The Role of Mitochondrial Abnormalities in Diabetic Cardiomyopathy
Abstract
1. Introduction
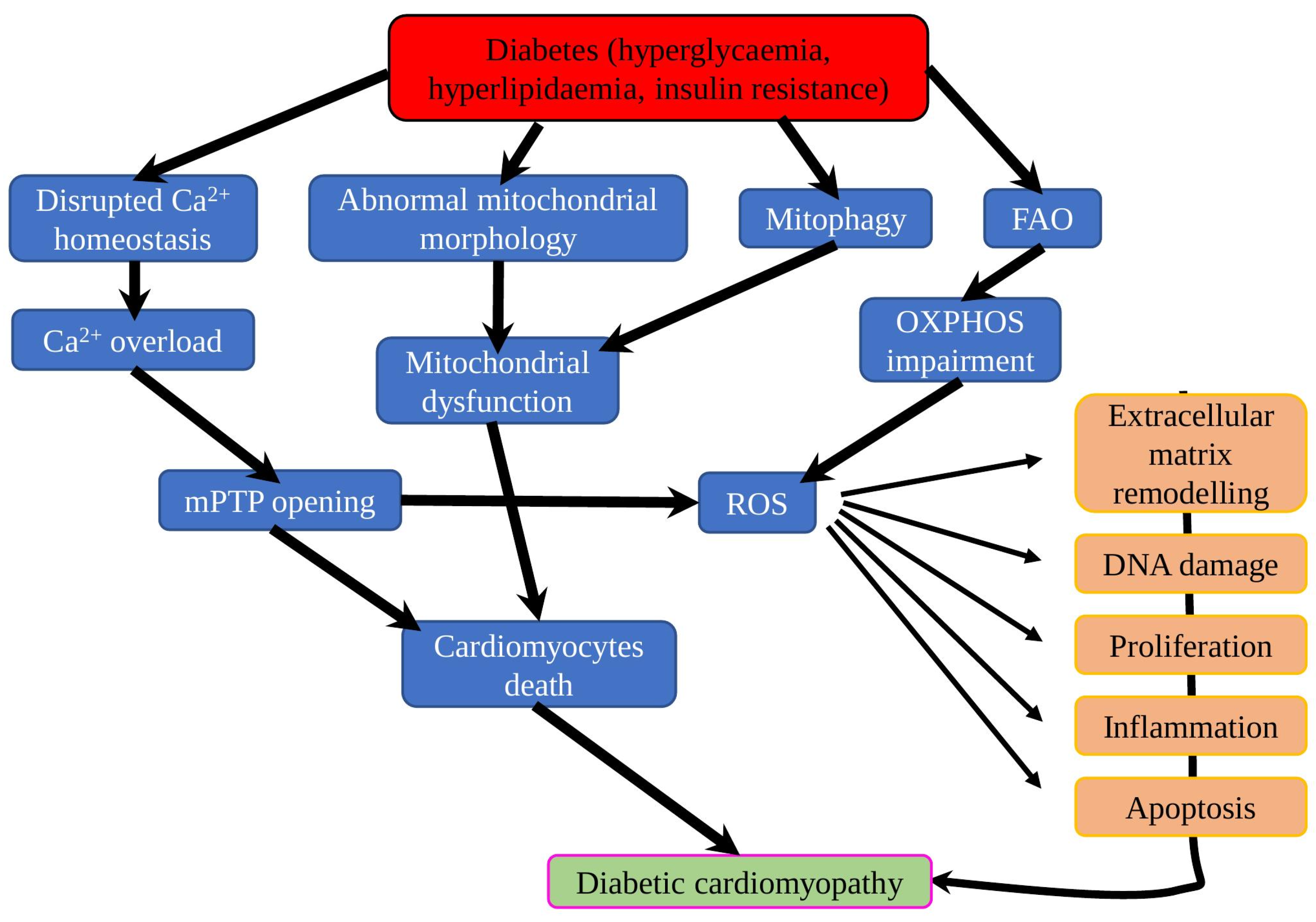
2. Structural and Functional Alterations in DCM
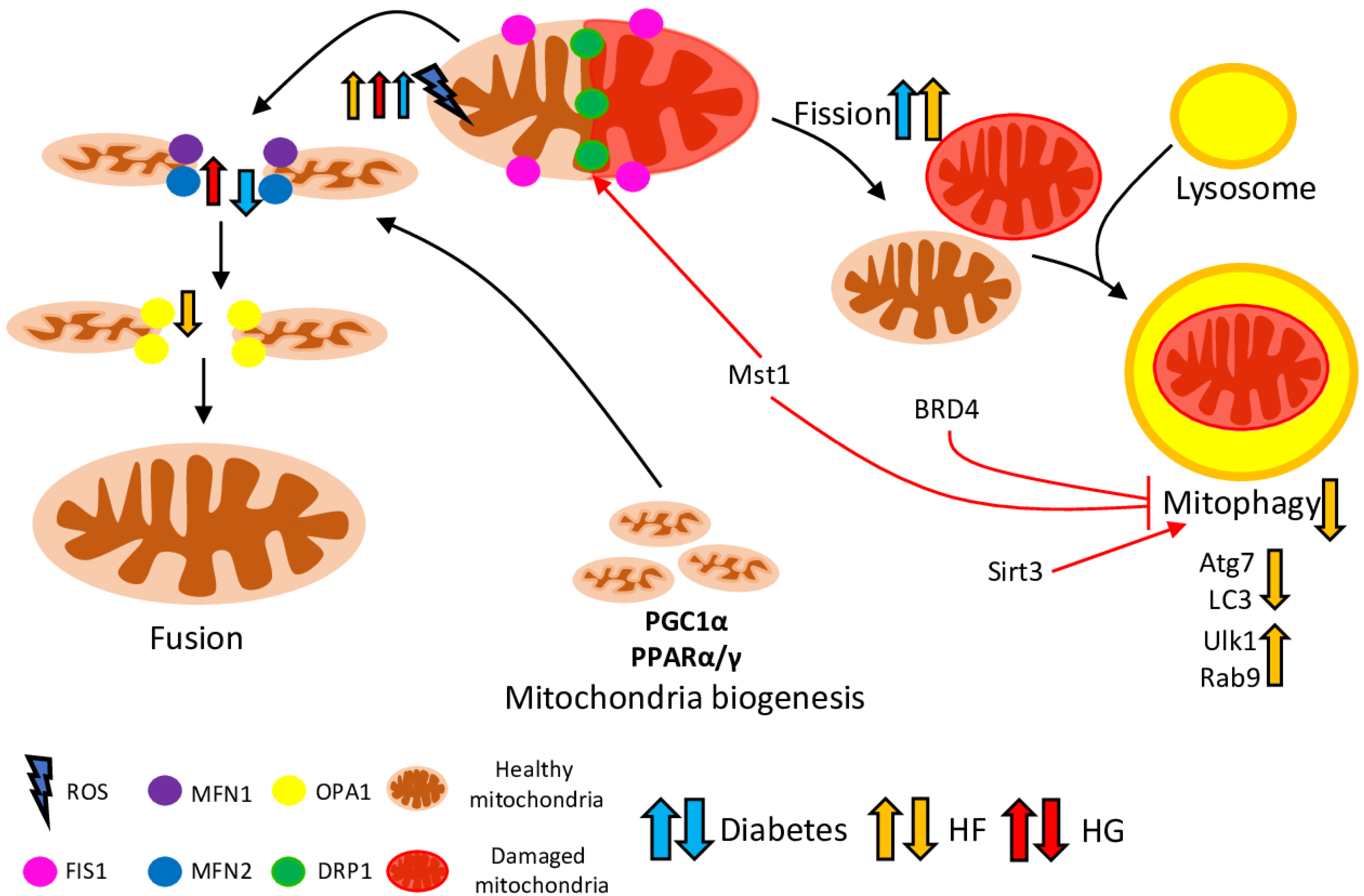
3. Mitochondrial Dynamics in DCM
4. Mitophagy
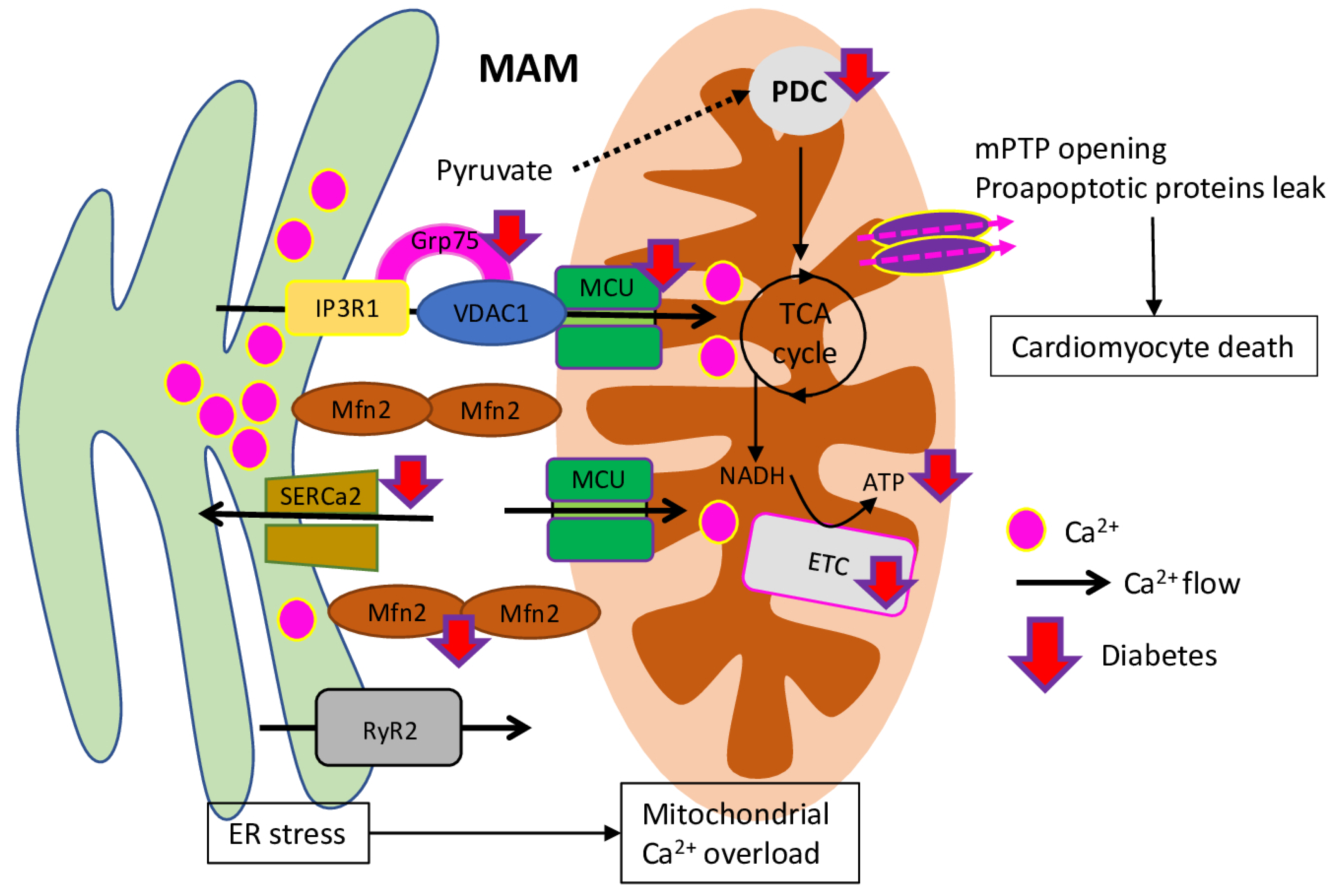
5. Mitochondrial Unbalanced Calcium Homeostasis
6. Mitochondrial Energy Metabolism in DCM
7. Mitochondria-Targeting Approach in DCM Treatment
8. Zinc Supplementation in DM Treatment and Cardioprotection
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC2 | acetyl-CoA carboxylase 2 |
| AF | atrial fibrillation |
| AKAP121 | A-kinase anchor protein |
| ANP | atrial natriuretic peptide |
| Atg7 | Autophagy-Related Protein 7 |
| BNIP3 | Bcl-2/adenovirus E1B 19-kDa protein-interacting protein 3 |
| BNP | brain natriuretic peptide |
| BRD4 | Bromodomain Containing 4 |
| DCM | Diabetic cardiomyopathy |
| DM | diabetes mellitus |
| DNM1L | Dynamin 1 like |
| Drp1 | Dynamic relative protein 1 |
| eNOS | endothelial nitric oxide synthase |
| ETC | electron transport chain |
| FAs | fatty acids |
| FIS1 | fission, mitochondrial 1 |
| FoxO1 | forkhead box protein O 1 |
| GLUT4 | glucose transporter type 4 |
| Grp75 | Stress-70 Protein, Mitochondrial |
| HF | high fat |
| HG | high glucose |
| IGFBP7 | Insulin Like Growth Factor Binding Protein 7 |
| IP3R | Inositol 1,4,5-Trisphosphate Receptor Type 3 |
| LACS1 | long chain acyl-CoA synthetase 1 |
| LC3 | Autophagy-Related Ubiquitin-Like Modifier LC3 A |
| LCAD | Acyl-CoA Dehydrogenase Long Chain |
| LTCCs | L-type calcium channels |
| LV | left ventricular |
| MAMs | mitochondrial associated membranes |
| MCU | mitochondrial Ca2+ uniporter |
| MFN1, MFN2 | mitofusin 1 and 2 |
| MICU1 | mitochondrial calcium uptake 1 |
| MMPs | matrix metalloproteinases |
| Mst1 | Mammalian sterile 20-like kinase 1 |
| OPA1 | optic atrophy protein 1 |
| Orai1 | Ca2+ release-activated calcium channel protein 1 |
| Parkin | Parkin RBR E3 Ubiquitin Protein Ligase |
| PDC | pyruvate dehydrogenase complex |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PINK1 | PTEN Induced Kinase 1 |
| Rab9 | Ras-related protein Rab-9 |
| ROS | reactive oxygen species |
| SR | sarcoplasmic reticulum |
| STZ | streptozotocin |
| TGF-β1 | Transforming growth factor beta 1 |
| TSP-1 | thrombospondin-1 |
| Ulk1 | unc51 like kinase 1 |
| VDAC | Voltage-dependent anion-selective channel protein 1 |
| ZDF | Zucker diabetic fatty |
| β-MHC | β-myosin heavy chain |
References
- Cardiomyopathy. National Center for Chronic Disease Prevention and Health Promotion. Available online: https://www.cdc.gov/heartdisease/cardiomyopathy.htm (accessed on 20 February 2022).
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic cardiomyopathy: Definition, diagnosis, and therapeutic implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Grubić Rotkvić, P.; Planinić, Z.; Liberati Pršo, A.-M.; Šikić, J.; Galić, E.; Rotkvić, L. The mystery of diabetic cardiomyopathy: From early concepts and underlying mechanisms to novel therapeutic possibilities. IJMS 2021, 22, 5973. [Google Scholar] [CrossRef] [PubMed]
- Kumric, M.; Ticinovic Kurir, T.; Borovac, J.A.; Bozic, J. Role of novel biomarkers in diabetic cardiomyopathy. World J. Diabetes 2021, 12, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N. Strategies to deliver microRNAs as potential therapeutics in the treatment of cardiovascular pathology. Drug Deliv. 2012, 19, 392–405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avagimyan, A. The pathophysiological basis of diabetic cardiomyopathy development. Curr. Probl. Cardiol. 2022, 101156. [Google Scholar] [CrossRef]
