Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen?
Abstract
:1. Introduction
2. Results
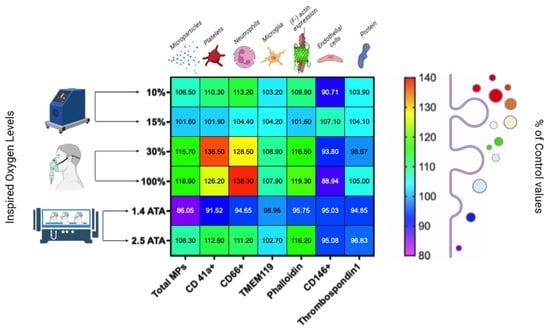
2.1. Microparticles Elicited after One Hour of Different Oxygen Exposures
2.2. Microparticles Expressing Proteins from Platelets, Neutrophils, Endothelial Cells, and Microglia after One Hour of Different Oxygen Exposures
2.3. Microparticles Expressing Proteins from Cell Activation after One Hour of Different Oxygen Exposures
2.4. Percentual and Absolute Changes of Microparticles Expressing Proteins after One Hour of Different Oxygen Exposures
3. Discussion
4. Materials and Methods
4.1. Experimental Protocol
4.2. Blood Sampling and Laboratory Procedure
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Blood Borne Microparticles |
| OPB | Oxygen Pre-Breathing |
| VGE | Vascular Gas Emboli |
| DCS | Decompression Sickness |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NRF2 | Nuclear Factor Erythroid 2 Related–Factor 2 |
| PO2 | Oxygen Partial Pressure |
| TSP | Thrombospondin 1 |
| NF-E2 | Nuclear Factor, Erythroid 2 |
| HIFs | Hypoxia Inducible Factors |
References
- Nakane, M. Biological effects of the oxygen molecule in critically ill patients. J. Intensive Care 2020, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Girault, C.; Boyer, D.; Jolly, G.; Carpentier, D.; Béduneau, G.; Frat, J.P. Operating principles, physiological effects and practical issues of high-flow nasal oxygen therapy. Rev. Mal. Respir. 2022, 39, 455–468. [Google Scholar] [CrossRef] [PubMed]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb. Med. 2017, 47, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Lafere, P.; Schubert, T.; De Bels, D.; Germonpre, P.; Balestra, C. Can the normobaric oxygen paradox (NOP) increase reticulocyte count after traumatic hip surgery? J. Clin. Anesth. 2013, 25, 129–134. [Google Scholar] [CrossRef] [Green Version]
- De Bels, D.; Corazza, F.; Germonpre, P.; Balestra, C. The normobaric oxygen paradox: A novel way to administer oxygen as an adjuvant treatment for cancer? Med. Hypotheses 2011, 76, 467–470. [Google Scholar] [CrossRef]
- Burk, R. Oxygen breathing may be a cheaper and safer alternative to exogenous erythropoietin (EPO). Med. Hypotheses 2007, 69, 1200–1204. [Google Scholar] [CrossRef]
- Balestra, C.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Levenez, M.; Germonpre, P.; Virgili, F.; Bosco, G.; Lafere, P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. Int. J. Mol. Sci. 2021, 22, 9600. [Google Scholar] [CrossRef]
- Bestavashvili, A.; Glazachev, O.; Bestavashvili, A.; Suvorov, A.; Zhang, Y.; Zhang, X.; Rozhkov, A.; Kuznetsova, N.; Pavlov, C.; Glushenkov, D.; et al. Intermittent Hypoxic-Hyperoxic Exposures Effects in Patients with Metabolic Syndrome: Correction of Cardiovascular and Metabolic Profile. Biomedicines 2022, 10, 566. [Google Scholar] [CrossRef]
- Matta, A.; Nader, V.; Lebrin, M.; Gross, F.; Prats, A.C.; Cussac, D.; Galinier, M.; Roncalli, J. Pre-Conditioning Methods and Novel Approaches with Mesenchymal Stem Cells Therapy in Cardiovascular Disease. Cells 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Lafère, P.; Lambrechts, K.; Germonpré, P.; Balestra, A.; Germonpré, F.L.; Marroni, A.; Cialoni, D.; Bosco, G.; Balestra, C. Heart Rate Variability During a Standard Dive: A Role for Inspired Oxygen Pressure? Front. Physiol. 2021, 12, 635132. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Theunissen, S.; Papadopoulou, V.; Le Mener, C.; Germonpré, P.; Guerrero, F.; Lafère, P. Pre-dive Whole-Body Vibration Better Reduces Decompression-Induced Vascular Gas Emboli than Oxygenation or a Combination of Both. Front. Physiol. 2016, 7, 586. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.T.; Pilmanis, A.A. Fifty years of decompression sickness research at Brooks AFB, TX: 1960–2010. Aviat. Space Environ. Med. 2011, 82, A1–A25. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, P.; Sushree, S.K.; Agarwal, A. Aeromedical Concerns and Lessons Learned during Oxygen Jump at Dolma Sampa. IJASM Indian J. Aerosp. Med. 2018, 62, 16–20. [Google Scholar]
- Kluis, L.; Diaz-Artiles, A. Revisiting decompression sickness risk and mobility in the context of the SmartSuit, a hybrid planetary spacesuit. NPJ Microgravity 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.H., 3rd; Schaefer, C.M.; Thompson, M.S.; Norcross, J.R.; Bekdash, O.S. Retrospective Evaluation of Clinical Symptoms Due to Mild Hypobaric Hypoxia Exposure in Microgravity. Aerosp. Med. Hum. Perform. 2018, 89, 792–797. [Google Scholar] [CrossRef]
- Imbert, J.P.; Egi, S.M.; Germonpre, P.; Balestra, C. Static Metabolic Bubbles as Precursors of Vascular Gas Emboli During Divers’ Decompression: A Hypothesis Explaining Bubbling Variability. Front. Physiol. 2019, 10, 807. [Google Scholar] [CrossRef] [Green Version]
- Mollerlokken, A.; Gaustad, S.E.; Havnes, M.B.; Gutvik, C.R.; Hjelde, A.; Wisloff, U.; Brubakk, A.O. Venous gas embolism as a predictive tool for improving CNS decompression safety. Eur. J. Appl. Physiol. 2012, 112, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Ånell, R.; Grönkvist, M.; Gennser, M.; Eiken, O. High-altitude decompression strain can be reduced by an early excursion to moderate altitude while breathing oxygen. Eur. J. Appl. Physiol. 2021, 121, 3225–3232. [Google Scholar] [CrossRef]
- Webb, J.T.; Pilmanis, A.A. Breathing 100% oxygen compared with 50% oxygen: 50% nitrogen reduces altitude-induced venous gas emboli. Aviat. Space Environ. Med. 1993, 64, 808–812. [Google Scholar] [PubMed]
- Webb, J.T.; Fischer, M.D.; Heaps, C.L.; Pilmanis, A.A. Exercise-enhanced preoxygenation increases protection from decompression sickness. Aviat. Space Environ. Med. 1996, 67, 618–624. [Google Scholar] [PubMed]
- Arieli, R.; Boaron, E.; Abramovich, A. Combined effect of denucleation and denitrogenation on the risk of decompression sickness in rats. J. Appl. Physiol. 2009, 106, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Arieli, Y.; Katsenelson, K.; Arieli, R. Bubble reduction after decompression in the prawn Palaemon elegans by pretreatment with hyperbaric oxygen. Undersea Hyperb. Med. 2007, 34, 369–378. [Google Scholar] [PubMed]
- Lambrechts, K.; Germonpre, P.; Vandenheede, J.; Delorme, M.; Lafere, P.; Balestra, C. Mini Trampoline, a New and Promising Way of SCUBA Diving Preconditioning to Reduce Vascular Gas Emboli? Int. J. Environ. Res. Public Health 2022, 19, 5410. [Google Scholar] [CrossRef]
- Thom, S.R.; Bennett, M.; Banham, N.D.; Chin, W.; Blake, D.F.; Rosen, A.; Pollock, N.W.; Madden, D.; Barak, O.; Marroni, A.; et al. Association of microparticles and neutrophil activation with decompression sickness. J. Appl. Physiol. 2015, 119, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Yang, M.; Bhopale, V.M.; Huang, S.; Milovanova, T.N. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J. Appl. Physiol. 2011, 110, 340–351. [Google Scholar] [CrossRef]
