Diabesity in Elderly Cardiovascular Disease Patients: Mechanisms and Regulators
Abstract
:1. Diabesity in Elderly Patients
2. Diabesity and Cardiovascular Disease
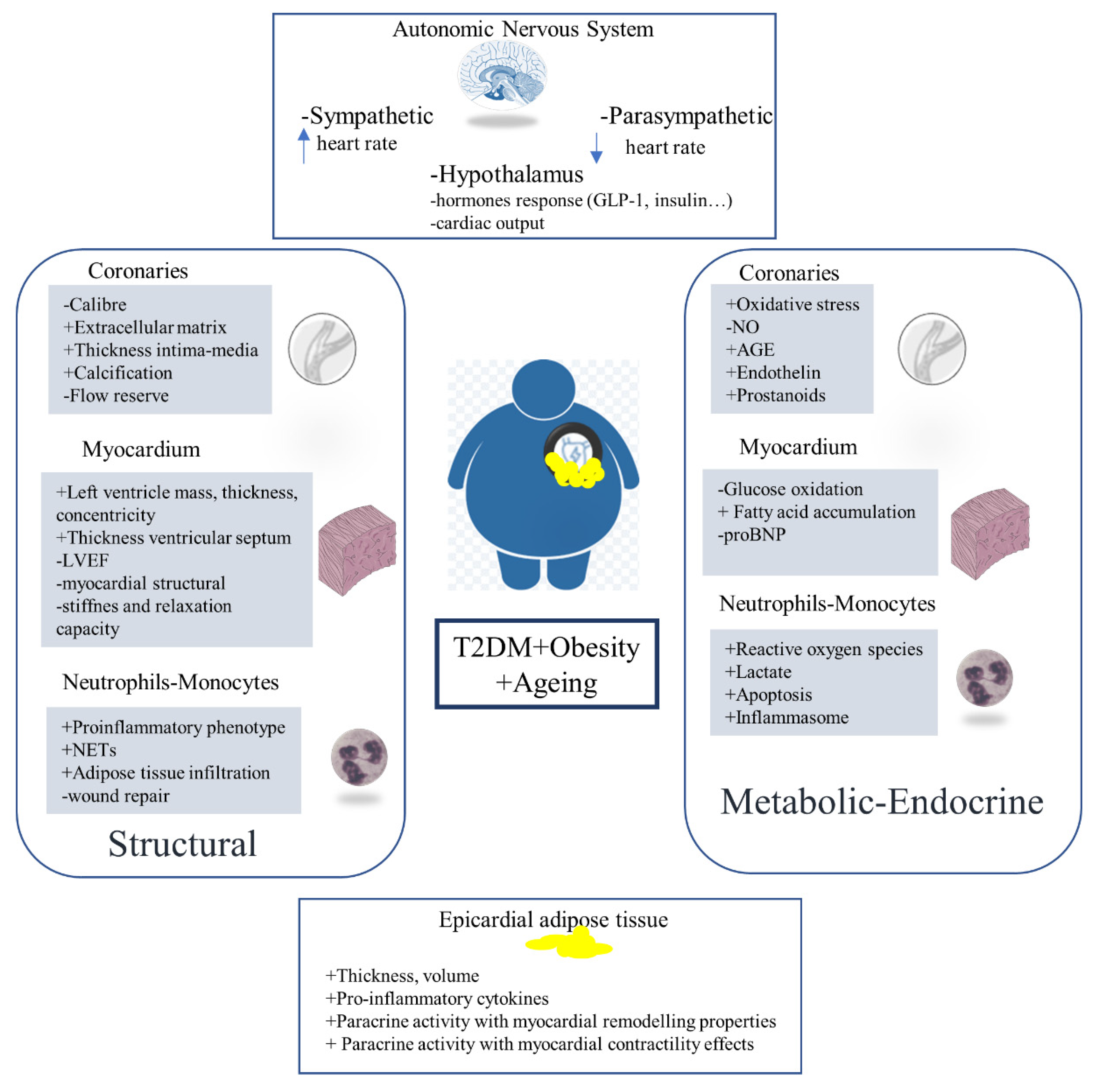
3. Physiopathological Mechanisms
3.1. Cardiac Structural Changes
3.1.1. Coronaries-CAD
3.1.2. Myocardium-HF
3.1.3. Nervous System (Cardiac Autonomic Neuropathy)-AF
3.1.4. Innate Immune System
3.2. Cardiometabolic Changes
3.2.1. Endothelial Metabolic Dysfunction
3.2.2. Cardiomyocytes Metabolic Dysfunction
3.2.3. Neurons and Metabolic Dysfunction
3.2.4. Innate Immune System Metabolism
3.3. Cardiac Endocrine Changes
3.3.1. Endothelium-Endocrine Activity
3.3.2. Myocardium-Endocrine Activity
3.4. Cardiac Adiposity
3.5. Inflammatory Changes
3.6. Electrophysiological Changes
4. Sex Differences Regarding Diabesity and Cardiovascular Disease
4.1. Structural Changes
4.2. Adiposity
5. Patient Management
5.1. Physical Activity
5.2. Novel Hypoglycaemic Drugs
5.2.1. SGLT2i
5.2.2. Incretins
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 16 June 2022).
- Ayah, R.; Joshi, M.D.; Wanjiru, R.; Njau, E.K.; Otieno, C.F.; Njeru, E.K.; Mutai, K.K. A population-based survey of prevalence of diabetes and correlates in an urban slum community in Nairobi, Kenya. BMC Public Health 2013, 13, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R.W. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjønneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Kaye, S.A.; Sellers, T.A.; Hong, C.P.; Cerhan, J.R.; Potter, J.D.; Prineas, R.J. Body fat distribution and 5-year risk of death in older women. JAMA 1993, 269, 483–487. [Google Scholar] [CrossRef]
- Slawik, M.; Vidal-Puig, A.J. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res. Rev. 2006, 5, 144–164. [Google Scholar] [CrossRef]
- Tchoukalova, Y.D.; Votruba, S.B.; Tchkonia, T.; Giorgadze, N.; Kirkland, J.L.; Jensen, M. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. USA 2010, 107, 18226–18231. [Google Scholar] [CrossRef] [Green Version]
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Thomou, T.; DePonte, M.; Koo, A.; Forse, R.A.; Chinnappan, D.; Martin-Ruiz, C.; von Zglinicki, T.; et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 2006, 55, 2571–2578. [Google Scholar] [CrossRef] [Green Version]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age (Dordr.) 2016, 38, 23. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, T.; Tanguay, J.F.; Bourassa, M.G. Management of coronary artery disease: Therapeutic options in patients with diabetes. J. Am. Coll. Cardiol. 2000, 36, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef] [PubMed]
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC National Diabetes Statistics Report 2020. Estimates of Diabetes and Its Burden in the United States. 2020. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 16 June 2022).