- Varma, U.; Koutsifeli, P.; Benson, V.L.; Mellor, K.M.; Delbridge, L.M.D. Molecular mechanisms of cardiac pathology in diabetes—Experimental Insights. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1949–1959. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Shah, A.K.; Tappia, P.S. Role of oxidative stress in metabolic and subcellular abnormalities in diabetic cardiomyopathy. Int. J. Mol. Sci. 2020, 21, E2413. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Liepinsh, E.; Murray, A.J.; Lemieux, H.; Dambrova, M.; Tepp, K.; Puurand, M.; Käämbre, T.; Han, W.H.; de Goede, P.; et al. Altered mitochondrial metabolism in the insulin-resistant heart. Acta Physiol. 2020, 228, e13430. [Google Scholar] [CrossRef]
- Smani, T.; Gallardo-Castillo, I.; Ávila-Médina, J.; Jimenez-Navarro, M.F.; Ordoñez, A.; Hmadcha, A. Impact of diabetes on cardiac and vascular disease: Role of calcium signaling. Curr. Med. Chem. 2019, 26, 4166–4177. [Google Scholar] [CrossRef]
- Kim, A.H.; Jang, J.E.; Han, J. Current status on the therapeutic strategies for heart failure and diabetic cardiomyopathy. Biomed. Pharm. 2022, 145, 112463. [Google Scholar] [CrossRef]
- Katogiannis, K.; Vlastos, D.; Kousathana, F.; Thymis, J.; Kountouri, A.; Korakas, E.; Plotas, P.; Papadopoulos, K.; Ikonomidis, I.; Lambadiari, V. Echocardiography, an indispensable tool for the management of diabetics, with or without coronary artery disease, in clinical practice. Medicina 2020, 56, E709. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Calicchio, F.; Grassi, G.; Mancia, G. Diabetic cardiomyopathy: How can cardiac magnetic resonance help? Acta Diabetol. 2020, 57, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Alzahrani, T. Cardiomyopathy imaging. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Soldatov, V.O.; Malorodova, T.N.; Balamutova, T.I.; Ksenofontov, A.O.; Dovgan, A.P.; Urozhevskaya, Z.S. Endothelial dysfunction: Comparative evaluation of ultrasound dopplerography, laser dopplerflowmetry and direct monitoring of arterial pressure for conducting pharmacological tests in rats. RRP 2018, 4, 73–80. [Google Scholar] [CrossRef]
- Tuleta, I.; Frangogiannis, N.G. Fibrosis of the diabetic heart: Clinical significance, molecular mechanisms, and therapeutic opportunities. Adv. Drug Deliv. Rev. 2021, 176, 113904. [Google Scholar] [CrossRef]
- Ibrahim, E.-S.H.; Dennison, J.; Frank, L.; Stojanovska, J. Diastolic cardiac function by MRI-imaging capabilities and clinical applications. Tomography 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C. Left atrial function in diabetes: Does it help? Acta Diabetol. 2021, 58, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Meng, K.; Pu, Y.; Zhang, X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2017, 133, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Chen, J.; Xu, Y. Thrombospondin-1: A key protein that induces fibrosis in diabetic complications. J. Diabetes Res. 2020, 2020, 8043135. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68–69, 490–506. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Chistiakov, D.; Revin, V.; Sobenin, I.; Orekhov, A.; Bobryshev, Y. Vascular endothelium: Functioning in norm, changes in atherosclerosis and current dietary approaches to improve endothelial function. MRMC 2015, 15, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mah, M.; Ritchie, R.H.; De Blasio, M.J. The adiponectin signalling pathway—A therapeutic target for the cardiac complications of type 2 diabetes? Pharmacol. Ther. 2022, 232, 108008. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, C.; Cheng, J. The role and molecular mechanism of FoxO1 in mediating cardiac hypertrophy. ESC Heart Fail. 2020, 7, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Cividini, F.; Scott, B.T.; Lehmann, K.; Diaz-Juarez, J.; Diemer, T.; Dai, A.; Suarez, J.A.; Jain, M.; Dillmann, W.H. Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J. Biol. Chem. 2018, 293, 8182–8195. [Google Scholar] [CrossRef]