- Vince, R.V.; McNaughton, L.R.; Taylor, L.; Midgley, A.W.; Laden, G.; Madden, L.A. Release of VCAM-1 associated endothelial microparticles following simulated SCUBA dives. Eur. J. Appl. Physiol. 2009, 105, 507–513. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Yu, K.; Yang, M. Provocative decompression causes diffuse vascular injury in mice mediated by microparticles containing interleukin-1beta. J. Appl. Physiol. 2018, 125, 1339–1348. [Google Scholar] [CrossRef]
- Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Bhopale, V.M.; Yang, M.; Thom, S.R. Microparticle and interleukin-1beta production with human simulated compressed air diving. Sci. Rep. 2019, 9, 13320. [Google Scholar] [CrossRef] [Green Version]
- Pontier, J.M.; Gempp, E.; Ignatescu, M. Blood platelet-derived microparticles release and bubble formation after an open-sea air dive. Appl. Physiol. Nutr. Metab. 2012, 37, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, J.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Extracellular vesicles as modulators of wound healing. Adv. Drug. Deliv. Rev. 2018, 129, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Slater, T.W.; Finkielsztein, A.; Mascarenhas, L.A.; Mehl, L.C.; Butin-Israeli, V.; Sumagin, R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J. Immunol. 2017, 198, 2886–2897. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Yang, M.; Bhopale, V.M.; Milovanova, T.N.; Bogush, M.; Buerk, D.G. Intramicroparticle nitrogen dioxide is a bubble nucleation site leading to decompression-induced neutrophil activation and vascular injury. J. Appl. Physiol. 2013, 114, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Eftedal, I.; Ljubkovic, M.; Flatberg, A.; Jorgensen, A.; Brubakk, A.O.; Dujic, Z. Acute and potentially persistent effects of scuba diving on the blood transcriptome of experienced divers. Physiol. Genom. 2013, 45, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.D.; Sureda, A.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free Radic. Res. 2007, 41, 274–281. [Google Scholar] [CrossRef]
- Morabito, C.; Bosco, G.; Pilla, R.; Corona, C.; Mancinelli, R.; Yang, Z.; Camporesi, E.M.; Fano, G.; Mariggio, M.A. Effect of pre-breathing oxygen at different depth on oxidative status and calcium concentration in lymphocytes of scuba divers. Acta Physiol. Oxf. 2011, 202, 69–78. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Capo, X.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014, 46, 647–654. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Ferrer, M.D.; Mestre-Alfaro, A.; Tur, J.A.; Pons, A. Scuba diving activates vascular antioxidant system. Int. J. Sports Med. 2012, 33, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving increases erythrocyte and plasma antioxidant defenses and spares NO without oxidative damage. Med. Sci. Sports Exerc. 2009, 41, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Madden, D.; Thom, S.R.; Milovanova, T.N.; Yang, M.; Bhopale, V.M.; Ljubkovic, M.; Dujic, Z. Exercise before scuba diving ameliorates decompression-induced neutrophil activation. Med. Sci. Sports Exerc. 2014, 46, 1928–1935. [Google Scholar] [CrossRef]
