Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci
Abstract
1. Introduction
2. Results
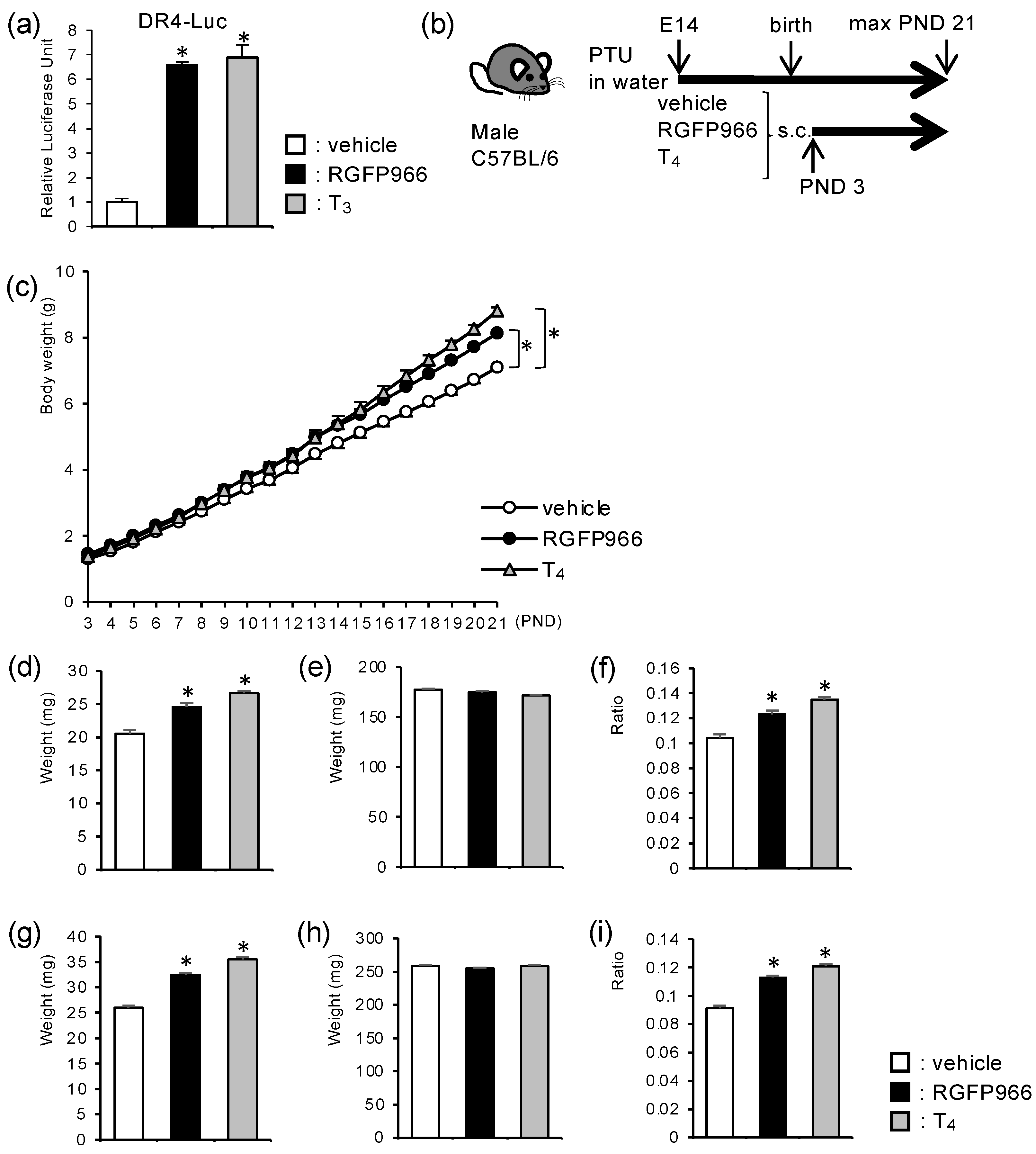
2.1. HDAC3-Inhibitor Treatment Increased Body Weight and Cerebellar Weight in Perinatal Hypothyroid Mice
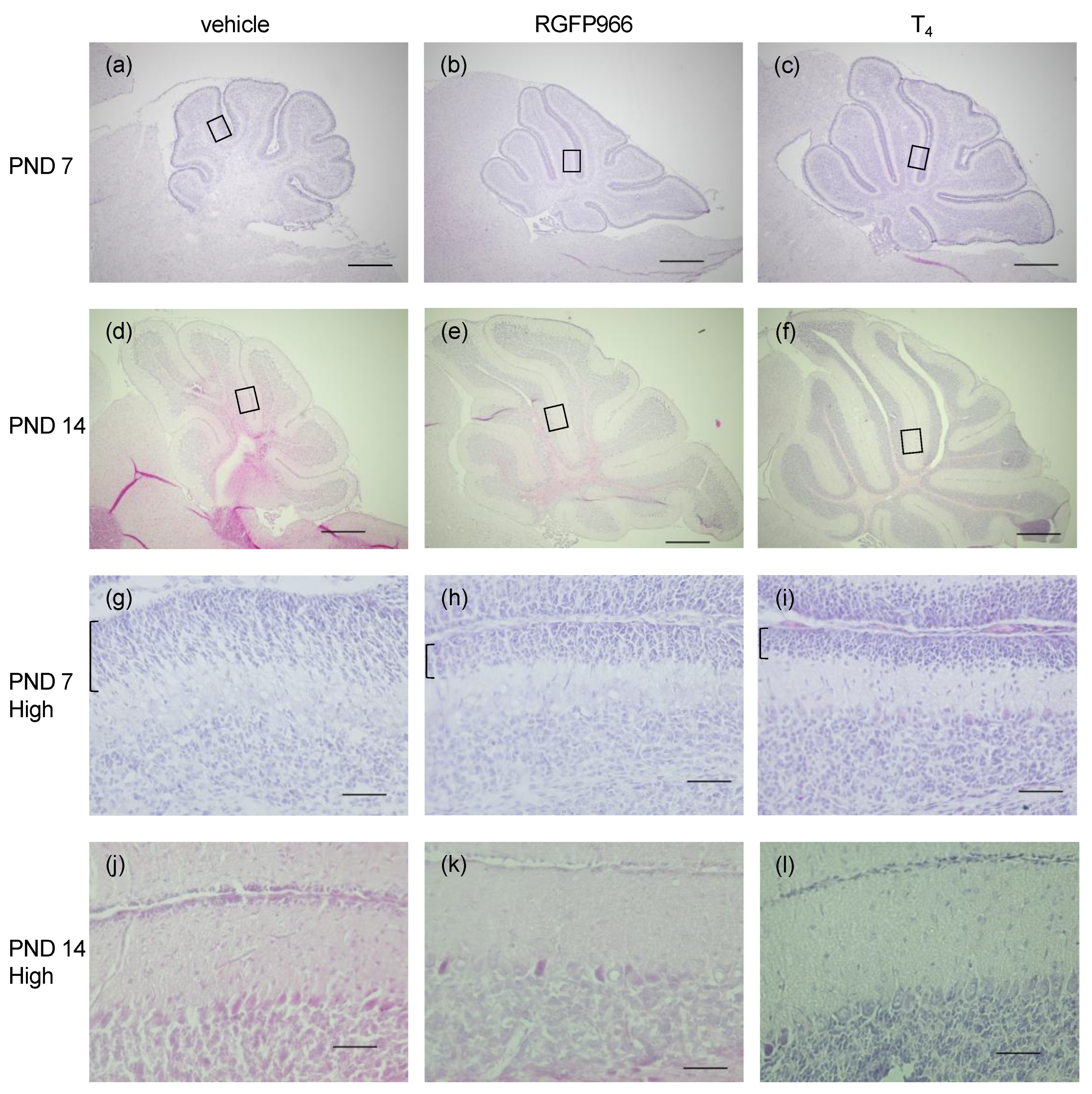
2.2. HDAC3 Inhibitor Alleviated Cerebellar Morphological Defects in Perinatal Hypothyroid Mice
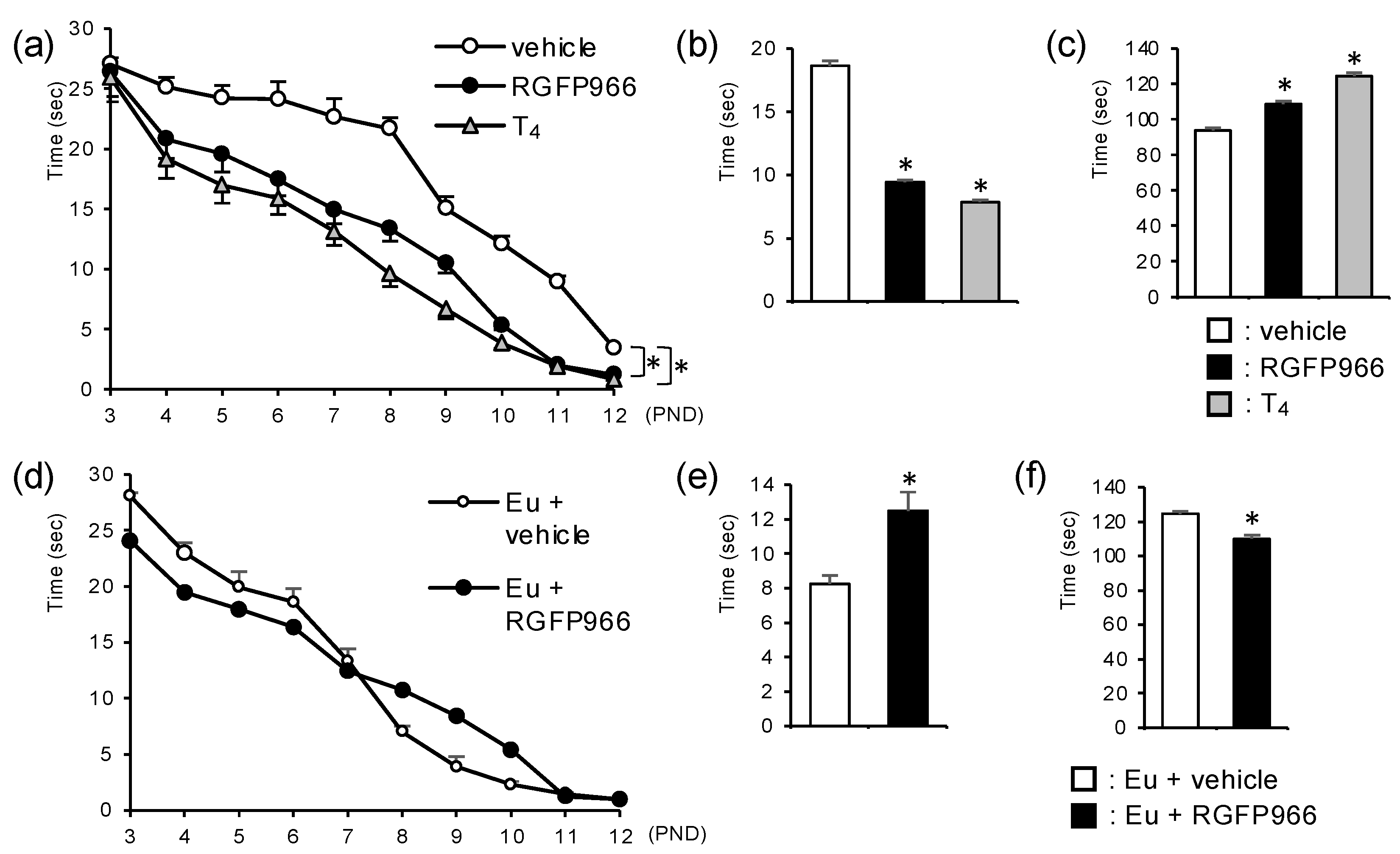
2.3. HDAC3 Inhibitor Alleviated Motor Coordination Defects in Perinatal Hypothyroid Mice
2.4. HDAC3 Inhibitor Alleviated Reduction in mRNA Levels of Cerebellar Thyroid Hormone Receptor-Target Genes in Perinatal Hypothyroid Mice
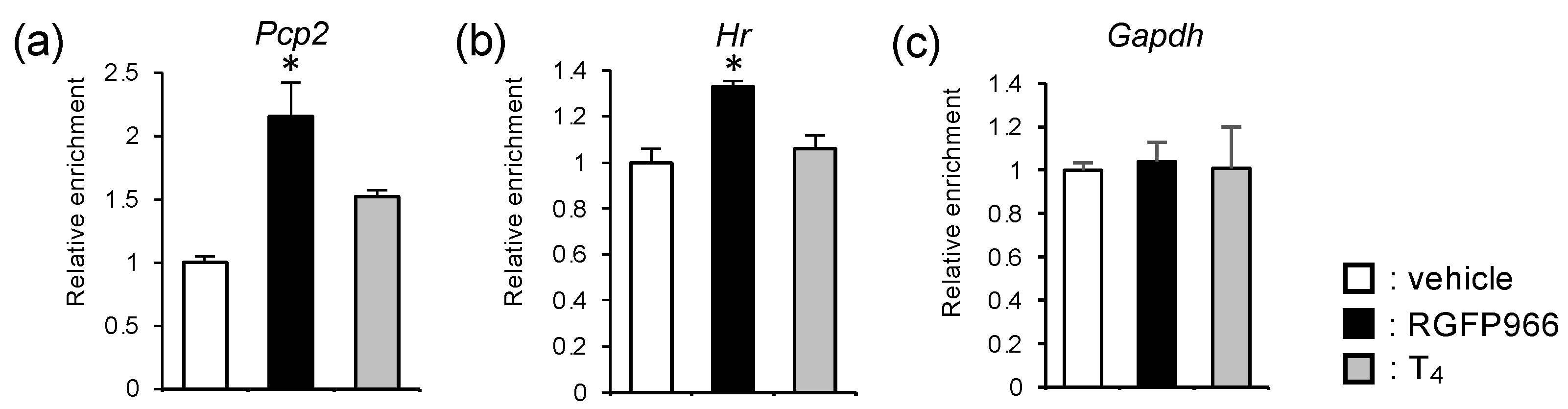
2.5. HDAC3-Inhibitor Treatment Increased Histone Acetylation Levels at Cerebellar Thyroid Hormone Receptor-Target Gene Loci in Perinatal Hypothyroid Mice
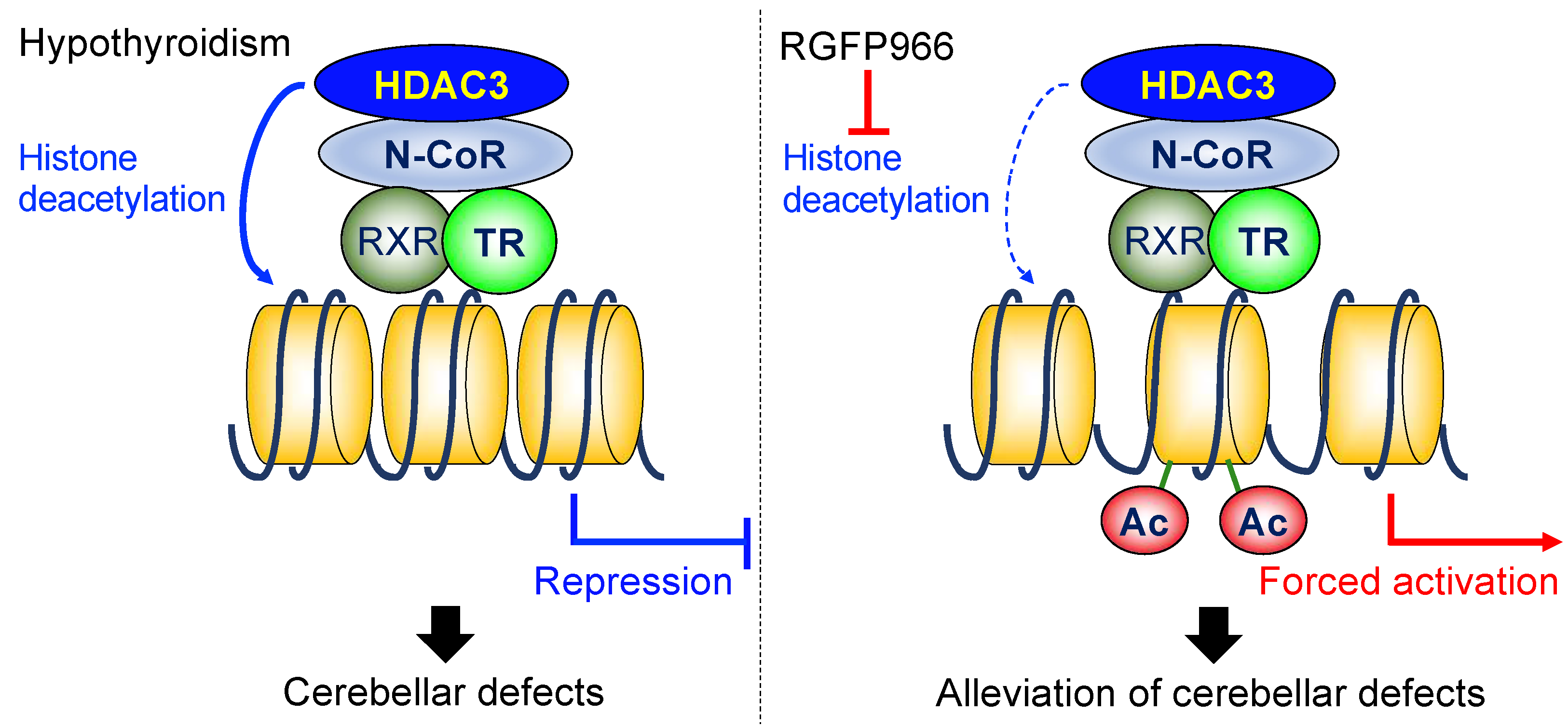
3. Discussion
4. Materials and Methods
4.1. Luciferase-Based Transcriptional Reporter Assay
4.2. Animals
4.3. Histological Studies
4.4. Behavioral Studies
4.5. Quantitative RT-PCR
4.6. In Vivo ChIP Assay
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Leto, K.; Arancillo, M.; Becker, E.B.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef] [PubMed]
- Farini, D.; Marazziti, D.; Geloso, M.C.; Sette, C. Transcriptome programs involved in the development and structure of the cerebellum. Cell Mol. Life Sci. 2021, 78, 6431–6451. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulos, I.; Sepp, M.; Frömel, R.; Leiss, K.; Trost, N.; Leushkin, E.; Okonechnikov, K.; Joshi, P.; Giere, P.; Kutscher, L.M.; et al. Developmental and evolutionary dynamics of cis-regulatory elements in mouse cerebellar cells. Science 2021, 373, eabg4696. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Schmahmann, J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef]
- Cendelin, J. From mice to men: Lessons from mutant ataxic mice. Cerebellum Ataxias 2014, 1, 4. [Google Scholar] [CrossRef]
- Wiebel, J. Cerebellar-ataxic syndrome in children and adolescents with hypothyroidism under treatment. Acta Pædiatrica 1976, 65, 201–205. [Google Scholar] [CrossRef]
- Adamaszek, M.; D’Agata, F.; Ferrucci, R.; Habas, C.; Keulen, S.; Kirkby, K.C.; Leggio, M.; Mariën, P.; Molinari, M.; Moulton, E.; et al. Consensus Paper: Cerebellum and Emotion. Cerebellum 2017, 16, 552–576. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Manto, M.; Cattaneo, Z.; Clausi, S.; Ferrari, C.; Gabrieli, J.D.E.; Guell, X.; Heleven, E.; Lupo, M.; Ma, Q.; et al. Consensus Paper: Cerebellum and Social Cognition. Cerebellum 2020, 19, 833–868. [Google Scholar] [CrossRef]
- Koibuchi, N.; Chin, W.W. Thyroid hormone action and brain development. Trends Endocrinol. Metab. 2000, 11, 123–128. [Google Scholar] [CrossRef]
- Ishii, S.; Amano, I.; Koibuchi, N. The Role of Thyroid Hormone in the Regulation of Cerebellar Development. Endocrinol. Metab. 2021, 36, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Wassner, A.J.; Brown, R.S. Hypothyroidism in the newborn period. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Vella, K.R.; Hollenberg, A.N. The actions of thyroid hormone signaling in the nucleus. Mol. Cell Endocrinol. 2017, 458, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. Thyroid hormone receptors: Multiple forms, multiple possibilities. Endocr. Rev. 1993, 14, 184–193. [Google Scholar] [CrossRef]
- Mellström, B.; Naranjo, J.R.; Santos, A.; Gonzalez, A.M.; Bernal, J. Independent expression of the alpha and beta c-erbA genes in developing rat brain. Mol. Endocrinol. 1991, 5, 1339–1350. [Google Scholar] [CrossRef]
- Strait, K.A.; Schwartz, H.L.; Seybold, V.S.; Ling, N.C.; Oppenheimer, J.H. Immunofluorescence localization of thyroid hormone receptor protein beta 1 and variant alpha 2 in selected tissues: Cerebellar Purkinje cells as a model for beta 1 receptor-mediated developmental effects of thyroid hormone in brain. Proc. Natl. Acad. Sci. USA 1991, 88, 3887–3891. [Google Scholar] [CrossRef]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Verdin, E.; Ott, M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015, 16, 258–264. [Google Scholar] [CrossRef]
- Ishizuka, T.; Lazar, M.A. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol. Cell Biol. 2003, 23, 5122–5131. [Google Scholar] [CrossRef]
- Ishii, S. The Role of Histone Deacetylase 3 Complex in Nuclear Hormone Receptor Action. Int. J. Mol. Sci. 2021, 22, 9138. [Google Scholar] [CrossRef]
