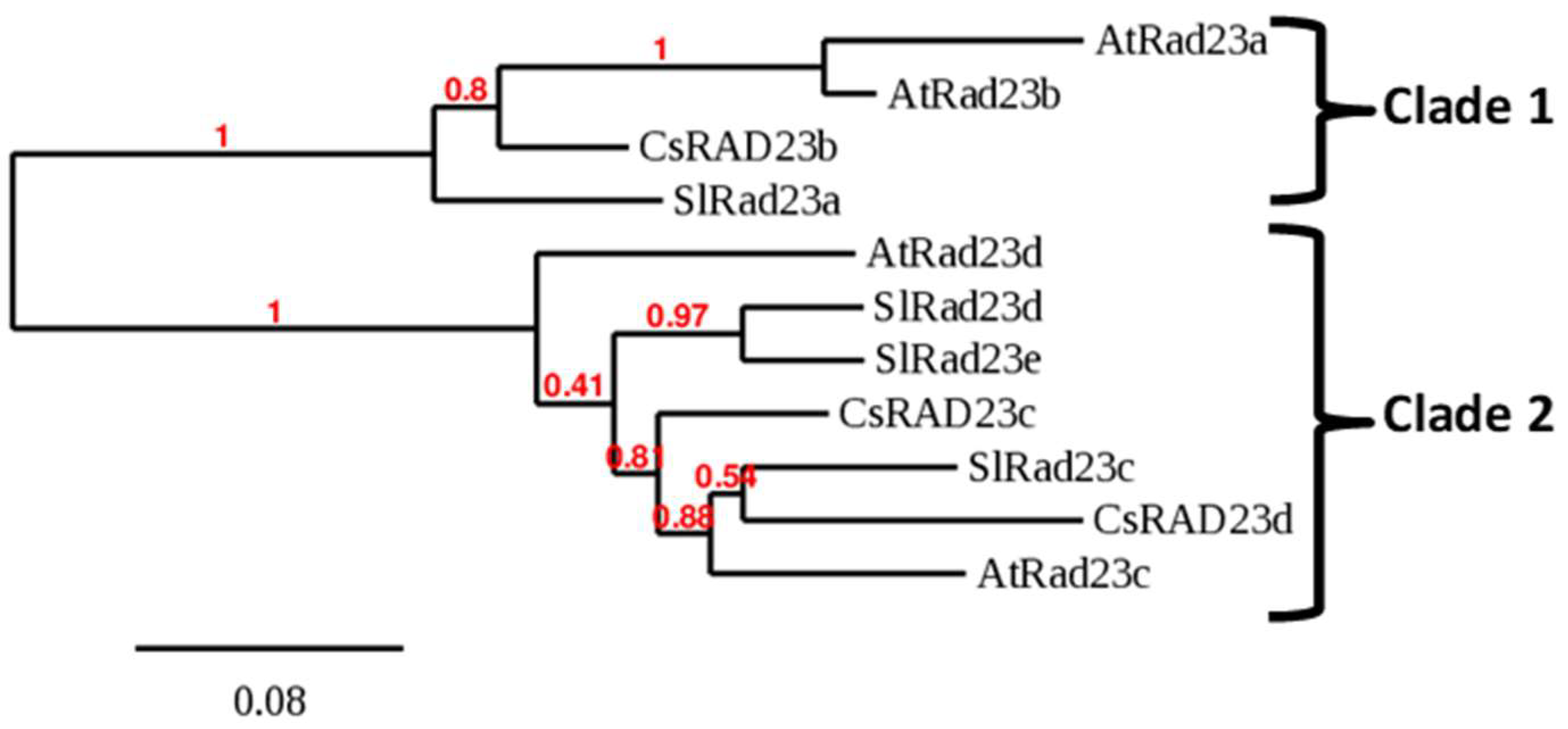
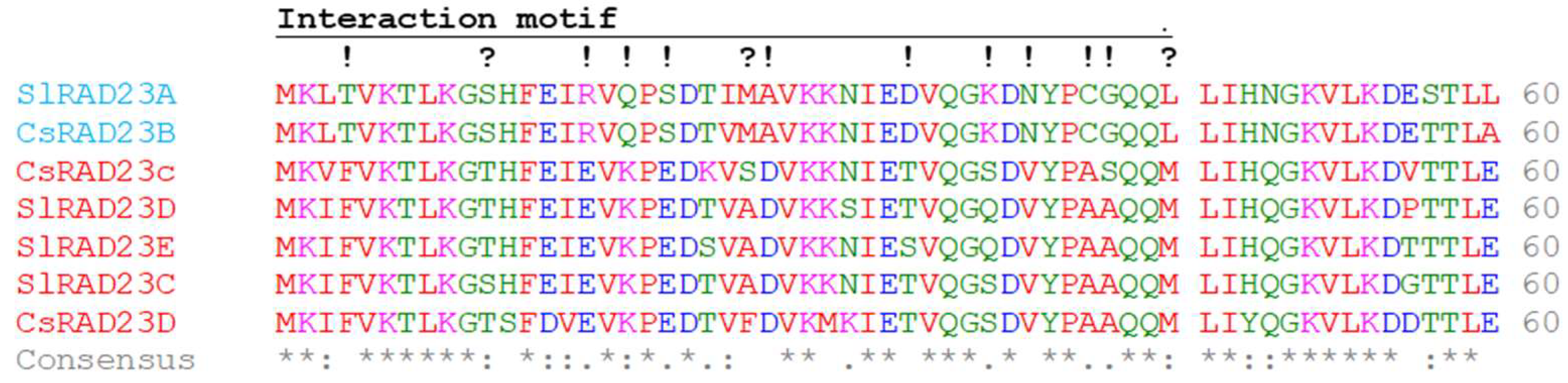
2.2. Identification of RAD23 Proteins Encoded in the Citrus Genome
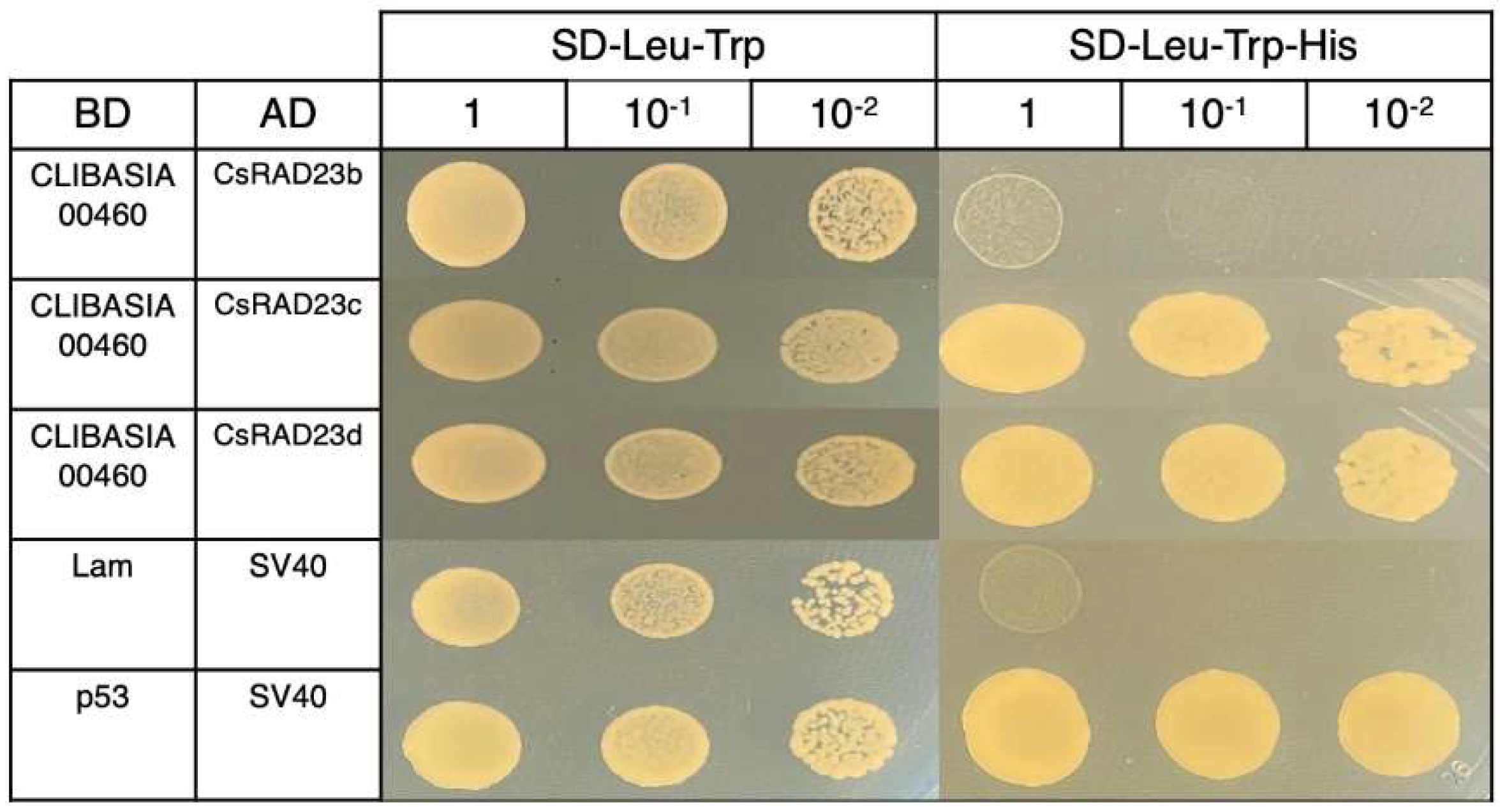
2.3. Yeast Two-Hybrid Interaction between CLIBASIA_00460 and Citrus RAD23
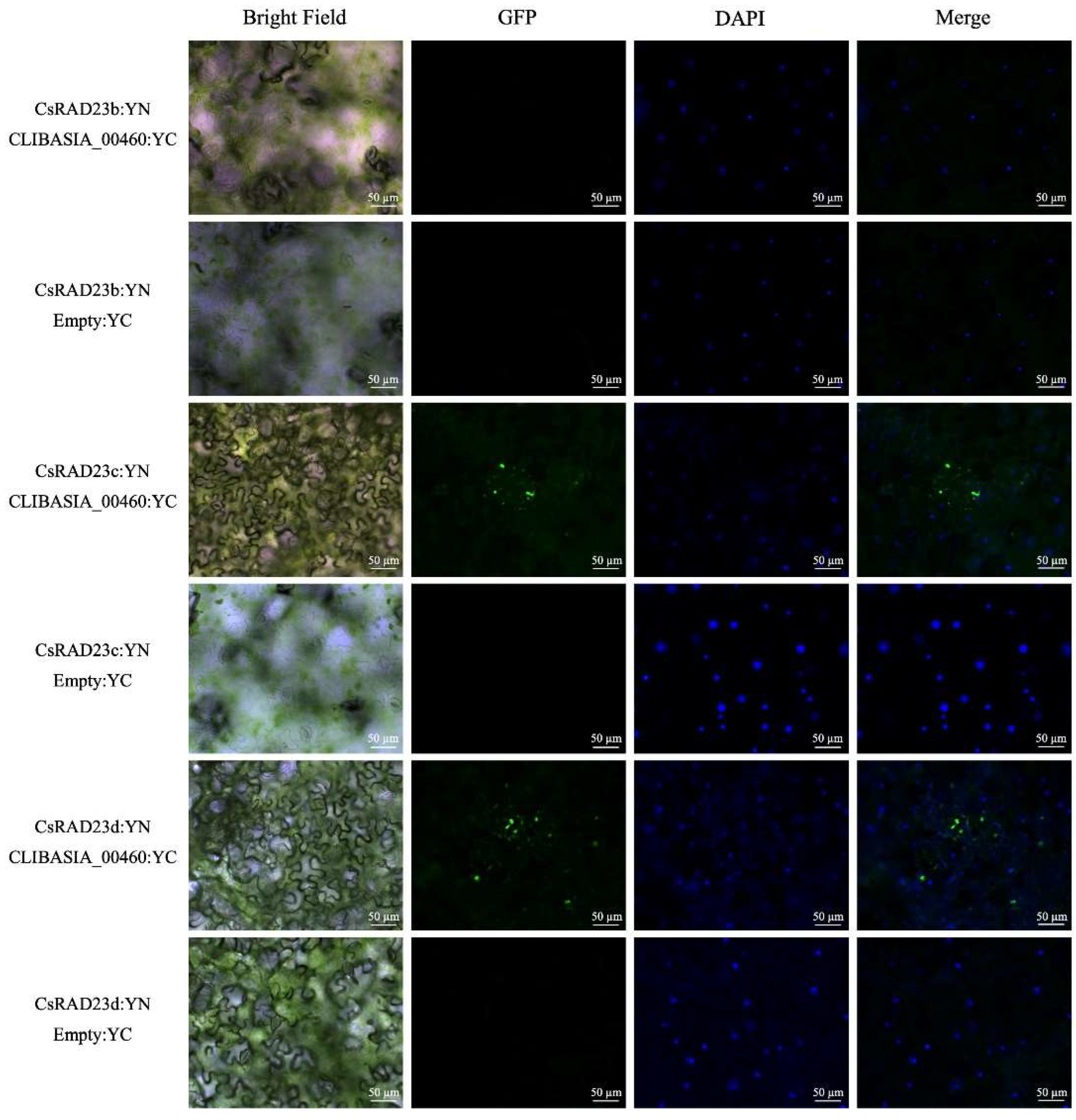
2.4. Validation of Interaction by Bi-Molecule Fluorescence Complementation
3. Discussion
4. Materials and Methods
4.1. Insect Colonies and Tomato Plants
4.2. Sequences Analysis
4.3. Directed Yeast Two-Hybrid (Y2H)
4.4. Bi-Molecular Fluorescence Complementation (BiFC)
4.5. Agrobacterium Tumefaciens-Mediated Transient Expression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.Z.; Li, J.Y.; Rangel, L.T.; Martins, J. The Candidatus Liberibacter-host interface: Insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef]
- Wang, N.; Trivedi, P. Citrus Huanglongbing: A newly relevant disease presents unprecedented challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, D.d.C.; Saillard, C.; Eveillard, S.; Danet, J.L.; Costa, P.I.d.; Ayres, A.J.; Bové, J. ‘Candidatus Liberibacter americanus’, associated with citrus Huanglongbing (greening disease) in São Paulo State, Brazil. Int. J. Syst. Evol. Microbiol. 2005, 55, 1857–1862. [Google Scholar] [CrossRef] [Green Version]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in São Paulo State: Sudden death, variegated chlorosis and Huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef]
- Wen, A.; Mallik, I.; Alvarado, V.Y.; Pasche, J.S.; Wang, X.; Li, W.; Levy, L.; Lin, H.; Scholthof, H.B.; Mirkov, T.E.; et al. Detection, distribution, and genetic variability of ‘Candidatus Liberibacter’ species associated with zebra complex disease of potato in North America. Plant Dis. 2009, 93, 1102–1115. [Google Scholar] [CrossRef] [Green Version]