- Ong, S.-B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef]
- Chengji, W.; Xianjin, F. Treadmill exercise alleviates diabetic cardiomyopathy by suppressing plasminogen activator inhibitor expression and enhancing ENOS in streptozotocin-induced male diabetic rats. Endocr. Connect. 2018, 7, 553–559. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Salonen, J.T.; Zhelankin, A.V.; Melnichenko, A.A.; Kaikkonen, J.; Bobryshev, Y.V.; Orekhov, A.N. Low density lipoprotein-containing circulating immune complexes: Role in atherosclerosis and diagnostic value. BioMed Res. Int. 2014, 2014, 205697. [Google Scholar] [CrossRef]
- Popov, L.-D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharm. 2021, 178, 2060–2076. [Google Scholar] [CrossRef]
- Onishi, M.; Okamoto, K. Mitochondrial clearance: Mechanisms and roles in cellular fitness. FEBS Lett. 2021, 595, 1239–1263. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Sazonova, M.A.; Postnov, A.Y.; Bobryshev, Y.V.; Orekhov, A.N. Changes of mitochondria in atherosclerosis: Possible determinant in the pathogenesis of the disease. Atherosclerosis 2013, 227, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Sazonova, M.A.; Postnov, A.Y.; Bobryshev, Y.V.; Orekhov, A.N. Mitochondrial mutations are associated with atherosclerotic lesions in the human aorta. Clin. Dev. Immunol. 2012, 2012, 832464. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Y.; Liu, Y.; Gao, J.; Feng, L.; Zhang, Y.; Shi, L.; Zhang, M.; Guo, D.; Qi, B.; et al. Mitochondrial quality control in the maintenance of cardiovascular homeostasis: The roles and interregulation of UPS, mitochondrial dynamics and mitophagy. Oxid. Med. Cell. Longev. 2021, 2021, 3960773. [Google Scholar] [CrossRef]
- Kaludercic, N.; Di Lisa, F. Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Sazonova, M.A.; Postnov, A.Y.; Salonen, J.T.; Bobryshev, Y.V.; Orekhov, A.N. Association of mitochondrial genetic variation with carotid atherosclerosis. PLoS ONE 2013, 8, e68070. [Google Scholar] [CrossRef]
- Hu, L.; Ding, M.; Tang, D.; Gao, E.; Li, C.; Wang, K.; Qi, B.; Qiu, J.; Zhao, H.; Chang, P.; et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics 2019, 9, 3687–3706. [Google Scholar] [CrossRef]
- Garvin, A.M.; Jackson, M.A.; Korzick, D.H. Inhibition of programmed necrosis limits infarct size through altered mitochondrial and immune responses in the aged female rat heart. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1434–H1442. [Google Scholar] [CrossRef]
- Apaijai, N.; Jinawong, K.; Singhanat, K.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. Necrostatin-1 reduces cardiac and mitochondrial dysfunction in prediabetic rats. J. Endocrinol. 2021, 251, 27–39. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; Yuan, M.; Liu, X.; et al. Hyperglycemia induces endoplasmic reticulum stress in atrial cardiomyocytes, and mitofusin-2 downregulation prevents mitochondrial dysfunction and subsequent cell death. Oxid. Med. Cell. Longev. 2020, 2020, 6569728. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Mitrofanov, K.Y.; Zhelankin, A.V.; Sazonova, M.A.; Postnov, A.Y.; Revin, V.V.; Bobryshev, Y.V.; Orekhov, A.N. Quantitative assessment of heteroplasmy of mitochondrial genome: Perspectives in diagnostics and methodological pitfalls. BioMed Res. Int. 2014, 2014, 292017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Wang, Y. Mitofusin-2 enhances mitochondrial contact with the endoplasmic reticulum and promotes diabetic cardiomyopathy. Front. Physiol. 