- Gustafsson, I.; Brendorp, B.; Seibaek, M.; Burchardt, H.; Hildebrandt, P.; Køber, L.; Torp-Pedersen, C.; Danish Investigatord of Arrhythmia and Mortality on Dofetilde Study Group. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J. Am. Coll. Cardiol. 2004, 43, 771–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet (Lond. Engl.) 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.W.; You, S.; Liu, Z.Y.; Hao, Q.Y.; Wang, J.F.; Vuitton, D.A.; Zhang, S.L.; Liu, P.M. Different Metabolic Phenotypes of Obesity and Risk of Coronary Artery Calcium Progression and Incident Cardiovascular Disease Events: The CARDIA Study. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 677–688. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A ‘set up’ for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Wu, C.; Odden, M.C.; Fisher, G.G.; Stawski, R.S. Association of retirement age with mortality: A population-based longitudinal study among older adults in the USA. J. Epidemiol. Community Health 2016, 70, 917–923. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Steg, P.G.; Smith, S.C., Jr.; Eagle, K.; Ohman, E.M.; Goto, S.; Kuder, J.; Im, K.; Wilson, P.W.; Bhatt, D.L. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015, 132, 923–931. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.A.; Read, S.H.; Kerssens, J.; Livingstone, S.; McGurnaghan, S.; Jhund, P.; Petrie, J.; Sattar, N.; Fischbacher, C.; Kristensen, S.L.; et al. Incidence of Hospitalization for Heart Failure and Case-Fatality among 3.25 Million People with and without Diabetes Mellitus. Circulation 2018, 138, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Cardiol. 2017, 120, S37–S47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Ostergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef] [Green Version]
- Echouffo-Tcheugui, J.B.; Ndumele, C.E.; Zhang, S.; Florido, R.; Matsushita, K.; Coresh, J.; Skali, H.; Shah, A.M.; Selvin, E. Diabetes and Progression of Heart Failure: The Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Coll. Cardiol. 2022, 79, 2285–2293. [Google Scholar] [CrossRef]
- Colilla, S.; Crow, A.; Petkun, W.; Singer, D.E.; Simon, T.; Liu, X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013, 112, 1142–1147. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fi brillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Echouffo-Tcheugui, J.B.; Shrader, P.; Thomas, L.; Gersh, B.J.; Kowey, P.R.; Mahaffey, K.W.; Singer, D.E.; Hylek, E.M.; Go, A.S.; Peterson, E.D.; et al. Care Patterns and Outcomes in Atrial Fibrillation Patients with and without Diabetes: ORBIT-AF Registry. J. Am. Coll. Cardiol. 2017, 70, 1325–1335. [Google Scholar] [CrossRef]
- Du, X.; Ninomiya, T.; de Galan, B.; Abadir, E.; Chalmers, J.; Pillai, A.; Woodward, M.; Cooper, M.; Harrap, S.; Hamet, P.; et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: Results of the ADVANCE study. Eur. Heart J. 2009, 30, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Zhi, H.; Yang, S.; Yu, E.Y.; Wang, L. Body Mass Index and the Risk of Atrial Fibrillation: A Mendelian Randomization Study. Nutrients 2022, 14, 1878. [Google Scholar] [CrossRef] [PubMed]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.J.; Akar, J.G. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanek, L.; Bordignon, S.; Chen, S.; Bologna, F.; Tohoku, S.; Dincher, M.; Schulte-Hahn, B.; Schmidt, B.; Chun, K.J. Impact of body mass index on cryoablation of atrial fibrillation: Patient characteristics, procedural data, and long-term outcomes. J. Cardiovasc. Electrophysiol. 2022, 33, 1106–1115. [Google Scholar] [CrossRef]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef] [Green Version]
- Mosseri, M.; Nahir, M.; Rozenman, Y.; Lotan, C.; Admon, D.; Raz, I.; Gotsman, M.S. Diffuse narrowing of coronary arteries in diabetic patients: The earliest phase of coronary artery disease. Cardiology 1998, 89, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Lüscher, T.F.; Cosentino, F.; Beckman, J.A. Diabetes and Vascular Disease. Circulation 2003, 108, 1527–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Ruiz, M.E.; Pérez-Torres, I.; Soto, M.E.; Pastelín, G.; Guarner-Lans, V. Aging in blood vessels. Medicinal agents FOR systemic arterial hypertension in the elderly. Ageing Res. Rev. 2014, 18, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Ferket, B.S.; Hunink, M.G.M.; Masharani, U.; Max, W.; Yeboah, J.; Fleischmann, K.E. Long-term Predictions of Incident Coronary Artery Calcium to 85 Years of Age for Asymptomatic Individuals with and without Type 2 Diabetes. Diabetes Care 2021, 44, 1664–1671. [Google Scholar] [CrossRef]
- Plakht, Y.; Elkis Hirsch, Y.; Shiyovich, A.; Abu Tailakh, M.; Liberty, I.F.; Gilutz, H. Heterogenicity of diabetes as a risk factor for all-cause mortality after acute myocardial infarction: Age and sex impact. Diabetes Res. Clin. Pract. 2021, 182, 109117. [Google Scholar] [CrossRef]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef] [Green Version]
- Assar, M.E.; Angulo, J.; Rodríguez-Mañas, L. Diabetes and ageing-induced vascular inflammation. J. Physiol. 2016, 594, 2125–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, B.; He, X.; Gu, N. Reassessing Revascularization Strategies in Coronary Artery Disease and Type 2 Diabetes Mellitus. Front. Cardiovasc. Med. 2021, 8, 738620. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, G.R.; Lu, J.; Faxon, D.P.; Kent, K.; Lago, R.M.; Lezama, C.; Hueb, W.; Weiss, M.; Slater, J.; Frye, R.L.; et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation 2011, 123, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Alcalá-Diaz, J.F.; Gutierrez-Mariscal, F.M.; Arenas-de Larriva, A.P.; Peña-Orihuela, P.J.; Blanco-Rojo, R.; Martinez-Botas, J.; Torres-Peña, J.D.; Perez-Martinez, P.; Ordovas, J.M.; et al. Association between cholesterol efflux capacity and peripheral artery disease in coronary heart disease patients with and without type 2 diabetes: From the CORDIOPREV study. Cardiovasc. Diabetol. 2021, 20, 72. [Google Scholar] [CrossRef]
- Kaze, A.D.; Santhanam, P.; Musani, S.K.; Ahima, R.; Echouffo-Tcheugui, J.B. Metabolic Dyslipidemia and Cardiovascular Outcomes in Type 2 Diabetes Mellitus: Findings From the Look AHEAD Study. J. Am. Heart Assoc. 2021, 10, e016947. [Google Scholar] [CrossRef]