- Karagianni, P.; Wong, J. HDAC3: Taking the SMRT-N-CoRrect road to repression. Oncogene 2007, 26, 5439–5449. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.P.; Rochel, N.; Ruff, M.; Vivat, V.; Chambon, P.; Gronemeyer, H.; Moras, D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature 1995, 378, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Shabtai, Y.; Nagaraj, N.K.; Batmanov, K.; Cho, Y.W.; Guan, Y.; Jiang, C.; Remsberg, J.; Forrest, D.; Lazar, M.A. A coregulator shift, rather than the canonical switch, underlies thyroid hormone action in the liver. Genes Dev. 2021, 35, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Hagen, S.G.; Strait, K.A.; Oppenheimer, J.H. Identification of thyroid hormone response elements in rodent Pcp-2, a developmentally regulated gene of cerebellar Purkinje cells. J. Biol. Chem. 1994, 269, 13346–13352. [Google Scholar] [CrossRef]
- Dudazy-Gralla, S.; Nordström, K.; Hofmann, P.J.; Meseh, D.A.; Schomburg, L.; Vennström, B.; Mittag, J. Identification of thyroid hormone response elements in vivo using mice expressing a tagged thyroid hormone receptor α1. Biosci. Rep. 2013, 33, e00027. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; Mangelsdorf, D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 2003, 1, e012. [Google Scholar] [CrossRef]
- Heyser, C.J. Assessment of developmental milestones in rodents. Curr. Protoc. Neurosci. 2004, 8, 8–18. [Google Scholar] [CrossRef]
- Jones, B.J.; Roberts, D.J. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968, 259, 211. [Google Scholar] [CrossRef]
- Amano, I.; Takatsuru, Y.; Toya, S.; Haijima, A.; Iwasaki, T.; Grasberger, H.; Refetoff, S.; Koibuchi, N. Aberrant Cerebellar Development in Mice Lacking Dual Oxidase Maturation Factors. Thyroid 2016, 26, 741–752. [Google Scholar] [CrossRef]