2021, 12, 707634. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.J.; Qi, X. Drp1 phosphorylation by MAPK1 causes mitochondrial dysfunction in cell culture model of Huntington’s disease. Biochem. Biophys. Res. Commun. 2018, 496, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-R.; Zheng, D.-L.; Liu, P.-M.; Yang, H.; Li, L.-A.; Kuang, S.-J.; Lai, Y.-Y.; Rao, F.; Xue, Y.-M.; Lin, J.-J.; et al. High glucose induces Drp1-mediated mitochondrial fission via the orai1 calcium channel to participate in diabetic cardiomyocyte hypertrophy. Cell Death Dis. 2021, 12, 216. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Ma, Z.; Hu, W.; Sun, D. Understanding the role of mammalian sterile 20-like kinase 1 (MST1) in cardiovascular disorders. J. Mol. Cell. Cardiol. 2018, 114, 141–149. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Yang, X.; Lin, J.; Man, W.; Dong, Y.; Zhang, Y.; Zhao, Z.; Wang, H.; Sun, D. Mst1 knockout alleviates mitochondrial fission and mitigates left ventricular remodeling in the development of diabetic cardiomyopathy. Front. Cell Dev. Biol. 2021, 8, 628842. [Google Scholar] [CrossRef]
- Czachor, A.; Failla, A.; Lockey, R.; Kolliputi, N. Pivotal role of AKAP121 in mitochondrial physiology. Am. J. Physiol. Cell Physiol. 2016, 310, C625–C628. [Google Scholar] [CrossRef]
- Tsushima, K.; Bugger, H.; Wende, A.R.; Soto, J.; Jenson, G.A.; Tor, A.R.; McGlauflin, R.; Kenny, H.C.; Zhang, Y.; Souvenir, R.; et al. Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circ. Res. 2018, 122, 58–73. [Google Scholar] [CrossRef]
- Koncsos, G.; Varga, Z.V.; Baranyai, T.; Boengler, K.; Rohrbach, S.; Li, L.; Schlüter, K.-D.; Schreckenberg, R.; Radovits, T.; Oláh, A.; et al. Diastolic dysfunction in prediabetic male rats: Role of mitochondrial oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H927–H943. [Google Scholar] [CrossRef]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.-I.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ. Res. 2019, 124, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Z.; Fan, Y.; Zhang, M.; Feng, X.; Lin, J.; Hu, J.; Cheng, Z.; Sun, C.; Liu, T.; et al. Mst1 inhibits Sirt3 expression and contributes to diabetic cardiomyopathy through inhibiting parkin-dependent mitophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-parkin-mediated mitophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and type 2 diabetes: Role in inflammation, oxidative stress, and mitochondrial function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Kolwicz, S.C.; Wang, P.; Roe, N.D.; Villet, O.; Nishi, K.; Hsu, Y.-W.A.; Flint, G.V.; Caudal, A.; Wang, W.; et al. Increasing fatty acid oxidation prevents high-fat diet-induced cardiomyopathy through regulating parkin-mediated mitophagy. Circulation 2020, 142, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Alexanian, M.; Linares-Saldana, R.; González-Terán, B.; Andreoletti, G.; Huang, Y.; Connolly, A.J.; Kim, W.; Hsu, A.; Duan, Q.; et al. BRD4 (bromodomain-containing protein 4) interacts with GATA4 (GATA binding protein 4) to govern mitochondrial homeostasis in adult cardiomyocytes. Circulation 2020, 142, 2338–2355. [Google Scholar] [CrossRef]
- Duan, Q.; McMahon, S.; Anand, P.; Shah, H.; Thomas, S.; Salunga, H.T.; Huang, Y.; Zhang, R.; Sahadevan, A.; Lemieux, M.E.; et al. BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci. Transl. Med. 2017, 9, eaah5084. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.-X.; Chen, W.-J. BET-inhibition by JQ1 alleviates streptozotocin-induced diabetic cardiomyopathy. Toxicol. Appl. Pharmacol. 2018, 352, 9–18. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, D.; Tian, Y.; Xie, Z.; Zou, M.-H. BRD4 inhibition by JQ1 prevents high-fat diet-induced diabetic cardiomyopathy by activating PINK1/parkin-mediated mitophagy in vivo. J. Mol. Cell. Cardiol. 2020, 149, 1–14. [Google Scholar] [CrossRef]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.-I.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ. Res. 2021, 129, 1105–1121. [Google Scholar] [CrossRef]