- Madden, D.; Thom, S.R.; Yang, M.; Bhopale, V.M.; Ljubkovic, M.; Dujic, Z. High intensity cycling before SCUBA diving reduces post-decompression microparticle production and neutrophil activation. Eur. J. Appl. Physiol. 2014, 114, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.A.; Chrismas, B.C.; Mellor, D.; Vince, R.V.; Midgley, A.W.; McNaughton, L.R.; Atkin, S.L.; Laden, G. Endothelial function and stress response after simulated dives to 18 msw breathing air or oxygen. Aviat. Space Environ. Med. 2010, 81, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Bhopale, V.M.; Yang, M.; Bushmann, K.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Dujic, Z. Microparticle production, neutrophil activation, and intravascular bubbles following open-water SCUBA diving. J. Appl. Physiol. 2012, 112, 1268–1278. [Google Scholar] [CrossRef]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Yang, M.; Bhopale, V.M.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Madden, D.; Lozo, M.; et al. Bubbles, microparticles, and neutrophil activation: Changes with exercise level and breathing gas during open-water SCUBA diving. J. Appl. Physiol. 2013, 114, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Bhopale, V.M.; Yang, M. Neutrophils generate microparticles during exposure to inert gases due to cytoskeletal oxidative stress. J. Biol. Chem. 2014, 289, 18831–18845. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Duan, R.; Sun, Y.; Li, Q. Hyperbaric oxygen therapy for healthy aging: From mechanisms to therapeutics. Redox Biol. 2022, 53, 102352. [Google Scholar] [CrossRef]
- Balestra, C.; Guerrero, F.; Theunissen, S.; Germonpre, P.; Lafere, P. Physiology of repeated mixed gas 100-m wreck dives using a closed-circuit rebreather: A field bubble study. Eur. J. Appl. Physiol. 2022, 122, 515–522. [Google Scholar] [CrossRef]
- Dugrenot, E.; Balestra, C.; Gouin, E.; L’Her, E.; Guerrero, F. Physiological effects of mixed-gas deep sea dives using a closed-circuit rebreather: A field pilot study. Eur. J. Appl. Physiol. 2021, 121, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guerrero, F.; Lambrechts, K.; Mazur, A.; Buzzacott, P.; Belhomme, M.; Theron, M. Simulated air dives induce superoxide, nitric oxide, peroxynitrite, and Ca2+ alterations in endothelial cells. J. Physiol. Biochem. 2020, 76, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bhopale, V.M.; Ruhela, D.; Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Levinson, S.L.; DiNubile, M.J.; Thom, S.R. Plasma gelsolin modulates the production and fate of IL-1beta-containing microparticles following high-pressure exposure and decompression. J. Appl. Physiol. 2021, 130, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Zhan, Y.J.; Yang, C.S.; Tzeng, S.F. Oxidative stress-induced attenuation of thrombospondin-1 expression in primary rat astrocytes. J. Cell. Biochem. 2011, 112, 59–70. [Google Scholar] [CrossRef]
- Novelli, E.M.; Kato, G.J.; Ragni, M.V.; Zhang, Y.; Hildesheim, M.E.; Nouraie, M.; Barge, S.; Meyer, M.P.; Hassett, A.C.; Gordeuk, V.R.; et al. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am. J. Hematol. 2012, 87, 326–330. [Google Scholar] [CrossRef]
- Yang, M.; Kosterin, P.; Salzberg, B.M.; Milovanova, T.N.; Bhopale, V.M.; Thom, S.R. Microparticles generated by decompression stress cause central nervous system injury manifested as neurohypophysial terminal action potential broadening. J. Appl. Physiol. 2013, 115, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Foster, P.P.; Pollock, N.W.; Conkin, J.; Dervay, J.P.; Caillot, N.; Chhikara, R.S.; Vann, R.D.; Butler, B.D.; Gernhardt, M.L. Protective mechanisms in hypobaric decompression. Aviat. Space Environ. Med. 2013, 84, 212–225. [Google Scholar] [CrossRef]