- Munyaneza, J.E.; Fisher, T.W.; Sengoda, V.G.; Garczynski, S.F.; Nissinen, A.; Lemmetty, A. First report of “Candidatus Liberibacter solanacearum” associated with psyllid-affected carrots in Europe. Plant Dis. 2010, 94, 639. [Google Scholar] [CrossRef]
- Teresani, G.R.; Bertolini, E.; Alfaro-Fernández, A.; Martínez, C.; Tanaka, F.A.O.; Kitajima, E.W.; Roselló, M.; Sanjuán, S.; Ferrándiz, J.C.; López, M.M.; et al. Association of ‘Candidatus Liberibacter solanacearum’ with a vegetative disorder of celery in Spain and development of a Real-Time PCR method for its detection. Phytopathology 2014, 104, 804–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, W.R.; Sengoda, V.G.; Alfaro-Fernandez, A.O.; Font, M.I.; Crosslin, J.M.; Munyaneza, J.E. A new haplotype of “Candidatus Liberibacter solanacearum” identified in the Mediterranean region. Eur. J. Plant Pathol. 2013, 135, 633–639. [Google Scholar] [CrossRef]
- Tahzima, R.; Maes, M.; Achbani, E.H.; Swisher, K.D.; Munyaneza, J.E.; De Jonghe, K. First report of ‘Candidatus Liberibacter solanacearum’ on carrot in Africa. Plant Dis. 2014, 98, 1426. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Wickström, A.; Wang, J.; Pirhonen, M.; Nissinen, A.I. A novel haplotype of ‘Candidatus Liberibacter solanacearum’ found in Apiaceae and Polygonaceae family plants. Eur. J. Plant Pathol. 2020, 156, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Haapalainen, M.L.; Wang, J.; Latvala, S.; Lehtonen, M.T.; Pirhonen, M.; Nissinen, A.I. Genetic variation of ‘Candidatus Liberibacter solanacearum’ haplotype C and identification of a novel haplotype from Trioza urticae and stinging nettle. Phytopathology 2018, 108, 925–934. [Google Scholar] [CrossRef] [Green Version]