- Feather-Schussler, D.N.; Ferguson, T.S. A Battery of Motor Tests in a Neonatal Mouse Model of Cerebral Palsy. J. Vis. Exp. 2016, 117, e53569. [Google Scholar] [CrossRef]
- Schönfeld, L.M.; Dooley, D.; Jahanshahi, A.; Temel, Y.; Hendrix, S. Evaluating rodent motor functions: Which tests to choose? Neurosci. Biobehav. Rev. 2017, 83, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Emmett, M.J.; Lazar, M.A. Integrative regulation of physiology by histone deacetylase 3. Nat. Rev. Mol. Cell Biol. 2019, 20, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Bhaskara, S.; Chyla, B.J.; Amann, J.M.; Knutson, S.K.; Cortez, D.; Sun, Z.W.; Hiebert, S.W. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol. Cell 2008, 30, 61–72. [Google Scholar] [CrossRef]
- Montgomery, R.L.; Potthoff, M.J.; Haberland, M.; Qi, X.; Matsuzaki, S.; Humphries, K.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J. Clin. Investig. 2008, 118, 3588–3597. [Google Scholar] [CrossRef]
- Poleshko, A.; Shah, P.P.; Gupta, M.; Babu, A.; Morley, M.P.; Manderfield, L.J.; Ifkovits, J.L.; Calderon, D.; Aghajanian, H.; Sierra-Pagán, J.E.; et al. Genome-Nuclear Lamina Interactions Regulate Cardiac Stem Cell Lineage Restriction. Cell 2017, 171, 573–587.e514. [Google Scholar] [CrossRef]
- Nguyen, H.C.B.; Adlanmerini, M.; Hauck, A.K.; Lazar, M.A. Dichotomous engagement of HDAC3 activity governs inflammatory responses. Nature 2020, 584, 286–290. [Google Scholar] [CrossRef]