- Prieur, X.; Mok, C.Y.; Velagapudi, V.R.; Núñez, V.; Fuentes, L.; Montaner, D.; Ishikawa, K.; Camacho, A.; Barbarroja, N.; O’Rahilly, S.; et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 2011, 60, 797–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Couillard, C.; Ruel, G.; Archer, W.R.; Pomerleau, S.; Bergeron, J.; Couture, P.; Lamarche, B.; Bergeron, N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J. Clin. Endocrinol. Metab. 2005, 90, 6454–6459. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro, M.; Hirata, Y.; Tabata, M.; Dagvasumberel, M.; Sato, H.; Kurobe, H.; Fukuda, D.; Soeki, T.; Kitagawa, T.; Takanashi, S.; et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Imai, A.; Komatsu, S.; Ohara, T.; Kamata, T.; Yoshida, J.; Miyaji, K.; Takewa, M.; Kodama, K. Visceral abdominal fat accumulation predicts the progression of noncalcified coronary plaque. Atherosclerosis 2012, 222, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Badoz, M.; Serzian, G.; Favoulet, B.; Sellal, J.M.; De Chillou, C.; Hammache, N.; Laurent, G.; Mebazaa, A.; Ecarnot, F.; Bardonnet, K.; et al. Impact of Midregional N-Terminal Pro-Atrial Natriuretic Peptide and Soluble Suppression of Tumorigenicity 2 Levels on Heart Rhythm in Patients Treated with Catheter Ablation for Atrial Fibrillation: The Biorhythm Study. J. Am. Heart Assoc. 2021, 10, e020917. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Badano, L.P.; Lang, R.M.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; Baroni, M.; Cardim, N.; Gomez de Diego, J.J.; Derumeaux, G.; et al. Normal Reference Ranges for Echocardiography: Rationale, study design, and methodology (NORRE Study). Eur. Heart J. Cardiovasc. Imaging 2013, 14, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilner, B.; Garg, S.; Ayers, C.R.; Maroules, C.D.; McColl, R.; Matulevicius, S.A.; de Lemos, J.A.; Drazner, M.H.; Peshock, R.; Neeland, I.J. Dynamic Relation of Changes in Weight and Indices of Fat Distribution with Cardiac Structure and Function: The Dallas Heart Study. J. Am. Heart Assoc. 2017, 6, e005897. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, E.B.; McClelland, R.L.; Kronmal, R.A.; Burke, G.L.; Bild, D.E.; Tracy, R.P.; Arai, A.E.; Lima, J.A.; Bluemke, D.A. The impact of obesity on the left ventricle: The Multi-Ethnic Study of Atherosclerosis (MESA). JACC. Cardiovasc. Imaging 2010, 3, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Gradman, A.H.; Alfayoumi, F. From left ventricular hypertrophy to congestive heart failure: Management of hypertensive heart disease. Prog. Cardiovasc. Dis. 2006, 48, 326–341. [Google Scholar] [CrossRef]
- Force, T.; Rosenzweig, A.; Choukroun, G.; Hajjar, R. Calcineurin inhibitors and cardiac hypertrophy. Lancet (Lond. Engl.) 1999, 353, 1290–1292. [Google Scholar] [CrossRef]
- Kramer, S.P.; Powell, D.K.; Haggerty, C.M.; Binkley, C.M.; Mattingly, A.C.; Cassis, L.A.; Epstein, F.H.; Fornwalt, B.K. Obesity reduces left ventricular strains, torsion, and synchrony in mouse models: A cine displacement encoding with stimulated echoes (DENSE) cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2013, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Karwi, Q.G.; Sun, Q.; Lopaschuk, G.D. The Contribution of Cardiac Fatty Acid Oxidation to Diabetic Cardiomyopathy Severity. Cells 2021, 10, 3259. [Google Scholar] [CrossRef]
- Lewis, A.J.M.; Abdesselam, I.; Rayner, J.J.; Byrne, J.; Borlaug, B.A.; Neubauer, S.; Rider, O.J. Adverse right ventricular remodelling, function, and stress responses in obesity: Insights from cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2021, jeab175. [Google Scholar] [CrossRef]
- Siurana, J.M.; Ventura, P.S.; Yeste, D.; Riaza-Martin, L.; Arciniegas, L.; Clemente, M.; Torres, M.; Amigó, N.; Giralt, G.; Roses-Noguer, F.; et al. Myocardial Geometry and Dysfunction in Morbidly Obese Adolescents (BMI 35–40 kg/m2). Am. J. Cardiol. 2021, 157, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Fung, K.; Aung, N.; Sanghvi, M.M.; Chadalavada, S.; Paiva, J.M.; Khanji, M.Y.; de Knegt, M.C.; Lukaschuk, E.; Lee, A.M.; et al. Changes in Cardiac Morphology and Function in Individuals with Diabetes Mellitus: The UK Biobank Cardiovascular Magnetic Resonance Substudy. Circ. Cardiovasc. Imaging 2019, 12, e009476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.S. Diabetic cardiomyopathy: An expression of stage B heart failure with preserved ejection fraction. Diabetes Vasc. Dis. Res. 2015, 12, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallinoro, E.; Paolisso, P.; Candreva, A.; Bermpeis, K.; Fabbricatore, D.; Esposito, G.; Bertolone, D.; Fernandez Peregrina, E.; Munhoz, D.; Mileva, N.; et al. Microvascular Dysfunction in Patients with Type II Diabetes Mellitus: Invasive Assessment of Absolute Coronary Blood Flow and Microvascular Resistance Reserve. Front. Cardiovasc. Med. 2021, 8, 765071. [Google Scholar] [CrossRef]
- Shen, M.T.; Guo, Y.K.; Liu, X.; Ren, Y.; Jiang, L.; Xie, L.J.; Gao, Y.; Zhang, Y.; Deng, M.Y.; Li, Y.; et al. Impact of BMI on Left Atrial Strain and Abnormal Atrioventricular Interaction in Patients with Type 2 Diabetes Mellitus: A Cardiac Magnetic Resonance Feature Tracking Study. J. Magn. Reson. Imaging 2022, 55, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Tahsili-Fahadan, P.; Geocadin, R.G. Heart–Brain Axis. Circ. Res. 2017, 120, 559–572. [Google Scholar] [CrossRef]
- Tedoldi, A.; Argent, L.; Montgomery, J.M. The role of the tripartite synapse in the heart: How glial cells may contribute to the physiology and pathophysiology of the intracardiac nervous system. Am. J. Physiol. Cell Physiol. 2021, 320, C1–C14. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, G.W.; Masuo, K.; Dawood, T.; Eikelis, N.; Nestel, P.J.; McGrane, M.T.; Mariani, J.A.; Socratous, F.; Chopra, R.; et al. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am. J. Clin. Nutr. 2009, 89, 27–36. [Google Scholar] [CrossRef]
- Piestrzeniewicz, K.; Łuczak, K.; Lelonek, M.; Wranicz, J.K.; Goch, J.H. Obesity and heart rate variability in men with myocardial infarction. Cardiol. J. 2008, 15, 43–49. [Google Scholar]