- Diaz-Juarez, J.; Suarez, J.A.; Dillmann, W.H. Mitochondrial calcium handling and heart disease in diabetes mellitus. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2021, 1867, 165984. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Trong, M.T.; Ullah, A.; Lederer, W.J.; Jafri, M.S. Cardiac alternans occurs through the synergy of voltage- and calcium-dependent mechanisms. Membranes 2021, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Kettlewell, S.; Saxena, P.; Dempster, J.; Colman, M.A.; Myles, R.C.; Smith, G.L.; Workman, A.J. Dynamic clamping human and rabbit atrial calcium current: Narrowing ICaL window abolishes early afterdepolarizations. J. Physiol. 2019, 597, 3619–3638. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Juarez, J.; Suarez, J.; Cividini, F.; Scott, B.T.; Diemer, T.; Dai, A.; Dillmann, W.H. Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. Am. J. Physiol. Cell Physiol. 2016, 311, C1005–C1013. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Liu, F.; Jing, Z.; Huang, Q.; Zhao, Y.; Cao, H.; Li, J.; Yin, C.; Xing, J.; Li, F. MICU1 alleviates diabetic cardiomyopathy through mitochondrial Ca2+-dependent antioxidant response. Diabetes 2017, 66, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Crisosto, C.; Pennanen, C.; Vasquez-Trincado, C.; Morales, P.E.; Bravo-Sagua, R.; Quest, A.F.G.; Chiong, M.; Lavandero, S. Sarcoplasmic reticulum–mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol. 2017, 14, 342–360. [Google Scholar] [CrossRef]
- Dia, M.; Gomez, L.; Thibault, H.; Tessier, N.; Leon, C.; Chouabe, C.; Ducreux, S.; Gallo-Bona, N.; Tubbs, E.; Bendridi, N.; et al. Reduced reticulum–mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res. Cardiol. 2020, 115, 74. [Google Scholar] [CrossRef]
- Federico, M.; Portiansky, E.L.; Sommese, L.; Alvarado, F.J.; Blanco, P.G.; Zanuzzi, C.N.; Dedman, J.; Kaetzel, M.; Wehrens, X.H.T.; Mattiazzi, A.; et al. Calcium-calmodulin-dependent protein kinase mediates the intracellular signalling pathways of cardiac apoptosis in mice with impaired glucose tolerance. J. Physiol. 2017, 595, 4089–4108. [Google Scholar] [CrossRef]
- Federico, M.; Zavala, M.; Vico, T.; López, S.; Portiansky, E.; Alvarez, S.; Abrille, M.C.V.; Palomeque, J. CaMKII activation in early diabetic hearts induces altered sarcoplasmic reticulum-mitochondria signaling. Sci. Rep. 2021, 11, 20025. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Xie, A.; Kim, T.-Y.; Terentyeva, R.; Liu, M.; Shi, G.; Feng, F.; Choi, B.-R.; Terentyev, D.; et al. Interleukin-1β, oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. JACC Basic Transl. Sci. 2021, 6, 42–52. [Google Scholar] [CrossRef]
- Joseph, L.C.; Subramanyam, P.; Radlicz, C.; Trent, C.M.; Iyer, V.; Colecraft, H.M.; Morrow, J.P. Mitochondrial oxidative stress during cardiac lipid overload causes intracellular calcium leak and arrhythmia. Heart Rhythm 2016, 13, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Wang, W.; Chen, J.; Zhang, Z.; Zheng, Q.; Liu, Q.; Cai, L. Protection against diabetic cardiomyopathy is achieved using a combination of sulforaphane and zinc in type 1 diabetic OVE26 mice. J. Cell. Mol. Med. 2019, 23, 6319–6330. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz-Icöz, S.; Al Said, S.; Radovits, T.; Li, S.; Brune, M.; Hegedűs, P.; Atmanli, A.; Ruppert, M.; Brlecic, P.; Lehmann, L.H.; et al. Oral treatment with a zinc complex of acetylsalicylic acid prevents diabetic cardiomyopathy in a rat model of type-2 diabetes: Activation of the akt pathway. Cardiovasc. Diabetol. 2016, 15, 75. [Google Scholar] [CrossRef]