- Malvaez, M.; McQuown, S.C.; Rogge, G.A.; Astarabadi, M.; Jacques, V.; Carreiro, S.; Rusche, J.R.; Wood, M.A. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc. Natl. Acad. Sci. USA 2013, 110, 2647–2652. [Google Scholar] [CrossRef]
- Bates, S.E. Epigenetic Therapies for Cancer. N. Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef]
- Kumar, P.; Mohan, V.; Sinha, R.A.; Chagtoo, M.; Godbole, M.M. Histone deacetylase inhibition reduces hypothyroidism-induced neurodevelopmental defects in rats. J. Endocrinol. 2015, 227, 83–92. [Google Scholar] [CrossRef]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Daujat, S.; Schneider, R. Lateral Thinking: How Histone Modifications Regulate Gene Expression. Trends Genet. 2016, 32, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Davis, P.J.; Mousa, S.A.; Lin, H.Y. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol. Rev. 2021, 101, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Yamada, M.; Satoh, T.; Monden, T.; Hashimoto, K.; Shibusawa, N.; Onigata, K.; Morikawa, A.; Mori, M. Aberrant dynamics of histone deacetylation at the thyrotropin-releasing hormone gene in resistance to thyroid hormone. Mol. Endocrinol. 2004, 18, 1708–1720. [Google Scholar] [CrossRef][Green Version]
- Koibuchi, N.; Yamaoka, S.; Chin, W.W. Effect of altered thyroid status on neurotrophin gene expression during postnatal development of the mouse cerebellum. Thyroid 2001, 11, 205–210. [Google Scholar] [CrossRef]

| Gene Symbols | Forward | Reverse |
|---|---|---|
| Pcp2 | GGATGGAATGCAGAAACGAC | GTTCCTGCGGAAGCTGAGT |
| Hr | TTGGCCCTTGTAGGAAATGC | TTTCAGCTTGGTGTGATGGC |