- Lambert, E.; Sari, C.I.; Dawood, T.; Nguyen, J.; McGrane, M.; Eikelis, N.; Chopra, R.; Wong, C.; Chatzivlastou, K.; Head, G.; et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 2010, 56, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.N.; Hart, E.C.; Curry, T.B.; Nicholson, W.T.; Eisenach, J.H.; Wallin, B.G.; Charkoudian, N.; Joyner, M.J. Aging enhances autonomic support of blood pressure in women. Hypertension 2014, 63, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcıoğlu, A.S.; Müderrisoğlu, H. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J. Diabetes 2015, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bergenstal, R.; Bonow, R.O.; Buse, J.; Deedwania, P.; Gale, E.A.; Howard, B.V.; Kirkman, M.S.; Kosiborod, M.; Reaven, P.; et al. Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes TrialsA position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009, 32, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaul, D.S.; Stein, S.; Matter, C.M. Neutrophils in cardiovascular disease. Eur. Heart J. 2017, 38, 1702–1704. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Poitou, C.; Dalmas, E.; Renovato, M.; Benhamo, V.; Hajduch, F.; Abdennour, M.; Kahn, J.-F.; Veyrie, N.; Rizkalla, S.; Fridman, W.-H.; et al. CD14dimCD16+ and CD14+CD16+ Monocytes in Obesity and During Weight Loss. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2322–2330. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Mori, K.; Okuma, H.; Sekine, T.; Miyazaki, A.; Tsuchiya, K. Age-associated decline of monocyte insulin sensitivity in diabetic and healthy individuals. Diab. Vasc. Dis. Res. 2021, 18, 1479164121989281. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Kim, I.; Weintraub, N.L.; Tang, Y. The Impaired Bioenergetics of Diabetic Cardiac Microvascular Endothelial Cells. Front. Endocrinol. (Lausanne) 2021, 12, 642857. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—Dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puddu, A.; Sanguineti, R.; Maggi, D.; Nicolò, M.; Traverso, C.E.; Cordera, R.; Viviani, G.L. Advanced Glycation End-Products and Hyperglycemia Increase Angiopoietin-2 Production by Impairing Angiopoietin-1-Tie-2 System. J Diabetes Res. 2019, 11, 6198495. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Fuh, H.; Donnelly, T.; Cybulsky, M. Advanced glycation end products promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol. Med. 1995, 1, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruberg, F.L.; Loscalzo, J. Prothrombotic determinants of coronary atherothrombosis. Vasc. Med. 2002, 7, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial Cell Metabolism in Normal and Diseased Vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Yozgatli, K.; Lefrandt, J.D.; Noordzij, M.J.; Oomen, P.H.N.; Brouwer, T.; Jager, J.; Castro Cabezas, M.; Smit, A.J. Accumulation of advanced glycation end products is associated with macrovascular events and glycaemic control with microvascular complications in type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1242. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Castiglione, S.; Macrì, F.; Giuliani, A.; Ramini, D.; Vinci, M.C.; Tortato, E.; Bonfigli, A.R.; Olivieri, F.; Raucci, A. Circulating levels of AGEs and soluble RAGE isoforms are associated with all-cause mortality and development of cardiovascular complications in type 2 diabetes: A retrospective cohort study. Cardiovasc. Diabetol. 2022, 21, 95. [Google Scholar] [CrossRef]
- Rider, O.J.; Francis, J.M.; Ali, M.K.; Holloway, C.; Pegg, T.; Robson, M.D.; Tyler, D.; Byrne, J.; Clarke, K.; Neubauer, S. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation 2012, 125, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Rayner, J.J.; Peterzan, M.A.; Watson, W.D.; Clarke, W.T.; Neubauer, S.; Rodgers, C.T.; Rider, O.J. Myocardial Energetics in Obesity: Enhanced ATP Delivery Through Creatine Kinase with Blunted Stress Response. Circulation 2020, 141, 1152–1163. [Google Scholar] [CrossRef]
- Rayner, J.J.; Peterzan, M.A.; Clarke, W.T.; Rodgers, C.T.; Neubauer, S.; Rider, O.J. Obesity modifies the energetic phenotype of dilated cardiomyopathy. Eur. Heart J. 2021, 43, 868–877. [Google Scholar] [CrossRef]
- Rijzewijk, L.J.; van der Meer, R.W.; Lamb, H.J.; de Jong, H.W.; Lubberink, M.; Romijn, J.A.; Bax, J.J.; de Roos, A.; Twisk, J.W.; Heine, R.J.; et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: Studies with cardiac positron emission tomography and magnetic resonance imaging. J. Am. Coll. Cardiol. 2009, 54, 1524–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carley, A.N.; Severson, D.L. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim. Biophys. Acta 2005, 1734, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Paradela-Dobarro, B.; Fernández-Trasancos, Á.; Bou-Teen, D.; Eiras, S.; González-Ferreiro, R.; Agra, R.M.; Varela-Román, A.; Castro-Pais, A.I.; Carreira, M.C.; Casanueva, F.F.; et al. Evolution and bad prognostic value of advanced glycation end products after acute heart failure: Relation with body composition. Cardiovasc. Diabetol. 2017, 16, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartog, J.W.; Voors, A.A.; Bakker, S.J.; Smit, A.J.; van Veldhuisen, D.J. Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. Eur. J. Heart Fail. 2007, 9, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Review A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Do some nerve cells release more than one transmitter? Neuroscience 1976, 1, 239–248. [Google Scholar] [CrossRef]
- Garcia-Serrano, A.M.; Duarte, J.M.N. Brain Metabolism Alterations in Type 2 Diabetes: What Did We Learn From Diet-Induced Diabetes Models? Front. Neurosci. 2020, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Imai, J.; Katagiri, H. Regulation of systemic metabolism by the autonomic nervous system consisting of afferent and efferent innervation. Int. Immunol. 2022, 34, 67–79. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Rodiño-Janeiro, B.K.; Grigorian-Shamagian, L.; Seoane-Blanco, A.; Moure-González, M.; Varela-Román, A.; Álvarez, E.; González-Juanatey, J.R. Evidence for a role of advanced glycation end products in atrial fibrillation. Int. J. Cardiol. 2012, 157, 397–402. [Google Scholar] [CrossRef]
- Delmastro-Greenwood, M.M.; Piganelli, J.D. Changing the energy of an immune response. Am. J. Clin. Exp. Immunol. 2013, 2, 30–54. [Google Scholar] [PubMed]