- Wamonje, F.O.; Zhou, N.; Bamrah, R.; Wist, T.; Prager, S.M. Detection and identification of a ‘Candidatus Liberibacter solanacearum’ species from ash tree infesting psyllids. Phytopathology 2022, 112, 76–80. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, L.; Hall, D.G.; Li, W.; Doddapaneni, H.; Lin, H.; Liu, L.; Vahling, C.M.; Gabriel, D.W.; Williams, K.P.; et al. Complete genome sequence of citrus Huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol. Plant Microbe. Interact. 2009, 22, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Doddapaneni, H.; Bai, X.; Yao, J.; Zhao, X.; Civerolo, E.L. Acquisition of uncharacterized sequences from Candidatus liberibacter, an unculturable bacterium, using an improved genomic walking method. Mol. Cell. Probes. 2008, 22, 30–37. [Google Scholar] [CrossRef]
- Lin, H.; Lou, B.; Glynn, J.M.; Doddapaneni, H.; Civerolo, E.L.; Chen, C.; Duan, Y.; Zhou, L.; Vahling, C.M. The complete genome sequence of ‘Candidatus Liberibacter solanacearum’, the bacterium associated with potato zebra chip disease. PLoS ONE 2011, 6, e19135. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Haapalainen, M.; Schott, T.; Thompson, S.M.; Smith, G.R.; Nissinen, A.I.; Pirhonen, M. Genomic sequence of ‘Candidatus Liberibacter solanacearum’ haplotype C and its comparison with haplotype A and B genomes. PLoS ONE 2017, 12, e0171531. [Google Scholar] [CrossRef] [Green Version]
- Wulff, N.A.; Zhang, S.; Setubal, J.C.; Almeida, N.F.; Martins, E.C.; Harakava, R.; Kumar, D.; Rangel, L.T.; Foissac, X.; Bové, J.M. The complete genome sequence of ‘Candidatus Liberibacter americanus’, associated with Citrus Huanglongbing. Mol. Plant Microbe. Interact. 