| Ntf3 | TGCCACGATCTTACAGGTGA | AGTCTTCCGGCAAACTCCTT |
| Rora | TCCTTCACCAACGGAGAGAC | CCAGGTGGGATTTGGATATG |
| Bdnf | ATCCAAAGGCCAACTGAAGC | ATTGGGTAGTTCGGCATTGC |
| Grm1 | CGAAGGCTATGAGGTGGAAG | AAGGATTCCTCGTGTTGGTG |
| Gapdh | TGCGACTTCCAACAGCAACTC | ATGTAGGCCATGAGGTCCAC |
| Rn18s | CGGCTACCACATCCAAGGAA | GGGCCTCGAAAGAGTCCTGT |
| Targets | Forward | Reverse |
|---|---|---|
| Pcp2 (TRE) | CTCCCCCTACACCCTAGCCTTTTA | CTAGAAGGTCTGAGCCTCCCTCTC |
| Hr (TRE) | TCCTGAGAGCTCTGGTCTAGC | CCTGACCTCTGGCTCCTG |
| Gapdh (TSS) | CTAGGACTGGATAAGCAGGGCGG | TGGAACAGGGAGGAGCAGAGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susetyo, A.; Ishii, S.; Fujiwara, Y.; Amano, I.; Koibuchi, N. Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci. Int. J. Mol. Sci. 2022, 23, 7869. https://doi.org/10.3390/ijms23147869
Susetyo A, Ishii S, Fujiwara Y, Amano I, Koibuchi N. Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci. International Journal of Molecular Sciences. 2022; 23(14):7869. https://doi.org/10.3390/ijms23147869
Chicago/Turabian StyleSusetyo, Alvin, Sumiyasu Ishii, Yuki Fujiwara, Izuki Amano, and Noriyuki Koibuchi. 2022. "Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci" International Journal of Molecular Sciences 23, no. 14: 7869. https://doi.org/10.3390/ijms23147869
APA StyleSusetyo, A., Ishii, S., Fujiwara, Y., Amano, I., & Koibuchi, N. (2022). Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci. International Journal of Molecular Sciences, 23(14), 7869. https://doi.org/10.3390/ijms23147869