- Alba-Loureiro, T.C.; Hirabara, S.M.; Mendonça, J.R.; Curi, R.; Pithon-Curi, T.C. Diabetes causes marked changes in function and metabolism of rat neutrophils. J. Endocrinol. 2006, 188, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.M.; Stern, D. Atherosclerosis and diabetes: The rage connection. Curr. Atheroscler. Rep. 2000, 2, 430–436. [Google Scholar] [CrossRef] [PubMed]
- de Vriese, A.S.; Verbeuren, T.J.; van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopfner, R.L.; Gopalakrishnan, V. Endothelin: Emerging role in diabetic vascular complications. Diabetologia 1999, 42, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R., Jr.; Lerman, A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef]
- James, K.B.; Troughton, R.W.; Feldschuh, J.; Soltis, D.; Thomas, D.; Fouad-Tarazi, F. Blood volume and brain natriuretic peptide in congestive heart failure: A pilot study. Am. Heart J. 2005, 150, e1–e984. [Google Scholar] [CrossRef]
- Lugnier, C.; Meyer, A.; Charloux, A.; Andrès, E.; Gény, B.; Talha, S. The Endocrine Function of the Heart: Physiology and Involvements of Natriuretic Peptides and Cyclic Nucleotide Phosphodiesterases in Heart Failure. J. Clin. Med. 2019, 8, 1746. [Google Scholar] [CrossRef] [Green Version]
- Mehra, M.R.; Uber, P.A.; Park, M.H.; Scott, R.L.; Ventura, H.O.; Harris, B.C.; Frohlich, E.D. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. Coll. Cardiol. 2004, 43, 1590–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen-Tournoux, A.; Khan, A.M.; Baggish, A.L.; Castro, V.M.; Semigran, M.J.; McCabe, E.L.; Moukarbel, G.; Reingold, J.; Durrani, S.; Lewis, G.D.; et al. Effect of weight loss after weight loss surgery on plasma N-terminal pro-B-type natriuretic peptide levels. Am. J. Cardiol. 2010, 106, 1450–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.Y.; Abdullah, S.M.; Jain, T.; Stanek, H.G.; Das, S.R.; McGuire, D.K.; Auchus, R.J.; de Lemos, J.A. Associations among androgens, estrogens, and natriuretic peptides in young women: Observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 2007, 49, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Standeven, K.F.; Hess, K.; Carter, A.M.; Rice, G.I.; Cordell, P.A.; Balmforth, A.J.; Lu, B.; Scott, D.J.; Turner, A.J.; Hooper, N.M.; et al. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. (Lond.) 2011, 35, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef]
- Khan, A.M.; Cheng, S.; Magnusson, M.; Larson, M.G.; Newton-Cheh, C.; McCabe, E.L.; Coviello, A.D.; Florez, J.C.; Fox, C.S.; Levy, D.; et al. Cardiac natriuretic peptides, obesity, and insulin resistance: Evidence from two community-based studies. J. Clin. Endocrinol. Metab. 2011, 96, 3242–3249. [Google Scholar] [CrossRef]
- Verboven, K.; Hansen, D.; Jocken, J.W.E.; Blaak, E.E. Natriuretic peptides in the control of lipid metabolism and insulin sensitivity. Obes. Rev. 2017, 18, 1243–1259. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Winders, B.R.; Ayers, C.R.; Das, S.R.; Chang, A.Y.; Berry, J.D.; Khera, A.; McGuire, D.K.; Vega, G.L.; de Lemos, J.A.; et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J. Am. Coll. Cardiol. 2013, 62, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Antoniades, C. The role of epicardial adipose tissue in cardiac biology: Classic concepts and emerging roles. J. Physiol. 2017, 595, 3907–3917. [Google Scholar] [CrossRef]
- Corradi, D.; Maestri, R.; Callegari, S.; Pastori, P.; Goldoni, M.; Luong, T.V.; Bordi, C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004, 13, 313–316. [Google Scholar] [CrossRef]
- Iacobellis, G.; Leonetti, F. Epicardial Adipose Tissue and Insulin Resistance in Obese Subjects. J. Clin. Endocrinol. Metab. 2005, 90, 6300–6302. [Google Scholar] [CrossRef] [PubMed]
- Roubíček, T.; Dolinková, M.; Bláha, J.; Haluzíková, D.; Bošanská, L.; Mráz, M.; Kremen, J.; Haluzík, M. Increased angiotensinogen production in epicardial adipose tissue during cardiac surgery: Possible role in a postoperative insulin resistance. Physiol. Res. 2007, 57, 911–917. [Google Scholar] [CrossRef]
- Iacobellis, G.; Leonetti, F.; Singh, N.; Sharma, A.M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007, 115, 272–273. [Google Scholar] [CrossRef]
- Kankaanpää, M.; Lehto, H.-R.; Pärkkä, J.P.; Komu, M.; Viljanen, A.; Ferrannini, E.; Knuuti, J.; Nuutila, P.; Parkkola, R.; Iozzo, P. Myocardial Triglyceride Content and Epicardial Fat Mass in Human Obesity: Relationship to Left Ventricular Function and Serum Free Fatty Acid Levels. J. Clin. Endocrinol. Metab. 2006, 91, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Trasancos, Á.; Fandiño-Vaquero, R.; Agra, R.M.R.M.; Fernández, Á.L.; Viñuela, J.E.J.E.; González-Juanatey, J.R.J.R.; Eiras, S.; Fernández-Trasancos, A.; Fandiño-Vaquero, R.; Agra, R.M.R.M.; et al. Impaired Adipogenesis and Insulin Resistance in Epicardial Fat-Mesenchymal Cells From Patients with Cardiovascular Disease. J. Cell. Physiol. 2014, 229, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Lun, M.; Wang, M.; Senyo, S.E.; Guillermier, C.; Patwari, P.; Steinhauser, M.L. Loss of White Adipose Hyperplastic Potential Is Associated with Enhanced Susceptibility to Insulin Resistance. Cell Metab. 2014, 20, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of DiabetesEstimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Ouwens, D.M.; Sell, H.; Greulich, S.; Eckel, J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 2010, 14, 2223–2234. [Google Scholar] [CrossRef] [Green Version]
- Malavazos, A.E.; Ermetici, F.; Coman, C.; Corsi, M.M.; Morricone, L.; Ambrosi, B. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int. J. Cardiol. 2007, 121, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Shibata, A.; Yoshida, T.; Tanihata, A.; Hayashi, H.; Kitada, R.; Ehara, S.; Izumiya, Y.; Fukuda, D. Epicardial adipose tissue volume is an independent predictor of left ventricular reverse remodeling in patients with non-ischemic cardiomyopathy. Int. J. Cardiol. 2022, 356, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Park, S.M.; Quon, M.J. Leptin and Cardiovascular Disease. Circulation 2008, 9, E34. [Google Scholar] [CrossRef] [Green Version]