- Puchenkova, O.A.; Nadezhdin, S.V.; Soldatov, V.O.; Zhuchenko, M.A.; Korshunova, D.S.; Kubekina, M.V.; Korshunov, E.N.; Korokina, L.V.; Golubinskaya, P.A.; Kulikov, A.L.; et al. Study of antiatherosclerotic and endothelioprotective activity of peptide agonists of EPOR/CD131 heteroreceptor. Pharm. Pharmacol. 2020, 8, 100–111. [Google Scholar] [CrossRef]
- Shams, A.S.; Mohammed, M.H.; Loka, M.M.; Abdel Rahman, G.M. Assessment of the protective role of prenatal zinc versus insulin supplementation on fetal cardiac damage induced by maternal diabetes in rat using caspase-3 and KI67 immunohistochemical stains. Cardiol. Res. Pract. 2016, 2016, 7469549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Amarsingh, G.V.; Cheung, C.C.H.; Wu, D.; Narayanan, U.; Zhang, L.; Cooper, G.J.S. Restoration of myocellular copper-trafficking proteins and mitochondrial copper enzymes repairs cardiac function in rats with diabetes-evoked heart failure. Metallomics 2020, 12, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Aaseth, J. The role of zinc and copper in insulin resistance and diabetes mellitus. Curr. Med. Chem. 2020, 27, 6643–6657. [Google Scholar] [CrossRef]
- Honka, H.; Solis-Herrera, C.; Triplitt, C.; Norton, L.; Butler, J.; DeFronzo, R.A. Therapeutic manipulation of myocardial metabolism: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 2022–2039. [Google Scholar] [CrossRef]
- Mereweather, L.J.; Montes Aparicio, C.N.; Heather, L.C. Positioning metabolism as a central player in the diabetic heart. J. Lipid Atheroscler. 2020, 9, 92–109. [Google Scholar] [CrossRef]
- Lou, P.-H.; Lucchinetti, E.; Scott, K.Y.; Huang, Y.; Gandhi, M.; Hersberger, M.; Clanachan, A.S.; Lemieux, H.; Zaugg, M. Alterations in fatty acid metabolism and sirtuin signaling characterize early type-2 diabetic hearts of fructose-fed rats. Physiol. Rep. 2017, 5, e13388. [Google Scholar] [CrossRef]
- Parker, A.M.; Tate, M.; Prakoso, D.; Deo, M.; Willis, A.M.; Nash, D.M.; Donner, D.G.; Crawford, S.; Kiriazis, H.; Granata, C.; et al. Characterisation of the myocardial mitochondria structural and functional phenotype in a murine model of diabetic cardiomyopathy. Front. Physiol. 2021, 12, 672252. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.R.; Schell, J.C.; Ha, C.-M.; Pepin, M.E.; Khalimonchuk, O.; Schwertz, H.; Pereira, R.O.; Brahma, M.K.; Tuinei, J.; Contreras-Ferrat, A.; et al. Maintaining myocardial glucose utilization in diabetic cardiomyopathy accelerates mitochondrial dysfunction. Diabetes 2020, 69, 2094–2111. [Google Scholar] [CrossRef] [PubMed]
- Bombicino, S.S.; Iglesias, D.E.; Rukavina-Mikusic, I.A.; Buchholz, B.; Gelpi, R.J.; Boveris, A.; Valdez, L.B. Hydrogen peroxide, nitric oxide and ATP are molecules involved in cardiac mitochondrial biogenesis in diabetes. Free Radic. Biol. Med. 2017, 112, 267–276. [Google Scholar] [CrossRef]
- Rukavina-Mikusic, I.A.; Rey, M.; Martinefski, M.; Tripodi, V.; Valdez, L.B. Temporal evolution of cardiac mitochondrial dysfunction in a type 1 diabetes model. mitochondrial complex i impairment, and H2O2 and NO productions as early subcellular events. Free Radic. Biol. Med. 2021, 162, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Bombicino, S.S.; Iglesias, D.E.; Mikusic, I.A.R.; D’Annunzio, V.; Gelpi, R.J.; Boveris, A.; Valdez, L.B. Diabetes impairs heart mitochondrial function without changes in resting cardiac performance. Int. J. Biochem. Cell Biol. 2016, 81, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; He, Y.-L.; Li, X.-M.; Nie, F.; Zhou, X.-K. Endogenous asymmetric dimethylarginine accumulation precipitates the cardiac and mitochondrial dysfunctions in type 1 diabetic rats. Eur. J. Pharmacol. 2021, 902, 174081. [Google Scholar] [CrossRef]