2014, 27, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bento, F.M.M.; Darolt, J.C.; Merlin, B.L.; Penã, L.; Wulff, N.A.; Cônsoli, F.L. The molecular interplay of the establishment of an infection – gene expression of Diaphorina citri gut and Candidatus Liberibacter asiaticus. BMC Genom. 2021, 22, 677. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, F.; Levy, J.; Tamborindeguy, C. Transcriptome analysis of “Candidatus Liberibacter solanacearum” in its psyllid vector, Bactericera cockerelli. PLoS ONE 2014, 9, e100955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toruño, T.Y.; Stergiopoulos, I.; Coaker, G. Plant-pathogen effectors: Cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 2016, 54, 419–441. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.P.; De Francesco, A.; Trinh, J.; Gurung, F.B.; Pang, Z.; Vidalakis, G.; Wang, N.; Ancona, V.; Ma, W.; Coaker, G. Genome-wide analyses of Liberibacter species provides insights into evolution, phylogenetic relationships, and virulence factors. Mol. Plant Pathol. 2020, 21, 716–731. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Xu, J.; Zhang, Y.; Wang, N. SEC-translocon dependent extracytoplasmic proteins of Candidatus Liberibacter asiaticus. Front. Microbiol. 2016, 7, 1989. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.G.; Gross, R.; Mendoza-Herrera, A.; Tang, X.; Babilonia, K.; Shan, L.; Kuhl, J.C.; Dibble, M.S.; Xiao, F.; Tamborindeguy, C. Lso-HPE1, an effector of ‘Candidatus Liberibacter solanacearum’, can repress plant immune response. Phytopathology 2020, 110, 648–655. [Google Scholar] [CrossRef]
- Kan, C.-C.; Mendoza-Herrera, A.; Levy, J.; Hull, J.J.; Fabrick, J.A.; Tamborindeguy, C. HPE1, an effector from zebra chip pathogen interacts with tomato proteins and perturbs ubiquitinated protein accumulation. Int. J. Mol. Sci. 2021, 22, 9003. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Zhang, C.; Dai, M.; Wang, X.; Li, W. Nuclear import of a secreted “Candidatus Liberibacter asiaticus” protein is temperature dependent and contributes to pathogenicity in Nicotiana benthamiana. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Pitino, M.; Zhang, S.; Krystel, J.; Cano, L.M.; Shatters, R.G., Jr.; Hall, D.G.; Stover, E. Temporal and spatial detection of Candidatus Liberibacter asiaticus putative effector transcripts during interaction with Huanglongbing-susceptible, -tolerant, and -resistant citrus hosts. BMC Plant Biol. 2019, 19, 122. [Google Scholar] [CrossRef] [Green Version]
- Farmer, L.M.; Book, A.J.; Lee, K.H.; Lin, Y.L.; Fu, H.; Vierstra, R.D. The RAD23 family provides an essential connection between the 26S proteasome and ubiquitylated proteins in Arabidopsis. Plant Cell 2010, 22, 124–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Üstün, S.; Sheikh, A.; Gimenez-Ibanez, S.; Jones, A.; Ntoukakis, V.; Börnke, F. The proteasome acts as a hub for plant immunity and is targeted by Pseudomonas type III effectors. Plant Physiol. 2016, 172, 1941–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLean, A.M.; Orlovskis, Z.; Kowitwanich, K.; Zdziarska, A.M.; Angenent, G.C.; Immink, R.G.H.; Hogenhout, S.A. Phytoplasma effector SAP54 hijacks plant reproduction by degrading MADS-box proteins and promotes insect colonization in a RAD23-dependent manner. PLoS Biol. 2014, 12, e1001835. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Tamborindeguy, C.; Scheuring, D.C.; Herrera, A.M.; Silva, A.; Badillo-Vargas, I.E.; Miller, J.C.; Levy, J.G. Differences in zebra chip severity between ‘Candidatus Liberibacter solanacearum’ haplotypes in Texas. Am. J. Potato Res. 2019, 96, 86–93. [Google Scholar] [CrossRef]