- Barouch, L.A.; Berkowitz, D.E.; Harrison, R.W.; O’Donnell, C.P.; Hare, J.M. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 2003, 108, 754–759. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Luo, C.; Zhu, D.; Song, Y.; Cao, L.; Luan, H.; Gao, L.; Zheng, S.; Li, H.; Tian, G. Pericardial Adipose Tissue-Derived Leptin Promotes Myocardial Apoptosis in High-Fat Diet-Induced Obese Rats Through Janus Kinase 2/Reactive Oxygen Species/Na+/K+-ATPase Signaling Pathway. J. Am. Heart Assoc. 2021, 10, e021369. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Fernández, Á.L.; González-Juanatey, J.R.; Eiras, S. Changes in lipid transport-involved proteins of epicardial adipose tissue associated with coronary artery disease. Atherosclerosis 2012, 224, 492–499. [Google Scholar] [CrossRef]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Rubio, J.; Couso, E.; González-Juanatey, J.R.; Eiras, S. Coronary artery disease is associated with higher epicardial Retinol-binding protein 4 (RBP4) and lower glucose transporter (GLUT) 4 levels in epicardial and subcutaneous adipose tissue. Clin. Endocrinol. (Oxf.) 2012, 76, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Joo, H.J.; Kim, M.N.; Lim, D.S.; Shim, W.J.; Park, S.M. Association between epicardial adipose tissue, high-sensitivity C-reactive protein and myocardial dysfunction in middle-aged men with suspected metabolic syndrome. Cardiovasc. Diabetol. 2018, 17, 95. [Google Scholar] [CrossRef]
- Cione, E.; Caroleo, M.C.; Cannataro, R.; Perri, M.; Pingitore, A.; Genchi, G. Vitamin A and Diabesity: New Insight for Drug Discovery. Mini. Rev. Med. Chem. 2016, 16, 738–742. [Google Scholar] [CrossRef]
- Silaghi, A.; Piercecchi-Marti, M.-D.; Grino, M.; Leonetti, G.; Alessi, M.C.; Clement, K.; Dadoun, F.; Dutour, A. Epicardial Adipose Tissue Extent: Relationship with Age, Body Fat Distribution, and Coronaropathy. Obesity 2008, 16, 2424–2430. [Google Scholar] [CrossRef]
- Gorter, P.M.; van Lindert, A.S.; de Vos, A.M.; Meijs, M.F.; van der Graaf, Y.; Doevendans, P.A.; Prokop, M.; Visseren, F.L. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 2008, 197, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, N.; Toschke, A.M.; Leite, D.; Rocha, J.; Carvalho, M.; Sampaio, F.; Xará, S.; Leite-Moreira, A.; Nagel, E.; Gama, V. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int. J. Cardiol. 2012, 158, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial Changes in Epicardial Fat Thickness After Weight Loss in Severely Obese Subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Tomita, T.; Kim, M.-J.; Sasai, H.; Maeda, S.; Tanaka, K. Aerobic exercise training reduces epicardial fat in obese men. J. Appl. Physiol. 2009, 106, 5–11. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Manintveld, O.C.; van Empel, V.P.M.; Willems, T.P.; de Boer, R.A.; Rienstra, M.; Westenbrink, B.D.; Gorter, T.M. Epicardial Adipose Tissue and Outcome in Heart Failure with Mid-Range and Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, E009238. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Bryant, J.A.; Jin, X.; van Woerden, G.; Asali, S.; Yiying, H.; Liew, O.W.; Ching, J.C.P.; Jaufeerally, F.; Loh, S.Y.; et al. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 835–838. [Google Scholar] [CrossRef]
- Elsanhoury, A.; Nelki, V.; Kelle, S.; Van Linthout, S.; Tschöpe, C. Epicardial Fat Expansion in Diabetic and Obese Patients with Heart Failure and Preserved Ejection Fraction—A Specific HFpEF Phenotype. Front. Cardiovasc. Med. 2021, 1031. [Google Scholar] [CrossRef]
- He, S.; Zhu, H.; Zhang, J.; Wu, X.; Zhao, L.; Yang, X. Proteomic analysis of epicardial adipose tissue from heart disease patients with concomitant heart failure with preserved ejection fraction. Int. J. Cardiol. 2022, 362, 118–125. [Google Scholar] [CrossRef]
- Packer, M. Disease-treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: The potential mediating influence of epicardial adipose tissue. Cardiovasc. Diabetol. 2019, 18, 121. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2017, 38, 1294–1302. [Google Scholar] [CrossRef] [Green Version]
- Ernault, A.C.; Verkerk, A.O.; Bayer, J.D.; Aras, K.; Montañés-Agudo, P.; Mohan, R.A.; Veldkamp, M.; Rivaud, M.R.; de Winter, R.; Kawasaki, M.; et al. Secretome of atrial epicardial adipose tissue facilitates reentrant arrhythmias by myocardial remodeling. Heart Rhythm. 2022, 12, S1547–S5271. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021, 18, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Pierzynová, A.; Šrámek, J.; Cinkajzlová, A.; Kratochvílová, H.; Lindner, J.; Haluzík, M.; Kučera, T. The number and phenotype of myocardial and adipose tissue CD68+ cells is associated with cardiovascular and metabolic disease in heart surgery patients. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 946–955. [Google Scholar] [CrossRef]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Jia, G.; Habibi, J.; Bostick, B.P.; Ma, L.; DeMarco, V.G.; Aroor, A.R.; Hayden, M.R.; Whaley-Connell, A.T.; Sowers, J.R. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 2015, 65, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Z.; Singh, N.; Howe, C.L.; Low, S.W.; Huang, J.J.; Ortega, G.; Lee, K.S.; Pandit, A. Colchicine for prevention of post-operative atrial fibrillation: A meta-analysis. JACC Clin. Electrophysiol. 2016, 2, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.; Giannopoulos, G.; Panagopoulou, V.; Bouras, G.; Raisakis, K.; Kossyvakis, C.; Karageorgiou, S.; Papadimitriou, C.; Vastaki, M.; Kaoukis, A.; et al. Anti-inflammatory treatment with colchicine in stable chronic heart failure: A prospective, randomized study. JACC. Heart Fail. 2014, 2, 131–137. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef]
- Henson, S.M.; Aksentijevic, D. Senescence and Type 2 Diabetic Cardiomyopathy: How Young Can You Die of Old Age? Front. Pharmacol. 2021, 12, 2692. [Google Scholar] [CrossRef]
- Liu, C.; Fu, H.; Li, J.; Yang, W.; Cheng, L.; Liu, T.; Li, G. Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu Kardiyol. Derg. 2012, 12, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Yokoshiki, H.; Mitsuyama, H.; Mizukami, K.; Ono, T.; Tsutsui, H. Conduction and refractory disorders in the diabetic atrium. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H86–H95. [Google Scholar] [CrossRef]