- Eckmann, D.M.; Zhang, J.; Lampe, J.; Ayyaswamy, P.S. Gas embolism and surfactant-based intervention: Implications for long-duration space-based activity. Ann. N. Y. Acad. Sci. 2006, 1077, 256–269. [Google Scholar] [CrossRef]
- Molenat, F.; Boussuges, A. Operation Everest III (Comex’97): Altitude-induced decompression sickness during a hypobaric chamber experiment: Necessity for circulating venous gas emboli monitoring for the investigators. Chest 2002, 121, 173–177. [Google Scholar] [CrossRef]
- Ross, J.; Duncan, M. Prebreathe protocol for extravehicular activity: Technical consultation report. NASA Eng. Saf. Cent. Tech. Consult. Rep. Doc. 2008, RP-05-91, 1–169. [Google Scholar]
- Arieli, R. Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease-An Overview. Front. Physiol. 2017, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Magri, K.; Eftedal, I.; Petroni Magri, V.; Matity, L.; Azzopardi, C.P.; Muscat, S.; Pace, N.P. Acute Effects on the Human Peripheral Blood Transcriptome of Decompression Sickness Secondary to Scuba Diving. Front. Physiol. 2021, 12, 660402. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Germonpré, P.; Poortmans, J.R.; Marroni, A. Serum erythropoietin levels in healthy humans after a short period of normobaric and hyperbaric oxygen breathing: The “normobaric oxygen paradox”. J. Appl. Physiol. 2006, 100, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimino, F.; Balestra, C.; Germonpré, P.; De Bels, D.; Tillmans, F.; Saija, A.; Speciale, A.; Virgili, F. Pulsed high oxygen induces a hypoxic-like response in human umbilical endothelial cells and in humans. J. Appl. Physiol. 2012, 113, 1684–1689. [Google Scholar] [CrossRef] [Green Version]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 866. [Google Scholar] [CrossRef]
- Ferraris, V.A. Microparticles: The good, the bad, and the ugly. J. Thorac. Cardiovasc. Surg. 2015, 149, 312–313. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Barres, B.A. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Bhopale, V.M.; Yang, M. Microparticle-induced vascular injury in mice following decompression is inhibited by hyperbaric oxygen: Effects on microparticles and interleukin-1beta. J. Appl. Physiol. 2019, 126, 1006–1014. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Bhopale, V.M.; Yu, K.; Huang, W.; Kane, M.A.; Margolis, D.J. Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. J. Biol. Chem. 2017, 292, 18312–18324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thom, S.R.; Hampton, M.; Troiano, M.A.; Mirza, Z.; Malay, D.S.; Shannon, S.; Jennato, N.B.; Donohue, C.M.; Hoffstad, O.; Woltereck, D.; et al. Measurements of CD34+/CD45-dim Stem Cells Predict Healing of Diabetic Neuropathic Wounds. Diabetes 2016, 65, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, J.W.; Parks, D.R.; Moore, W.A.; Herzenberg, L.A.; Herzenberg, L.A. New approaches to fluorescence compensation and visualization of FACS data. Clin. Immunol. 2004, 110, 277–283. [Google Scholar] [CrossRef] [PubMed]









| Exposition | Baseline | After 120 min | p Value | n |
|---|---|---|---|---|
| Extreme Hypoxia 10% (0.1 ATA) | MPs/μL | Mps/μL | Paired t Test | |
| Total MPs | 2241 ± 77.5 | 2388 ± 101.4 | <0.0001 **** | 6 |
| Thrombospondin 1 | 12 ± 0.9338 | 12.44 ± 0.9422 | 0.2862 | 6 |
| TMEM119 | 26.97 ± 0.4024 | 27.84 ± 0.8396 | 0.0480 * | 6 |
| CD 146+ | 33.11 ± 2.934 | 30.31 ± 2.329 | 0.0260 * | 6 |
| CD 41a+ | 4.5 ± 0.1117 | 4.962 ± 0.2962 | 0.0125 * | 6 |