- Mendoza Herrera, A.; Levy, J.; Harrison, K.; Yao, J.; Ibanez, F.; Tamborindeguy, C. Infection by ‘Candidatus Liberibacter solanacearum’ haplotypes A and B in Solanum lycopersicum ‘Moneymaker’. Plant Dis. 2018, 102, 2009–2015. [Google Scholar] [CrossRef] [Green Version]
- Pitino, M.; Armstrong, C.M.; Cano, L.M.; Duan, Y. Transient expression of Candidatus Liberibacter asiaticus effector induces cell death in Nicotiana benthamiana. Front. Plant Sci. 2016, 7, 982. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus Huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Chevenet, F.; Brun, C.; Bañuls, A.L.; Jacq, B.; Christen, R. TreeDyn: Towards dynamic graphics and annotations for analyses of trees. BMC Bioinform. 2006, 7, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, X.; Tian, G.; Wang, F.; Liu, K.; Nguyen, V.; Kohalmi, S.E.; Keller, W.A.; Tsang, E.W.; Harada, J.J.; et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: Components and anchoring nucleoporin. Plant J. 2010, 61, 259–270. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Levy, J.G.; Kan, C.-C.; Ibanez-Carrasco, F.; Tamborindeguy, C. CLIBASIA_00460 Disrupts Hypersensitive Response and Interacts with Citrus Rad23 Proteins. Int. J. Mol. Sci. 2022, 23, 7846. https://doi.org/10.3390/ijms23147846
Oh J, Levy JG, Kan C-C, Ibanez-Carrasco F, Tamborindeguy C. CLIBASIA_00460 Disrupts Hypersensitive Response and Interacts with Citrus Rad23 Proteins. International Journal of Molecular Sciences. 2022; 23(14):7846. https://doi.org/10.3390/ijms23147846
Chicago/Turabian StyleOh, Junepyo, Julien G. Levy, Chia-Cheng Kan, Freddy Ibanez-Carrasco, and Cecilia Tamborindeguy. 2022. "CLIBASIA_00460 Disrupts Hypersensitive Response and Interacts with Citrus Rad23 Proteins" International Journal of Molecular Sciences 23, no. 14: 7846. https://doi.org/10.3390/ijms23147846