- Ozturk, N.; Uslu, S.; Ozdemir, S. Diabetes-induced changes in cardiac voltage-gated ion channels. World J. Diabetes 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M.; et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Huttin, O.; Magnusson, M.; Ferreira, J.P.; Bozec, E.; Huby, A.C.; Preud’homme, G.; Duarte, K.; Lamiral, Z.; Dalleau, K.; et al. Machine Learning-Derived Echocardiographic Phenotypes Predict Heart Failure Incidence in Asymptomatic Individuals. JACC Cardiovasc. Imaging 2022, 15, 193–208. [Google Scholar] [CrossRef]
- Beck, L.; Su, J.; Comerma-Steffensen, S.; Pinilla, E.; Carlsson, R.; Hernanz, R.; Sheykhzade, M.; Danielsen, C.C.; Simonsen, U. Endothelial Dysfunction and Passive Changes in the Aorta and Coronary Arteries of Diabetic db/db Mice. Front. Physiol. 2020, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- MacCannell, A.D.V.; Futers, T.S.; Whitehead, A.; Moran, A.; Witte, K.K.; Roberts, L.D. Sexual dimorphism in adipose tissue mitochondrial function and metabolic flexibility in obesity. Int. J. Obes. (Lond.) 2021, 45, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-C.; Arthur, R.; Iyengar, N.M.; Kamensky, V.; Xue, X.; Wassertheil-Smoller, S.; Allison, M.A.; Shadyab, A.H.; Wild, R.A.; Sun, Y.; et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur. Heart J. 2019, 40, 2849–2855. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Karlsson, T.; Rask-Andersen, M.; Pan, G.; Höglund, J.; Wadelius, C.; Ek, W.E.; Johansson, Å. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat. Med. 2019, 25, 1390–1395. [Google Scholar] [CrossRef]
- Norheim, F.; Hasin-Brumshtein, Y.; Vergnes, L.; Chella Krishnan, K.; Pan, C.; Seldin, M.M.; Hui, S.T.; Mehrabian, M.; Zhou, Z.; Gupta, S.; et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab. 2019, 29, 932–949.e4. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Yang, M.X.; Huang, S.; Yan, W.F.; Qian, W.L.; Li, Y.; Guo, Y.K.; Yang, Z.G. Effect of diabetes mellitus on the development of left ventricular contractile dysfunction in women with heart failure and preserved ejection fraction. Cardiovasc. Diabetol. 2021, 20, 185. [Google Scholar] [CrossRef]
- Maslov, P.Z.; Kim, J.K.; Argulian, E.; Ahmadi, A.; Narula, N.; Singh, M.; Bax, J.; Narula, J. Is Cardiac Diastolic Dysfunction a Part of Post-Menopausal Syndrome? JACC Heart Fail. 2019, 7, 192–203. [Google Scholar] [CrossRef]
- Upadhya, B.; Kitzman, D.W. Heart Failure with Preserved Ejection Fraction in Older Adults. Heart Fail. Clin. 2017, 13, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Aggio, D.; Papachristou, E.; Papacosta, O.; Lennon, L.T.; Ash, S.; Whincup, P.H.; Wannamethee, S.G.; Jefferis, B.J. Trajectories of self-reported physical activity and predictors during the transition to old age: A 20-year cohort study of British men. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Eghbalzadeh, K.; Brixius, K.; Bloch, W.; Brinkmann, C. Skeletal muscle nitric oxide (NO) synthases and NO-signaling in “diabesity”—What about the relevance of exercise training interventions? Nitric Oxide 2014, 37, 28–40. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; Patterson, N.L.; McMullen, J.R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: Current knowledge and emerging concepts. Physiol. Rev. 2018, 98, 419–475. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Balducci, S.; Zanuso, S.; Allgrove, J.; Bertiato, F.; Jimenez, A. Changes in insulin sensitivity in response to different modalities of exercise: A review of the evidence. Diabetes Metab. Res. Rev. 2014, 30, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppert, J.M.; Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Carraça, E.V.; Encantado, J.; Ermolao, A.; Pramono, A.; et al. Exercise training in the management of overweight and obesity in adults: Synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 2021, 22, e13273. [Google Scholar] [CrossRef]
- López-González, L.; Becerra-Tomás, N.; Babio, N.; Martínez-González, M.Á.; Nishi, S.K.; Corella, D.; Goday, A.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. One-year changes in fruit and vegetable variety intake and cardiometabolic risk factors changes in a middle-aged Mediterranean population at high cardiovascular risk. Eur. J. Clin. Nutr. 2022, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bassuk, S.S.; Manson, J.E. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J. Appl. Physiol. 2005, 99, 1193–1204. [Google Scholar] [CrossRef]
- Conraads, V.M.; Pattyn, N.; De Maeyer, C.; Beckers, P.J.; Coeckelberghs, E.; Cornelissen, V.A.; Denollet, J.; Frederix, G.; Goetschalckx, K.; Hoymans, V.Y.; et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX-CAD study. Int. J. Cardiol. 2015, 20, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Flynn, K.E.; Piña, I.L.; Whellan, D.J.; Lin, L.; Blumenthal, J.A.; Ellis, S.J.; Fine, L.J.; Howlett, J.G.; Keteyian, S.J.; Kitzman, D.W.; et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009, 301, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Malmo, V.; Nes, B.M.; Amundsen, B.H.; Tjonna, A.E.; Stoylen, A.; Rossvoll, O.; Wisloff, U.; Loennechen, J.P. Aerobic Interval Training Reduces the Burden of Atrial Fibrillation in the Short Term: A Randomized Trial. Circulation 2016, 133, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheen, A.J. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Tsapas, A. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 17–18. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 2097–2099. [Google Scholar] [CrossRef]
- Storgaard, H.; Gluud, L.L.; Bennett, C.; Grøndahl, M.F.; Christensen, M.B.; Knop, F.K.; Vilsbøll, T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166125. [Google Scholar] [CrossRef]