- Kanaan, G.N.; Patten, D.A.; Redpath, C.J.; Harper, M.-E. Atrial fibrillation is associated with impaired atrial mitochondrial energetics and supercomplex formation in adults with type 2 diabetes. Can. J. Diabetes 2019, 43, 67–75.e1. [Google Scholar] [CrossRef]
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxid. Med. Cell. Longev. 2016, 2016, 8470394. [Google Scholar] [CrossRef]
- Jayakumari, N.R.; Rajendran, R.S.; Sivasailam, A.; Vimala, S.S.; Nanda, S.; Manjunatha, S.; Pillai, V.V.; Karunakaran, J.; Gopala, S. Impaired substrate-mediated cardiac mitochondrial complex i respiration with unaltered regulation of fatty acid metabolism and oxidative stress status in type 2 diabetic Asian Indians. J. Diabetes 2020, 12, 542–555. [Google Scholar] [CrossRef]
- Mdaki, K.S.; Larsen, T.D.; Wachal, A.L.; Schimelpfenig, M.D.; Weaver, L.J.; Dooyema, S.D.R.; Louwagie, E.J.; Baack, M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H681–H692. [Google Scholar] [CrossRef]
- Raji, S.R.; Nandini, R.J.; Ashok, S.; Anand, R.C.; Vivek, P.V.; Karunakaran, J.; Sreelatha, H.V.; Manjunatha, S.; Gopala, S. Diminished substrate-mediated cardiac mitochondrial respiration and elevated autophagy in adult male offspring of gestational diabetic rats. IUBMB Life 2021, 73, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Gandy, T.C.T.; Baack, M.L. Mitochondrial transfer improves cardiomyocyte bioenergetics and viability in male rats exposed to pregestational diabetes. IJMS 2021, 22, 2382. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.-C.; Lacefield, J.C.; Lu, Y.; Tissier, S.L.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.; Higgins, G.C.; De Blasio, M.J.; Lindblom, R.; Prakoso, D.; Deo, M.; Kiriazis, H.; Park, M.; Baeza-Garza, C.D.; Caldwell, S.T.; et al. The mitochondria-targeted methylglyoxal sequestering compound, mitogamide, is cardioprotective in the diabetic heart. Cardiovasc. Drugs 2019, 33, 669–674. [Google Scholar] [CrossRef]
- Park, M.; Nishimura, T.; Baeza-Garza, C.D.; Caldwell, S.T.; Pun, P.B.L.; Prag, H.A.; Young, T.; Sauchanka, O.; Logan, A.; Forkink, M.; et al. Confirmation of the cardioprotective effect of mitogamide in the diabetic heart. Cardiovasc. Drugs 2020, 34, 823–834. [Google Scholar] [CrossRef]
- Belosludtseva, N.V.; Starinets, V.S.; Mikheeva, I.B.; Serov, D.A.; Astashev, M.E.; Belosludtsev, M.N.; Dubinin, M.V.; Belosludtsev, K.N. Effect of the MPT pore inhibitor alisporivir on the development of mitochondrial dysfunction in the heart tissue of diabetic mice. Biology 2021, 10, 839. [Google Scholar] [CrossRef]
- Yang, F.; Yu, X.; Li, T.; Wu, J.; Zhao, Y.; Liu, J.; Sun, A.; Dong, S.; Wu, J.; Zhong, X.; et al. Exogenous H 2 S regulates endoplasmic reticulum-mitochondria cross-talk to inhibit apoptotic pathways in stz-induced type i diabetes. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E190–E203. [Google Scholar] [CrossRef]
- Gasser, E.; Moutos, C.P.; Downes, M.; Evans, R.M. FGF1—A new weapon to control type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2017, 13, 599–609. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Metabolic messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- Tanajak, P.; Sa-Nguanmoo, P.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Chattipakorn, S.C.; Chattipakorn, N. Fibroblast growth factor 21 (FGF21) therapy attenuates left ventricular dysfunction and metabolic disturbance by improving FGF21 sensitivity, cardiac mitochondrial redox homoeostasis and structural changes in pre-diabetic rats. Acta Physiol. 2016, 217, 287–299. [Google Scholar] [CrossRef]