| CD 66b+ | 13.56 ± 1.006 | 15.33 ± 0.9346 | <0.0001 **** | 6 |
| Phalloidin | 14.24 ± 0.6746 | 15.66 ± 0.9320 | 0.0007 *** | 6 |
| Moderate Hypoxia 15% (0.15 ATA) | ||||
| Total MPs | 2085 ± 79.27 | 2114 ± 80.54 | 0.6174 | 8 |
| Thrombospondin 1 | 18.28 ± 0.6066 | 19.03 ± 0.9983 | 0.0473 * | 8 |
| TMEM119 | 33.96 ± 0.4660 | 35.36 ± 1.141 | 0.0234 * | 8 |
| CD 146+ | 33.94 ± 0.5551 | 36.32 ±2.880 | 0.0156 * | 8 |
| CD 41a+ | 10.60 ± 1.136 | 10.64 ± 1.008 | 0.9582 | 8 |
| CD 66b+ | 22.26 ± 0.5924 | 23.20 ± 1.216 | 0.1563 | 8 |
| Phalloidin | 20.60 ± 0.2195 | 20.93 ± 0.1674 | 0.0315 * | 8 |
| Moderate Hyperoxia 30% (0.3 ATA) | ||||
| Total MPs | 1838 ± 123.2 | 2116 ± 68.66 | 0.0159 * | 6 |
| Thrombospondin 1 | 17.86 ± 1.4 | 17.55 ± 0.8321 | 0.0473 * | 6 |
| TMEM119 | 30.98 ± 2.081 | 33.60 ± 0.3714 | 0.0203 * | 6 |
| CD 146+ | 33.13 ± 2.933 | 30.94 ± 1.841 | 0.0472 * | 6 |
| CD 41a+ | 8.227 ± 0.7471 | 11.06 ± 1.192 | 0.0149 * | 6 |
| CD 66b+ | 17 ± 1.849 | 21.64 ± 0.5708 | 0.001 *** | 6 |
| Phalloidin | 17.91 ± 2.438 | 20.54 ± 0.2030 | 0.0356 * | 6 |
| Hyperoxia 100% (1 ATA) | ||||
| Total MPs | 1786 ± 118.0 | 2072 ± 56.29 | <0.0001 **** | 14 |
| Thrombospondin 1 | 17.16 ± 0.5448 | 18.01 ± 1.218 | 0.0266 * | 14 |
| TMEM119 | 30.80 ± 1.993 | 33.10 ± 0.5293 | 0.0031 ** | 14 |
| CD 146+ | 42.38 ± 12.31 | 35.32 ± 4.333 | 0.0785 | 14 |
| CD 41a+ | 7.751 ± 0.9495 | 9.552 ± 1.086 | 0.0035 ** | 14 |
| CD 66b+ | 14.85 ± 1.662 | 20.30 ± 1.723 | <0.0001 **** | 14 |
| Phalloidin | 16.74 ± 2.008 | 19.65 ± 0.7498 | 0.0023 ** | 14 |
| Hyperbaric Hyperoxia 1.4 ATA | ||||
| Total MPs | 2766.69 ± 80.74 | 2381.7 ± 156.3 | 0.0004 *** | 6 |
| Thrombospondin 1 | 11.82 ± 0.26 | 11.21 ± 0.4 | 0.0354 * | 6 |
| TMEM119 | 31.27 ± 0.35 | 30.94 ± 0.36 | 0.087 | 6 |
| CD 146+ | 22.62 ± 0.3 | 21.5 ± 0.32 | 0.0011 ** | 6 |
| CD 41a+ | 6.56 ± 0.40 | 5.6 ± 0.22 | 0.0336 ** | 6 |
| CD 66b+ | 17.65 ± 0.18 | 16.7 ± 0.4 | 0.0077 ** | 6 |
| Phalloidin | 16.86 +/− 0.22 | 16.15 +/− 0.09 | 0.001 *** | 6 |
| Hyperbaric Hyperoxia 2.5 ATA | ||||
| Total MPs | 1846 ± 128.8 | 1989 ± 44.05 | 0.0206 * | 8 |
| Thrombospondin 1 | 17.08 ± 2.048 | 16.37 ± 0.865 | 0.6406 | 8 |
| TMEM119 | 32.13 ± 0.445 | 32.98 ± 0.5293 | 0.0347 * | 8 |
| CD 146+ | 39.47 ± 4.985 | 36.88 ± 6.047 | 0.3828 | 8 |
| CD 41a+ | 8.114 ± 0.6390 | 9.109 ± 0.5885 | 0.0078 ** | 8 |
| CD 66b+ | 17.64 ± 0.8149 | 19.59 ± 1.327 | 0.0057 ** | 8 |
| Phalloidin | 16.80 ± 1.893 | 19.22 ± 0.4839 | 0.0149 * | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpré, P.; Lambrechts, K.; Lafère, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. https://doi.org/10.3390/ijms23147888
Balestra C, Arya AK, Leveque C, Virgili F, Germonpré P, Lambrechts K, Lafère P, Thom SR. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen? International Journal of Molecular Sciences. 2022; 23(14):7888. https://doi.org/10.3390/ijms23147888
Chicago/Turabian StyleBalestra, Costantino, Awadhesh K. Arya, Clément Leveque, Fabio Virgili, Peter Germonpré, Kate Lambrechts, Pierre Lafère, and Stephen R. Thom. 2022. "Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen?" International Journal of Molecular Sciences 23, no. 14: 7888. https://doi.org/10.3390/ijms23147888
APA StyleBalestra, C., Arya, A. K., Leveque, C., Virgili, F., Germonpré, P., Lambrechts, K., Lafère, P., & Thom, S. R. (2022). Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen? International Journal of Molecular Sciences, 23(14), 7888. https://doi.org/10.3390/ijms23147888