- Scheen, A.J. Effect of SGLT2 Inhibitors on the Sympathetic Nervous System and Blood Pressure. Curr. Cardiol. Rep. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, N.S.R.; Fegan, P.G.; Yeap, B.B.; Dwivedi, G. The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: Current evidence and future directions. ESC Heart Fail. 2019, 6, 927–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Hirata, Y.; Ise, T.; Kusunose, K.; Yamada, H.; Fukuda, D.; Salim, H.M.; Maimaituxun, G.; Nishio, S.; Takagawa, Y.; et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Jeong, M.H.; Yun, K.H.; Oh, S.K.; Park, E.M.; Kim, Y.K.; Rhee, S.J.; Lee, E.M.; Lee, J.; Yoo, N.J.; et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ. J. 2007, 71, 536–539. [Google Scholar] [CrossRef] [Green Version]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Couselo-Seijas, M.; Agra-Bermejo, R.M.; Fernández, A.L.; Martínez-Cereijo, J.M.; Sierra, J.; Soto-Pérez, M.; Rozados-Luis, A.; González-Juanatey, J.R.; Eiras, S. High released lactate by epicardial fat from coronary artery disease patients is reduced by dapagliflozin treatment. Atherosclerosis 2020, 292, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Rodríguez, E.; Agra, R.M.; Fernández, Á.L.; Adrio, B.; García-Caballero, T.; González-Juanatey, J.R.; Eiras, S. Effects of dapagliflozin on human epicardial adipose tissue: Modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc. Res. 2018, 114, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Bernd, M.; Fuchs, L.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin Inhibits Basal and IL-1β-Mediated MCP-1/CCL2 and Endothelin-1 Expression in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2020, 21, 8189. [Google Scholar] [CrossRef]
- Tahara, A.; Kondo, Y.; Takasu, T.; Tomiyama, H. Effects of the SGLT2 inhibitor ipragliflozin on food intake, appetite-regulating hormones, and arteriovenous differences in postprandial glucose levels in type 2 diabetic rats. Biomed. Pharmacother. 2018, 105, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Elrick, H.; Stimmler, L.; Hlad, C.J., Jr.; Arai, Y. Plasma insulin response to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995, 358, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.; Vedtofte, L.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011, 60, 3103–3109. [Google Scholar] [CrossRef] [Green Version]
- Fisman, E.Z.; Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: A novel cardiometabolic therapeutic prospect. Cardiovasc. Diabetol. 2021, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Frandsen, K.B.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, L.A.; Mankad, S.; Sokos, G.G.; Miske, G.; Shah, A.; Elahi, D.; Shannon, R.P. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004, 109, 962–965. [Google Scholar] [CrossRef] [Green Version]
- Sokos, G.G.; Nikolaidis, L.A.; Mankad, S.; Elahi, D.; Shannon, R.P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Fail. 2006, 12, 694–699. [Google Scholar] [CrossRef]
- Thrainsdottir, I.; Malmberg, K.; Olsson, A.; Gutniak, M.; Rydén, L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diabetes Vasc. Dis. Res. 2004, 1, 40–43. [Google Scholar] [CrossRef]
- Bizino, M.B.; Jazet, I.M.; Westenberg, J.J.M.; van Eyk, H.J.; Paiman, E.H.M.; Smit, J.W.A.; Lamb, H.J. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: Randomized placebo-controlled trial. Cardiovasc. Diabetol. 2019, 18, 55. [Google Scholar] [CrossRef] [Green Version]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withaar, C.; Meems, L.M.G.; Markousis-Mavrogenis, G.; Boogerd, C.J.; Silljé, H.H.W.; Schouten, E.M.; Dokter, M.M.; Voors, A.A.; Westenbrink, B.D.; Lam, C.S.P.; et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Tian, H.; Wang, Y.; Lu, W.; Li, Y.; Ma, T.; Gao, X.; Yao, W. A novel oral glucagon-like peptide 1 receptor agonist protects against diabetic cardiomyopathy via alleviating cardiac lipotoxicity induced mitochondria dysfunction. Biochem. Pharmacol. 2020, 182, 114209. [Google Scholar] [CrossRef] [PubMed]
- Morano, S.; Romagnoli, E.; Filardi, T.; Nieddu, L.; Mandosi, E.; Fallarino, M.; Turinese, I.; Dagostino, M.P.; Lenzi, A.; Carnevale, V. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: An ultrasonography study. Acta Diabetol. 2015, 52, 727–732. [Google Scholar] [CrossRef]
- Bizino, M.B.; Jazet, I.M.; de Heer, P.; van Eyk, H.J.; Dekkers, I.A.; Rensen, P.C.N.; Paiman, E.H.M.; Lamb, H.J.; Smit, J.W. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: A pre-specified secondary study on ectopic fat accumulation. Diabetologia 2020, 63, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Iacobellis, G.; Camarena, V.; Sant, D.W.; Wang, G. Human Epicardial Fat Expresses Glucagon-Like Peptide 1 and 2 Receptors Genes. Horm. Metab. Res. 2017, 49, 625–630. [Google Scholar] [CrossRef]
- Dozio, E.; Vianello, E.; Malavazos, A.E.; Tacchini, L.; Schmitz, G.; Iacobellis, G.; Corsi Romanelli, M.M. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: A target to modulate cardiovascular risk? Int. J. Cardiol. 2019, 292, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Dokken, B.B.; La Bonte, L.R.; Davis-Gorman, G.; Teachey, M.K.; Seaver, N.; McDonagh, P.F. Glucagon-like peptide-1 (GLP-1), immediately prior to reperfusion, decreases neutrophil activation and reduces myocardial infarct size in rodents. Horm. Metab. Res. 2011, 43, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D.; Salter, B.M.; Oliveria, J.P.; El-Gammal, A.; Tworek, D.; Smith, S.G.; Sehmi, R.; Gauvreau, G.M.; Butler, M.; O’Byrne, P.M. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin. Exp. Allergy 2017, 47, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Moschovaki Filippidou, F.; Kirsch, A.H.; Thelen, M.; Kétszeri, M.; Artinger, K.; Aringer, I.; Schabhüttl, C.; Mooslechner, A.A.; Frauscher, B.; Pollheimer, M.; et al. Glucagon-Like Peptide-1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T-Cell Proliferation. Am. J. Pathol. 2020, 190, 400–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Vega, D.; González-Juanatey, J.R.; Eiras, S. Diabesity in Elderly Cardiovascular Disease Patients: Mechanisms and Regulators. Int. J. Mol. Sci. 2022, 23, 7886. https://doi.org/10.3390/ijms23147886
García-Vega D, González-Juanatey JR, Eiras S. Diabesity in Elderly Cardiovascular Disease Patients: Mechanisms and Regulators. International Journal of Molecular Sciences. 2022; 23(14):7886. https://doi.org/10.3390/ijms23147886
Chicago/Turabian StyleGarcía-Vega, David, José Ramón González-Juanatey, and Sonia Eiras. 2022. "Diabesity in Elderly Cardiovascular Disease Patients: Mechanisms and Regulators" International Journal of Molecular Sciences 23, no. 14: 7886. https://doi.org/10.3390/ijms23147886
APA StyleGarcía-Vega, D., González-Juanatey, J. R., & Eiras, S. (2022). Diabesity in Elderly Cardiovascular Disease Patients: Mechanisms and Regulators. International Journal of Molecular Sciences, 23(14), 7886. https://doi.org/10.3390/ijms23147886