- Yang, H.; Feng, A.; Lin, S.; Yu, L.; Lin, X.; Yan, X.; Lu, X.; Zhang, C. Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis. 2018, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, Y.; Wang, S.; Zhao, T.; Gong, F.; Zhao, Y.; Wang, B.; Huang, Y.; Cheng, Z.; Zhu, G.; et al. FGF1ΔHBS prevents diabetic cardiomyopathy by maintaining mitochondrial homeostasis and reducing oxidative stress via AMPK/Nur77 suppression. Signal Transduct. Target. Ther. 2021, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Hassany, M.A.; El-Ebidi, A.M.; Hafez, M.; Tawfik, N.M.; Wang, P.H. Role of mitochondria in diabetic cardiomyopathy and treatment challenges for mitochondrial dysfunction. IOSR J. Dent. Med. Sci. 2022, 21, 7–19. [Google Scholar] [CrossRef]
- Asghari, K.; Shargh, Z.; Fatehfar, S.; Chodari, L.; Sameei, P. The impact of zinc on the molecular signaling pathways in the diabetes disease. J. Trace Elem. Med. Biol. 2022, 72, 126985. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, E.S.; Checcaglini, F.; Fanò-Illic, G.; Fulle, S. H2O2/Ca2+/Zn2+ complex can be considered a “collaborative sensor” of the mitochondrial capacity? Antioxidants 2022, 11, 342. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, L. Zinc homeostasis plays an important role in the prevention of obesity-induced cardiac inflammation, remodeling and dysfunction. J. Trace Elem. Med. Biol. 2020, 62, 126615. [Google Scholar] [CrossRef]
- Tamura, Y. The role of zinc homeostasis in the prevention of diabetes mellitus and cardiovascular diseases. JAT 2021, 28, 1109–1122. [Google Scholar] [CrossRef]
- MacKenzie, S.; Bergdahl, A. Zinc homeostasis in diabetes mellitus and vascular complications. Biomedicines 2022, 10, 139. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Wathurapatha, W.S.; Galappatthy, P.; Katulanda, P.; Jayawardena, R.; Constantine, G.R. Zinc supplementation in prediabetes: A randomized double-blind placebo-controlled clinical trial. J. Diabetes 2018, 10, 386–397. [Google Scholar] [CrossRef]
- Peel, R.; Hure, A.; Wiggers, J.; McEvoy, M.; Holliday, E.; Searles, A.; Reeves, P.; Ranasinghe, P.; Jayawardena, R.; Samman, S.; et al. Zinc in preventing the progression of pre-diabetes (ZIPPeD Study)—Study protocol for a randomised placebo-controlled trial in Australia. Trials 2019, 20, 219. [Google Scholar] [CrossRef]
- Attia, J.R.; Holliday, E.; Weaver, N.; Peel, R.; Fleming, K.C.; Hure, A.; Wiggers, J.; McEvoy, M.; Searles, A.; Reeves, P.; et al. The effect of zinc supplementation on glucose homeostasis: A randomised double-blind placebo-controlled trial. Acta Diabetol. 2022, 59, 965–975. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Mitochondrial Abnormalities in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 7863. https://doi.org/10.3390/ijms23147863
Dabravolski SA, Sadykhov NK, Kartuesov AG, Borisov EE, Sukhorukov VN, Orekhov AN. The Role of Mitochondrial Abnormalities in Diabetic Cardiomyopathy. International Journal of Molecular Sciences. 2022; 23(14):7863. https://doi.org/10.3390/ijms23147863
Chicago/Turabian StyleDabravolski, Siarhei A., Nikolay K. Sadykhov, Andrey G. Kartuesov, Evgeny E. Borisov, Vasily N. Sukhorukov, and Alexander N. Orekhov. 2022. "The Role of Mitochondrial Abnormalities in Diabetic Cardiomyopathy" International Journal of Molecular Sciences 23, no. 14: 7863. https://doi.org/10.3390/ijms23147863
APA StyleDabravolski, S. A., Sadykhov, N. K., Kartuesov, A. G., Borisov, E. E., Sukhorukov, V. N., & Orekhov, A. N. (2022). The Role of Mitochondrial Abnormalities in Diabetic Cardiomyopathy. International Journal of Molecular Sciences, 23(14), 7863. https://doi.org/10.3390/ijms23147863







